Bacterial Tomato Pathogen Ralstonia solanacearum Invasion Modulates Rhizosphere Compounds and Facilitates the Cascade Effect of Fungal Pathogen Fusarium solani
Abstract
1. Introduction
2. Material and Methods
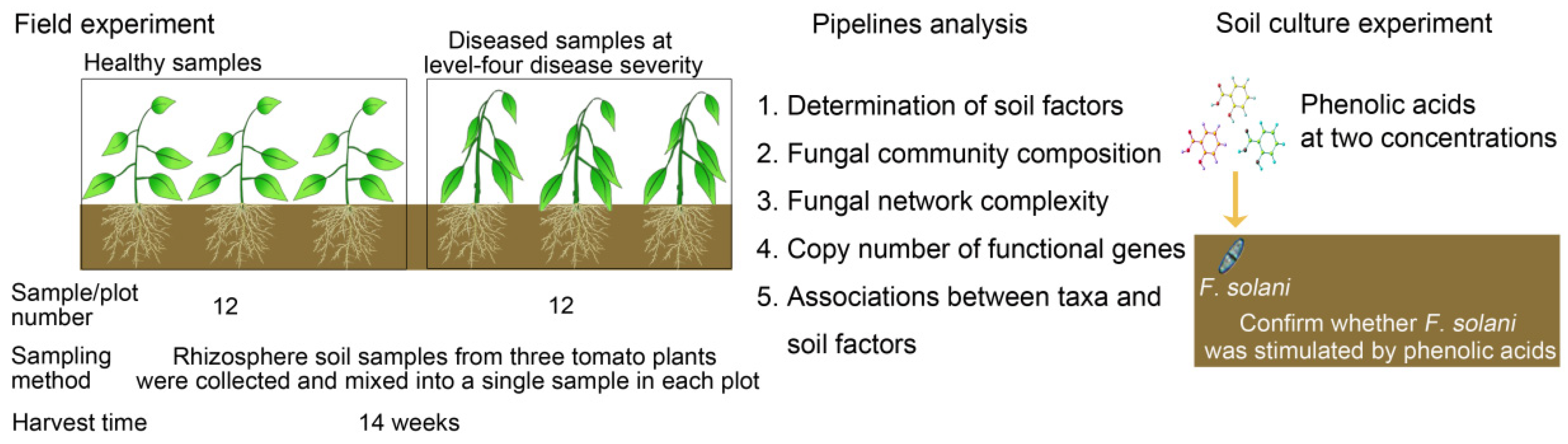
2.1. Experimental Field Site and Sampling Regime
2.2. Determination of Phenolic Acids in the Rhizosphere Soil
2.3. Determination of the Soil pH, Total Carbon and Nitrogen
2.4. DNA Extraction and Sequencing
2.5. Quantitative PCR
2.6. Statistical Analyses
3. Results
3.1. Identification of the Isolates from the Stem of Bacterial Wilt-Diseased Tomato Plant
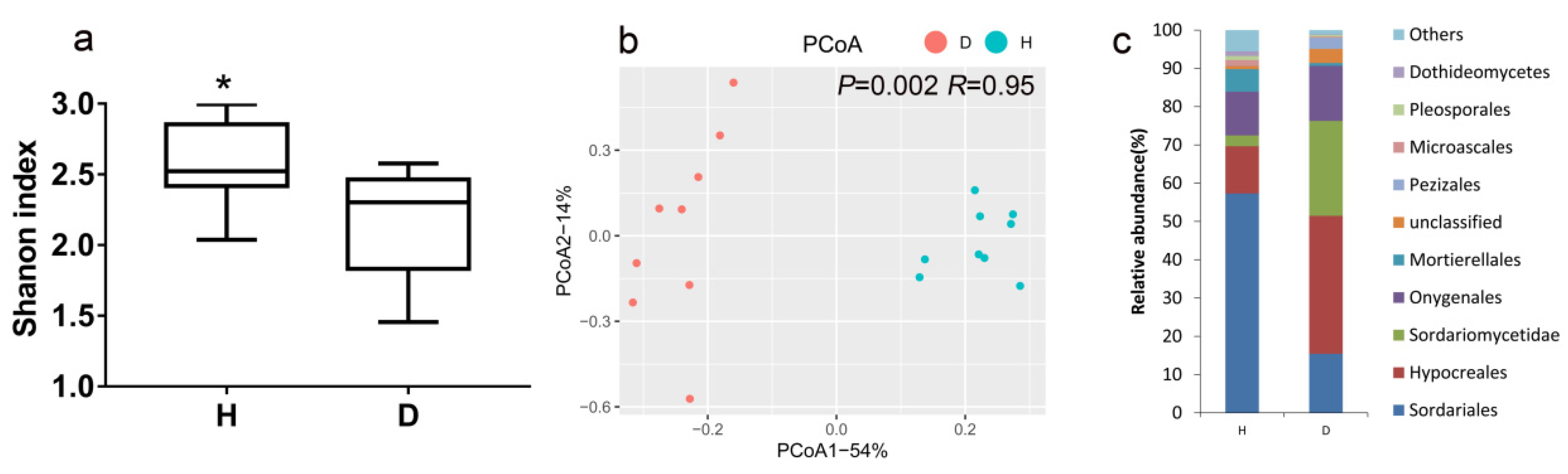
3.2. Fungal Community Diversity, Structure and Composition in Healthy and Bacterial Wilt-Diseased Tomato Plant Rhizospheres
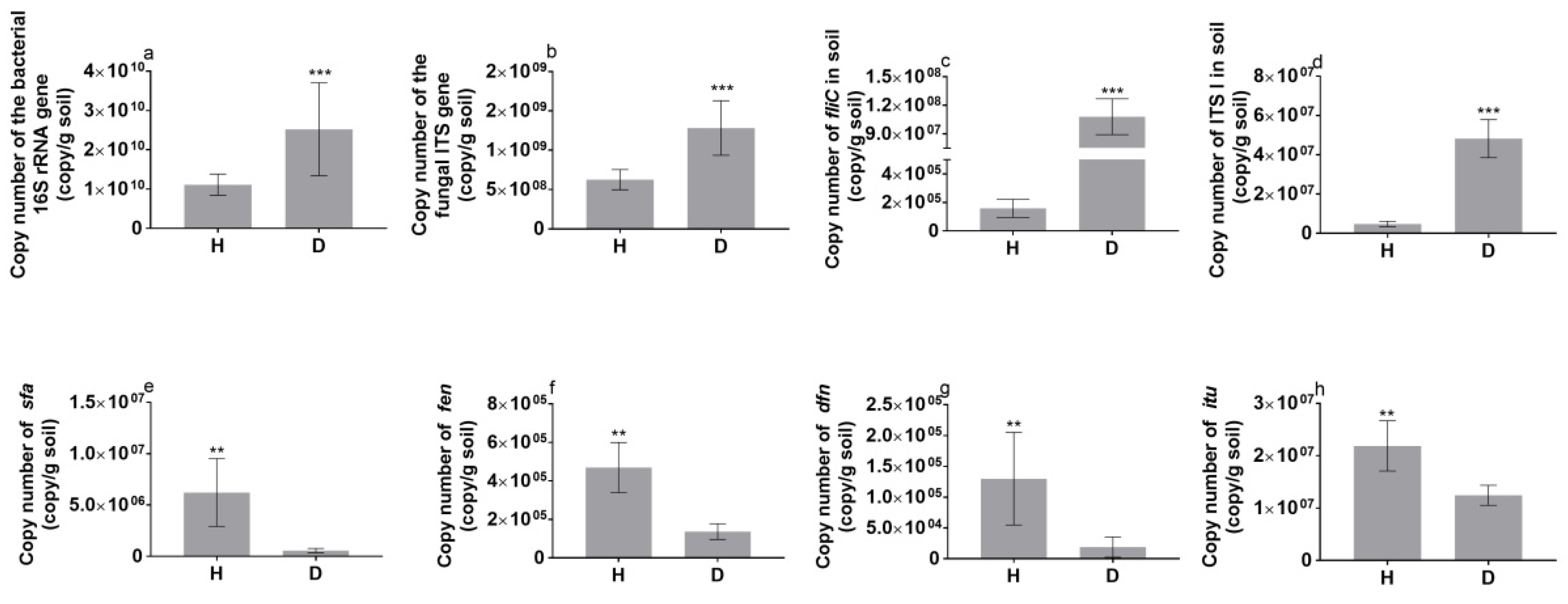
3.3. Functional Genes in the Tomato Rhizosphere
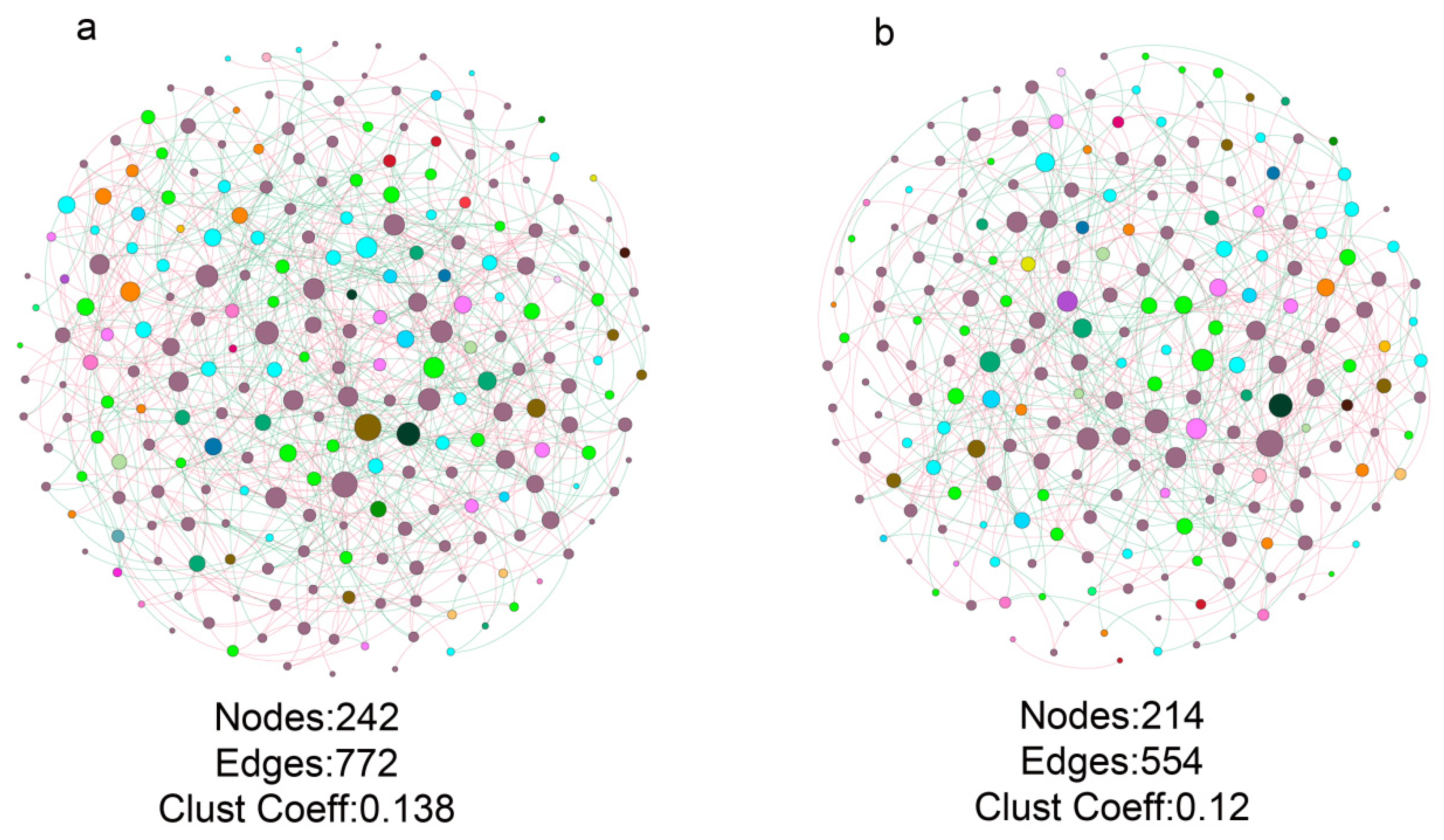
3.4. Fungal Co-Occurrence in Healthy and Bacterial Wilt-Diseased Tomato Plant Rhizospheres
3.5. Changes in Soil Factors in Healthy and Bacterial Wilt-Diseased Tomato Plant Rhizospheres
3.6. Identification of Soil Factors Determining Dominant OTUs
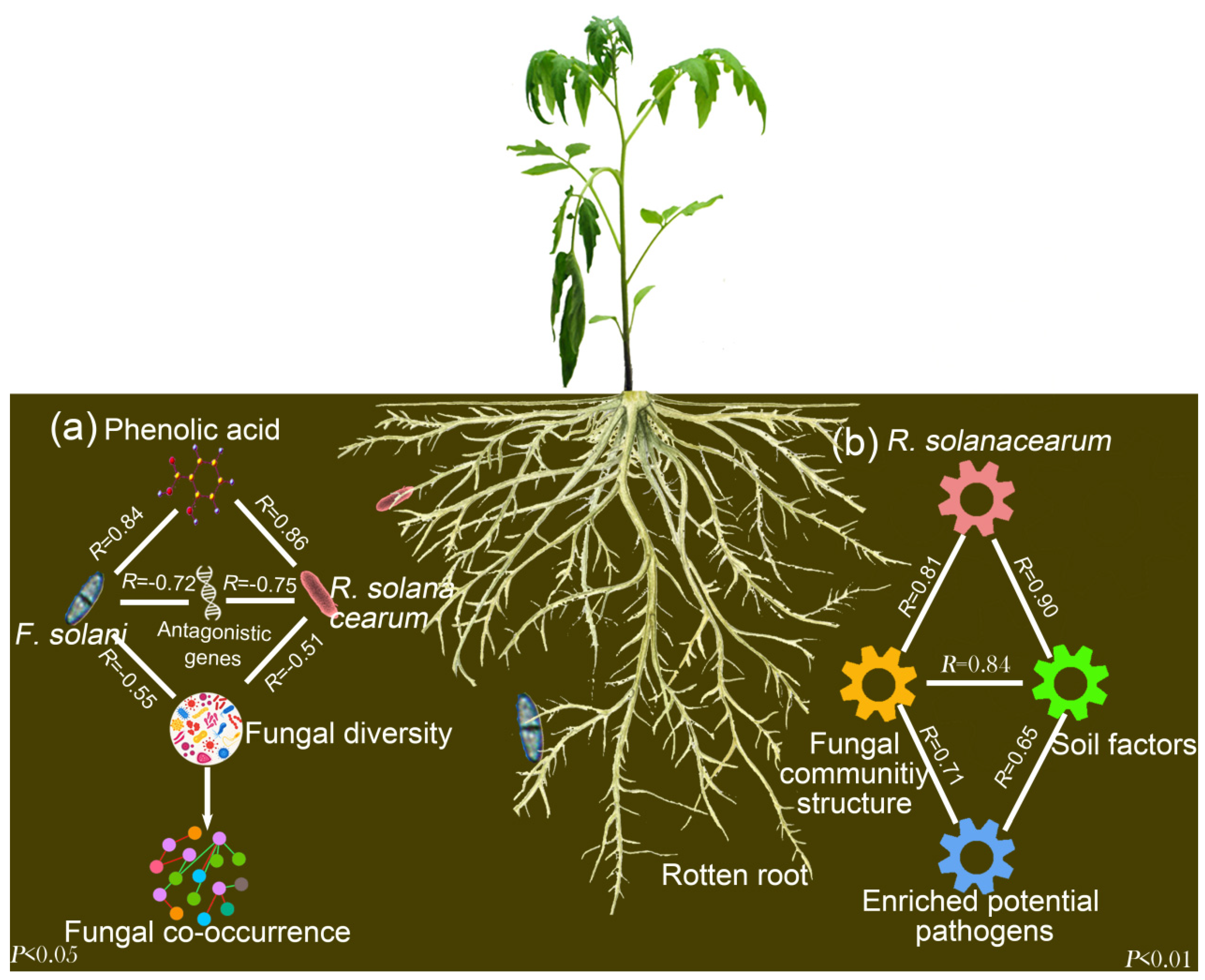
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huet, G. Breeding for resistances to Ralstonia solanacearum. Front. Plant. Sci. 2014, 5, 715. [Google Scholar] [CrossRef]
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas Solanacearum. Phytopathology 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Elphinstone, J.G.; Allen, C.; Prior, P.; Hayward, A.C. The current bacterial wilt situation: A global overview. In Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex; American Phytopathological Society Press: St. Paul, MN, USA, 2005. [Google Scholar]
