The First Step of Neurospora crassa Molybdenum Cofactor Biosynthesis: Regulatory Aspects under N-Derepressing and Nitrate-Inducing Conditions
Abstract
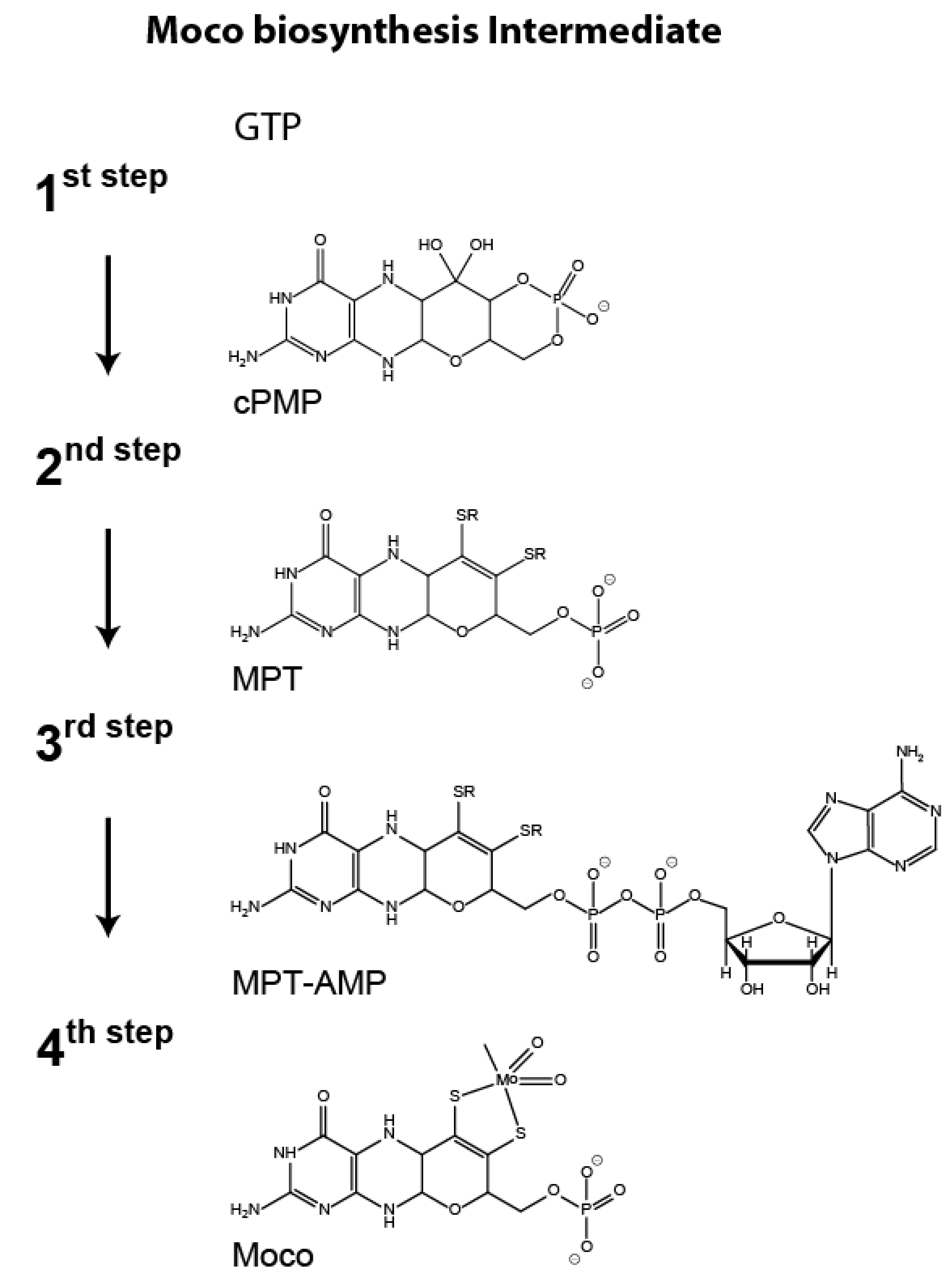
1. Introduction
2. Material and Methods
2.1. Neurospora crassa Strains and Growth Conditions
2.2. Transformation of Neurospora crassa
2.3. PCR-Based Cloning and Mutagenesis
2.4. RNA Extraction and cDNA Synthesis
2.5. Transcript Analysis
2.6. 5′ rapid amplification of cDNA ends
2.7. Protein and Molybdenum Cofactor Metabolites Extraction from N. crassa
2.8. Neurospora crassa Nitrate Reductase Activity Assay
2.9. Generation of Monoclonal Antibodies for NIT-7A and Nitrate Reductase
2.10. Detection of Enhanced Green Fluorescent Protein
2.11. Quantitative Detection of Molybdenum Cofactor and Its Metabolites
2.12. Bright Field Microscopy
2.13. Confocal Microscopy
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta 2012, 1823, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, E.I. The biogeochemistry of molybdenum and tungsten. Met. Ions Biol. Syst. 2002, 39, 1–29. [Google Scholar] [PubMed]
- Mendel, R.R. The molybdenum cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Teschner, J.; Lachmann, N.; Schulze, J.; Geisler, M.; Selbach, K.; Santamaria-Araujo, J.; Balk, J.; Mendel, R.R.; Bittner, F. A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis. Plant Cell 2010, 22, 468–480. [Google Scholar] [CrossRef]
- Reiss, J.; Christensen, E.; Kurlemann, G.; Zabot, M.T.; Dorche, C. Genomic structure and mutational spectrum of the bicistronic MOCS1 gene defective in molybdenum cofactor deficiency type A. Hum. Genet. 1998, 103, 639–644. [Google Scholar] [CrossRef]
- Reiss, J.; Cohen, N.; Dorche, C.; Mandel, H.; Mendel, R.R.; Stallmeyer, B.; Zabot, M.T.; Dierks, T. Mutations in a polycistronic nuclear gene associated with molybdenum cofactor deficiency. Nat. Genet. 1998, 20, 51–53. [Google Scholar] [CrossRef]
- Probst, C.; Ringel, P.; Boysen, V.; Wirsing, L.; Alexander, M.M.; Mendel, R.R.; Kruse, T. Genetic characterization of the Neurospora crassa molybdenum cofactor biosynthesis. Fungal Genet. Biol. 2014, 66, 69–78. [Google Scholar] [CrossRef]
- Hover, B.M.; Loksztejn, A.; Ribeiro, A.A.; Yokoyama, K. Identification of a cyclic nucleotide as a cryptic intermediate in molybdenum cofactor biosynthesis. J. Am. Chem. Soc. 2013, 135, 7019–7032. [Google Scholar] [CrossRef]
- Hover, B.M.; Tonthat, N.K.; Schumacher, M.A.; Yokoyama, K. Mechanism of pyranopterin ring formation in molybdenum cofactor biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 6347–6352. [Google Scholar] [CrossRef]
- Pitterle, D.M.; Johnson, J.L.; Rajagopalan, K.V. In vitro synthesis of molybdopterin from precursor Z using purified converting factor. Role of protein-bound sulfur in formation of the dithiolene. J. Biol. Chem. 1993, 268, 13506–13509. [Google Scholar]
- Johnson, M.E.; Rajagopalan, K.V. Involvement of chlA, E, M, and N loci in Escherichia coli molybdopterin biosynthesis. J. Bacteriol. 1987, 169, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Rajagopalan, K.V. In vitro system for molybdopterin biosynthesis. J. Bacteriol. 1987, 169, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Llamas, A.; Mendel, R.R.; Schwarz, G. Synthesis of adenylated molybdopterin: An essential step for molybdenum insertion. J. Biol. Chem. 2004, 279, 55241–55246. [Google Scholar] [CrossRef] [PubMed]
- Kuper, J.; Llamas, A.; Hecht, H.-J.; Mendel, R.R.; Schwarz, G. Structure of the molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism. Nature 2004, 430, 803–806. [Google Scholar] [CrossRef]
- Llamas, A.; Otte, T.; Multhaup, G.; Mendel, R.R.; Schwarz, G. The Mechanism of nucleotide-assisted molybdenum insertion into molybdopterin. A novel route toward metal cofactor assembly. J. Biol. Chem. 2006, 281, 18343–18350. [Google Scholar] [CrossRef]
- Wuebbens, M.M.; Rajagopalan, K.V. Structural characterization of a molybdopterin precursor. J. Biol. Chem. 1993, 268, 13493–13498. [Google Scholar]
- Leimkühler, S.; Wuebbens, M.M.; Rajagopalan, K.V. The history of the discovery of the molybdenum cofactor and novel aspects of its biosynthesis in bacteria. Coord. Chem. Rev. 2011, 255, 1129–1144. [Google Scholar] [CrossRef]
- Mendel, R.R.; Leimkühler, S. The biosynthesis of the molybdenum cofactors. JBIC J. Biol. Inorg. Chem. 2015, 20, 337–347. [Google Scholar] [CrossRef]
- Zupok, A.; Iobbi-Nivol, C.; Mejean, V.; Leimkuhler, S. The regulation of Moco biosynthesis and molybdoenzyme gene expression by molybdenum and iron in bacteria. Metallomics 2019, 11, 1602–1624. [Google Scholar] [CrossRef]
- Nason, A.; Evans, H.J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J. Biol. Chem. 1953, 202, 655–673. [Google Scholar]
- Marzluf, G.A. Metabolic Regulation in Fungi. Appl. Mycol. Biotechnol. 2001, 1, 55–72. [Google Scholar]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. A convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Tomsett, A.B.; Garrett, R.H. The isolation and characterization of mutants defective in nitrate assimilation in Neurospora crassa. Genetics 1980, 95, 649–660. [Google Scholar]
- Westergaard, M.; Mitchell, H.K.; Neurospora, V. A Synthetic Medium Favoring Sexual Reproduction. Am. J. Bot. 1947, 34, 573–577. [Google Scholar] [CrossRef]
- Davis, R.H.; de Serres, F.J. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970, 27, 79–143. [Google Scholar]
- Perkins, D.D.; Turner, B.C.; Pollard, V.C.; Fairfield, A. Neurospora strains incorporating fluffy, and their use as testers. Fungal Genet. Rep. 1989, 36, 64. [Google Scholar] [CrossRef][Green Version]