- Wei, Z.; Hu, J.; Gu, Y.; Yin, S.; Xu, Y.; Jousset, A.; Shen, Q.; Friman, V.-P. Ralstonia solanacearum pathogen disrupts bacterial rhizosphere microbiome during an invasion. Soil Biol. Biochem. 2018, 118, 8–17. [Google Scholar] [CrossRef]
- Li, J.G.; Ren, G.D.; Jia, Z.J.; Dong, Y.H. Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes. Plant. Soil 2014, 380, 337–347. [Google Scholar] [CrossRef]
- Shenashen, M.; Derbalah, A.; Hamza, A.; Mohamed, A.; El Safty, S. Recent trend in controlling root rot disease of tomato caused by Fusarium solani using aluminasilica nanoparticles. Int. J. Adv. Res. Biol. Sci. 2017, 4, 105–119. [Google Scholar]
- Vawdrey, L.L.; Peterson, R.A. Fusarium solani, the cause of foot rot of tomatoes in Central Queensland. Australas. Plant. Pathol. 1988, 17, 24–25. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F. Effects of amendments of ferulic acid on soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.). Eur. J. Soil Biol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Cheng, Y.-X.; Ma, Y.-N.; Chen, C.-J.; Xu, F.-R.; Dong, X. Role of phenolic acids from the rhizosphere soils of panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules 2018, 23, 819. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Jacobs, J.M.; Ailloud, F.; Fochs, B.; Prior, P.; Allen, C. Degradation of the plant defense signal salicylic acid protects Ralstonia solanacearum from toxicity and enhances virulence on tobacco. MBio 2016, 7, e00656-16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhang, W.; Wang, R.; Qiu, Q.; Luo, F.; Hikichi, Y.; Ohnishi, K.; Ding, W. Ferulic acid, but not all hydroxycinnamic acids, is a novel T3SS inducer of Ralstonia solanacearum and promotes its infection process in host plants under hydroponic condition. Front. Plant. Sci. 2017, 8, 1595. [Google Scholar] [CrossRef]
- Steinkellner, S.; Mammerler, R.; Vierheilig, H. Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J. Plant. Interact. 2005, 1, 23–30. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F. p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f. sp. cucumerinum Owen. PLoS ONE 2012, 7, e48288. [Google Scholar]
- Li, P.; Wang, X.; Li, Y.; Wang, H.; Liang, F.; Dai, C. The contents of phenolic acids in continuous cropping peanut and their allelopathy. Acta Ecol. Sin. 2010, 30, 2128–2134. [Google Scholar]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; Van Der Heijden, M.G. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.; Babujee, L.; Allen, C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS ONE 2011, 6, e15853. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Rashid, H. Isolation and characterization of Ralstonia solanacearum from infected tomato plants of Soan Skesar valley of Punjab. Pak. J. Bot. 2011, 43, 2979–2985. [Google Scholar]
- Dalton, B.R.; Weed, S.B.; Blum, U. Plant phenolic acids in soils: A comparison of extraction procedures. Soil Sci. Soc. Am. J. 1987, 51, 1515–1521. [Google Scholar] [CrossRef]
- Hao, W.-Y.; Ren, L.-X.; Ran, W.; Shen, Q.-R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant. Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Jia, Z.; Zhang, T.; Wang, X. Effect of benzoic acid on soil microbial communities associated with soilborne peanut diseases. Appl. Soil Ecol. 2016, 110, 34–42. [Google Scholar] [CrossRef]