- White, B.; Woodward, D. A simple method for making disposable race tubes. Fungal Genet. Rep. 1995, 42, 79. [Google Scholar] [CrossRef]
- Chakraborty, B.N.; Kapoor, M. Transformation of filamentous fungi by electroporation. Nucleic Acids Res. 1990, 22, 6737. [Google Scholar] [CrossRef]
- Xia, Y.; Chu, W.; Qi, Q.; Xun, L. New insights into the QuikChangeTM process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 2015, 43, e12. [Google Scholar] [CrossRef]
- Wirsing, L.; Klawonn, F.; Sassen, W.A.; Lünsdorf, H.; Probst, C.; Hust, M.; Mendel, R.R.; Kruse, T.; Jänsch, L. Linear discriminant analysis identifies mitochondrially localized proteins in Neurospora crassa. J. Proteome Res. 2015, 14, 3900–3911. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.J.; Nason, A. The effect of reduced triphosphopyridine nucleotide on nitrate reduction by purified nitrate reductase. Arch. Biochem. Biophys. 1952, 39, 234–235. [Google Scholar] [CrossRef]
- Ringel, P.; Krausze, J.; Van Heuvel, J.D.; Curth, U.; Pierik, A.J.; Herzog, S.; Mendel, R.R.; Kruse, T. Biochemical characterization of molybdenum cofactor-free nitrate reductase from Neurospora crassa. J. Biol. Chem. 2013, 288, 14657–14671. [Google Scholar] [CrossRef] [PubMed]
- Zupok, A.; Gorka, M.; Siemiatkowska, B.; Skirycz, A.; Leimkühler, S. Iron-dependent regulation of molybdenum cofactor biosynthesis genes in Escherichia coli. J. Bacteriol. 2019, 201, e00382-19. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.; Eisenreich, W.; O’Brien, J.; Richter, G.; Gotze, E.; Boyle, P.; Blanchard, S.; Bacher, A.; Simon, H. Rearrangement reactions in the biosynthesis of molybdopterin an NMR study with multiply 13C/15N labelled precursors. Eur. J. Biochem. 1998, 255, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Hainline, B.E.; Rajagopalan, K.V.; Arison, B.H. The Pterin Component of the Molybdenum Cofactor—Structural Characterization of two Fluorescent Derivatives. J. Biol. Chem. 1984, 259, 5414–5422. [Google Scholar]
- Hercher, T.W.; Krausze, J.; Hoffmeister, S.; Zwerschke, D.; Lindel, T.; Blankenfeldt, W.; Mendel, R.-R.; Kruse, T. Insights into the Cnx1E catalyzed MPT-AMP hydrolysis. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Arenas, M.; Fairbanks, L.D.; Vijayakumar, K.; Carr, L.; Escuredo, E.; Marinaki, A.M. An unusual genetic variant in the MOCS1 gene leads to complete missplicing of an alternatively spliced exon in a patient with molybdenum cofactor deficiency. J. Inherit. Metab. Dis. 2009, 32, 560–569. [Google Scholar] [CrossRef]
- Gross-Hardt, S.; Reiss, J. The bicistronic MOCS1 gene has alternative start codons on two mutually exclusive exons. Mol. Genet. Metab. 2002, 76, 340–343. [Google Scholar] [CrossRef]