- Sun, B.; Dong, Z.X.; Zhang, X.X.; Li, Y.; Cao, H.; Cui, Z.L. Rice to vegetables: Short-versus long-term impact of land-use change on the indigenous soil microbial community. Microb. Ecol. 2011, 62, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Wang, Q.; Greenfield, P.; Charleston, M.A.; Porrasalfaro, A.; Kuske, C.R.; Cole, J.R.; Midgley, D.J.; Trandinh, N. Fungal identification using a Bayesian classifier and the Warcup training set of internal transcribed spacer sequences. Mycologia 2016, 108, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Khan, N.; Maymon, M.; Hirsch, A. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 2017, 5, 75. [Google Scholar] [CrossRef]
- Perrier, A.; Barlet, X.; Rengel, D.; Prior, P.; Poussier, S.; Genin, S.; Guidot, A. Spontaneous mutations in a regulatory gene induce phenotypic heterogeneity and adaptation of Ralstonia solanacearum to changing environments. Environ. Microbiol. 2019, 21, 3140–3152. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Hendrich, C.G.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.L.; Ricca, P.; Naidoo, J.; Cook, D. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Karim, N.F.A.; Mohd, M.; Nor, N.M.I.M.; Zakaria, L. Saprophytic and potentially pathogenic Fusarium species from peat soil in Perak and Pahang. Trop. Life Sci. Res. 2016, 27, 1. [Google Scholar]
- Sadasivan, T. Succession of fungi decomposing wheat straw in different soils, with special reference to Fusarium culmorum. Ann. Appl. Biol. 1939, 26, 497–508. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils 2018, 54, 363–372. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, Z.; Wang, X.; Friman, V.-P.; Huang, J.; Wang, X.; Mei, X.; Xu, Y.; Shen, Q.; Jousset, A. Pathogen invasion indirectly changes the composition of soil microbiome via shifts in root exudation profile. Biol. Fertil. Soils 2016, 52, 997–1005. [Google Scholar] [CrossRef]