- Gray, T.A.; Nicholls, R.D. Diverse splicing mechanisms fuse the evolutionarily conserved bicistronic MOCS1A and MOCS1B open reading frames. RNA 2000, 6, 928–936. [Google Scholar] [CrossRef]
- Sorger, G.J.; Giles, N.H. Genetic control of nitrate reductase in Neurospora crassa. Genetics 1965, 52, 777–788. [Google Scholar] [PubMed]
- Reiss, J. Genetics of molybdenum cofactor deficiency. Hum. Genet. 2000, 2, 157–163. [Google Scholar] [CrossRef]
- Salamov, A.A.; Nishikawa, T.; Swindells, M.B. Assessing protein coding region integrity in cDNA sequencing projects. Bioinformatics 1998, 14, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.J.; Röper, J.; Schwarz, G. Alternative splicing of the bicistronic gene molybdenum cofactor synthesis 1 (MOCS1) uncovers a novel mitochondrial protein maturation mechanism. J. Biol. Chem. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.V. Novel aspects of the biochemistry of the molybdenum cofactor. Adv. Enzymol. Relat. Areas Mol. Biol. 1991, 64, 215–290. [Google Scholar]
- Kramer, S.P.; Johnson, J.L.; Ribeiro, A.A.; Millington, D.S.; Rajagopalan, K.V. The structure of the molybdenum cofactor. Characterization of di-(carboxamidomethyl) molybdopterin from sulfite oxidase and xanthine oxidase. J. Biol. Chem. 1987, 262, 16357–16363. [Google Scholar]
- Romao, M.J.; Archer, M.; Moura, I.; Moura, J.J.; LeGall, J.; Engh, R.; Schneider, M.; Hof, P.; Huber, R. Crystal structure of the xanthine oxidase-related aldehyde oxido- reductase from D. gigas. Science 1995, 270, 1170–1176. [Google Scholar] [CrossRef]
- Exley, G.E.; Colandene, J.D.; Garrett, R.H. Molecular cloning, characterization, and nucleotide sequence of nit-6, the structural gene for nitrite reductase in Neurospora crassa. J. Bacteriol. 1993, 175, 2379–2392. [Google Scholar] [CrossRef][Green Version]
- Gao-Rubinelli, F.; Marzluf, G.A. Identification and characterization of a nitrate transporter gene in Neurospora crassa. Biochem. Genet. 2004, 42, 21–34. [Google Scholar] [CrossRef]
- Pang, H.; Yokoyama, K. Lessons from the Studies of a C–C Bond Forming Radical SAM Enzyme in Molybdenum Cofactor Biosynthesis. Methods Enzymol. 2018, 606, 485–522. [Google Scholar]
- Reiss, J.; Hahnewald, R. Molybdenum cofactor deficiency: Mutations in GPHN, MOCS1, and MOCS2. Hum. Mutat. 2011, 32, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wainright, P.O.; Hinkle, G.; Sogin, M.L.; Stickel, S.K. Monophyletic origins of the metazoa: An evolutionary link with fungi. Science 1993, 260, 340–342. [Google Scholar] [CrossRef] [PubMed]







| Strain Number | Genotype | Origin |
|---|---|---|
| FGSC 2489 | 74-OR23-1V; mat A | FGSC |
| FGSC 4317 | fl; mat A | FGSC |
| FGSC 4347 | fl; mat a | FGSC |