- Ingelög, T.; Nohrstedt, H.Ö. Ammonia formation and soil pH increase caused by decomposing fruitbodies of macrofungi. Oecologia 1993, 93, 449–451. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.-F.; Liu, H.-Y.; Zhang, Y.-Q.; Yu, L.-Y. Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Wagg, C.; Dudenhöffer, J.H.; Widmer, F.; van der Heijden, M.G. Linking diversity, synchrony and stability in soil microbial communities. Funct. Ecol. 2018, 32, 1280–1292. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Der Heijden, M.G.A.V.; Hartmann, M. Networking in the plant microbiome. PLoS Biol. 2016, 14, e1002378. [Google Scholar] [CrossRef]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Tsai, S.M.; Mendes, L.W.; Faust, K.; de Hollander, M.; Cassman, N.A.; Raes, J.; van Veen, J.A.; Kuramae, E.E. Soil microbiome responses to the short-term effects of Amazonian deforestation. Mol. Ecol. 2015, 24, 2433–2448. [Google Scholar] [CrossRef]
- Eroshin, V.; Dedyukhina, E. Effect of lipids from Mortierella hygrophila on plant resistance to phytopathogens. World J. Microbiol. Biotechnol. 2002, 18, 165–167. [Google Scholar] [CrossRef]
- De Tender, C.; Mesuere, B.; Van der Jeugt, F.; Haegeman, A.; Ruttink, T.; Vandecasteele, B.; Dawyndt, P.; Debode, J.; Kuramae, E.E. Peat substrate amended with chitin modulates the N-cycle, siderophore and chitinase responses in the lettuce rhizobiome. Sci. Rep. 2019, 9, 9890. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Sato, H. Network hubs in root-associated fungal metacommunities. Microbiome 2018, 6, 116. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543. [Google Scholar] [CrossRef]
- Soytong, K.; Kanokmedhakul, S.; Kukongviriyapa, V.; Isobe, M. Application of Chaetomium species (Ketomium) as a new broad spectrum biological fungicide for plant disease control. Fungal Divers. 2001, 7, 1–15. [Google Scholar]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Chen, S.; Yu, H.; Zhou, X.; Wu, F. Cucumber (Cucumis sativus L.) seedling rhizosphere Trichoderma and Fusarium spp. communities altered by vanillic acid. Front. Microbiol. 2018, 9, 2195. [Google Scholar] [CrossRef]
- Huang, Y.; Busk, P.K.; Lange, L. Cellulose and hemicellulose-degrading enzymes in Fusarium commune transcriptome and functional characterization of three identified xylanases. Enzym. Microb. Technol. 2015, 73, 9–19. [Google Scholar] [CrossRef] [PubMed]

| Genus | Relative Abundance (%) | p | |
|---|---|---|---|
| H | B | ||
| Acremonium | 1.04 ± 0.74 | 0.15 ± 0.22 | 0.001 |
| Arthroderma | 3.1 ± 2.29 | 10.46 ± 9.97 | 0.041 |
| Chaetomium | 13.29 ± 10.32 | 4.34 ± 3.05 | 0.019 |
| Chrysosporium | 7.08 ± 3.5 | 0.52 ± 0.47 | 0.001 |
| Fusarium | 32.71 ± 12.81 | 8.93 ± 4.11 | 0.001 |
| Gibellulopsis | 0.58 ± 0.57 | 3.4 ± 2.31 | 0.004 |
| Humicola | 4.29 ± 3.94 | 0.29 ± 0.33 | 0.001 |
| Mortierella | 5.86 ± 6.73 | 0.73 ± 0.73 | 0.002 |
| Nectria | 0.2 ± 0.2 | 2.82 ± 3.18 | 0.002 |
| Phaeoacremonium | 1.02 ± 0.81 | 0.12 ± 0.17 | 0.002 |
| Plectosphaerella | 2.82 ± 4.04 | 24.82 ± 17.91 | 0.003 |
| Pseudogymnoascus | 1.32 ± 0.66 | 0.15 ± 0.12 | 0.001 |
| Sarocladium | 1.33 ± 1.39 | 0.18 ± 0.11 | 0.008 |