| FGSC 9720 | his-3-; Δmus-52::bar+; mat A | FGSC |
| G200 (ATP-1-Preseq-mCherryNC) | his-3::Pccg1-atp1.1_120-mcherry; Δmus-52::bar+; mat A | Wirsing et al., 2015 |
| G253 | Δmus-52::bar+; Δnit-7::hph+; mat a | Probst et al., 2014 |
| G546 | his-3-; Δmus-52::bar+; Δnit-7::hph+; mat A | This study |
| G554 | his-3::nit-7+(EC); Δmus52::bar+; Δnit-7::hph+; mat A | This study |
| G555 | his-3::nit-7.1461T>C(EC); Δmus52::bar+; Δnit-7::hph+; mat A | This study |
| G557 | his-3::nit-7.1470T>C(EC); Δmus52::bar+; Δnit-7::hph+; mat A | This study |
| G552 (NIT-7A) | his-3::Pccg1-nit-7.1_1467; Δnit-7::hph+; Δmus-52::bar+; mat A | This study |
| G553 (NIT-7AB) | his-3::Pccg1-nit-7.1_1459+1697_2664; Δnit-7::hph+; Δmus-52::bar+; mat A | This study |
| G558 (NIT-7B) | his-3::Pccg1-nit-7.2104_2664; Δnit-7::hph+; Δmus-52::bar+; mat A | This study |
| G542 (NIT-7A-eGFP) | his-3::Pccg1-nit-7.1_1464-egfp; Δmus-52::bar+; mat A | This study |
| G544 (NIT-7AB-eGFP) | his-3::Pccg1-nit-7.1_1459+1697_2661-egfp; Δmus-52::bar+; mat A | This study |
| G550 (NIT-7B-eGFP) | his-3::Pccg1-nit-7.2104_2661-egfp; Δmus-52::bar+; mat A | This study |
| G575 (NIT-7A without mts-eGFP) | his-3::Pccg1-nit-7.163_1464-egfp; Δmus-52::bar+; mat A | This study |
| G576 (NIT-7AB without mts-eGFP) | his-3::Pccg1-nit-7.163_1459+1697_2661-egfp; Δmus-52::bar+; mat A | This study |
| G577 (Linker-NIT-7B-eGFP) | his-3::Pccg1-nit-7.1459+1697_2661-egfp; Δmus-52::bar+; mat A | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajmann, S.; Hercher, T.W.; Buchmeier, S.; Hänsch, R.; Mendel, R.R.; Kruse, T. The First Step of Neurospora crassa Molybdenum Cofactor Biosynthesis: Regulatory Aspects under N-Derepressing and Nitrate-Inducing Conditions. Microorganisms 2020, 8, 534. https://doi.org/10.3390/microorganisms8040534
Wajmann S, Hercher TW, Buchmeier S, Hänsch R, Mendel RR, Kruse T. The First Step of Neurospora crassa Molybdenum Cofactor Biosynthesis: Regulatory Aspects under N-Derepressing and Nitrate-Inducing Conditions. Microorganisms. 2020; 8(4):534. https://doi.org/10.3390/microorganisms8040534
Chicago/Turabian StyleWajmann, Simon, Thomas W. Hercher, Sabine Buchmeier, Robert Hänsch, Ralf R. Mendel, and Tobias Kruse. 2020. "The First Step of Neurospora crassa Molybdenum Cofactor Biosynthesis: Regulatory Aspects under N-Derepressing and Nitrate-Inducing Conditions" Microorganisms 8, no. 4: 534. https://doi.org/10.3390/microorganisms8040534
APA StyleWajmann, S., Hercher, T. W., Buchmeier, S., Hänsch, R., Mendel, R. R., & Kruse, T. (2020). The First Step of Neurospora crassa Molybdenum Cofactor Biosynthesis: Regulatory Aspects under N-Derepressing and Nitrate-Inducing Conditions. Microorganisms, 8(4), 534. https://doi.org/10.3390/microorganisms8040534