| Thielavia | 32.09 ± 8.37 | 3.12 ± 1.72 | 0.001 |
| Sample Group | p-hydroxybenzoic Acid (μg g−1) | Vanillic Acid (μg g−1) | Ferulic Acid (μg g−1) | pH | C (g kg−1) | N (g kg−1) |
|---|---|---|---|---|---|---|
| H | 10.95 ± 2.13 | 4.38 ± 1.16 | 7.7 ± 0.99 | 6.66 ± 0.22 | 11.86 ± 2.85 | 1.69 ± 0.15 |
| D | 45.24 ± 3.93 *** | 8.27 ± 1.95 *** | 37.18 ± 4.83 *** | 7.47 ± 0.21 *** | 21.5 ± 3.76 *** | 2.57 ± 0.26 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Zhang, L.; Nie, D.; Kuramae, E.E.; Shen, B.; Shen, Q. Bacterial Tomato Pathogen Ralstonia solanacearum Invasion Modulates Rhizosphere Compounds and Facilitates the Cascade Effect of Fungal Pathogen Fusarium solani. Microorganisms 2020, 8, 806. https://doi.org/10.3390/microorganisms8060806
Su L, Zhang L, Nie D, Kuramae EE, Shen B, Shen Q. Bacterial Tomato Pathogen Ralstonia solanacearum Invasion Modulates Rhizosphere Compounds and Facilitates the Cascade Effect of Fungal Pathogen Fusarium solani. Microorganisms. 2020; 8(6):806. https://doi.org/10.3390/microorganisms8060806
Chicago/Turabian StyleSu, Lv, Lifan Zhang, Duoqian Nie, Eiko E. Kuramae, Biao Shen, and Qirong Shen. 2020. "Bacterial Tomato Pathogen Ralstonia solanacearum Invasion Modulates Rhizosphere Compounds and Facilitates the Cascade Effect of Fungal Pathogen Fusarium solani" Microorganisms 8, no. 6: 806. https://doi.org/10.3390/microorganisms8060806
APA StyleSu, L., Zhang, L., Nie, D., Kuramae, E. E., Shen, B., & Shen, Q. (2020). Bacterial Tomato Pathogen Ralstonia solanacearum Invasion Modulates Rhizosphere Compounds and Facilitates the Cascade Effect of Fungal Pathogen Fusarium solani. Microorganisms, 8(6), 806. https://doi.org/10.3390/microorganisms8060806






