Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs and Feeding
2.2. Experimental Design
2.3. Samples
2.4. Measures
2.4.1. Feces Characteristics
2.4.2. Blood Parameters
2.4.3. Fecal Concentrations of SCFA and BCFA
2.4.4. Microbiota Analysis
2.4.5. Metabolomic Analysis
2.5. Statistical Analysis
2.5.1. Univariate Analysis
2.5.2. Amplicon Sequencing
2.5.3. Global Relationships between Microbiota Composition and Activity, and Phenotypic Variables
3. Results
3.1. Effects of Dietary Protein Level and Prebiotic Supplementation on Host Phenotypic Parameters
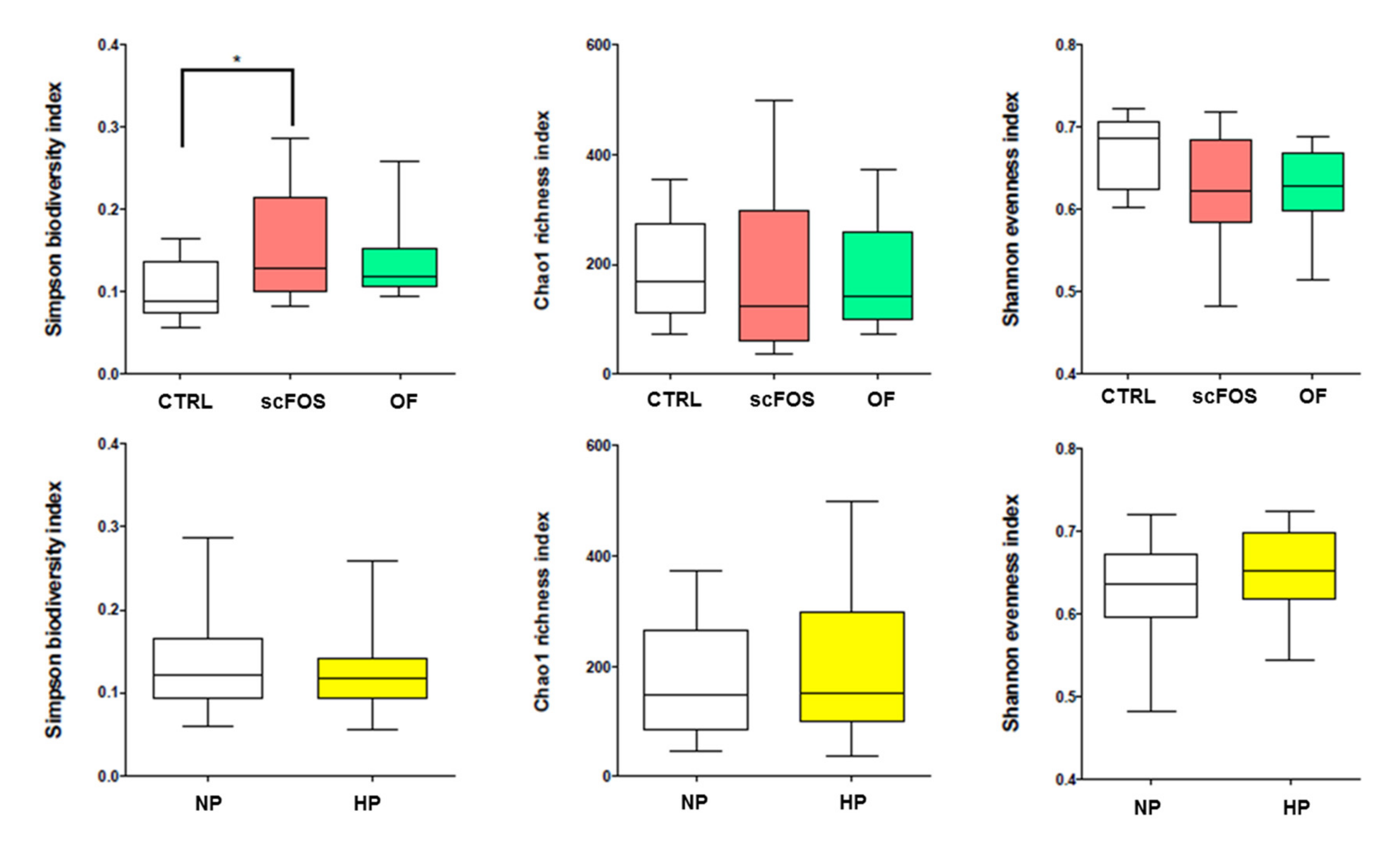
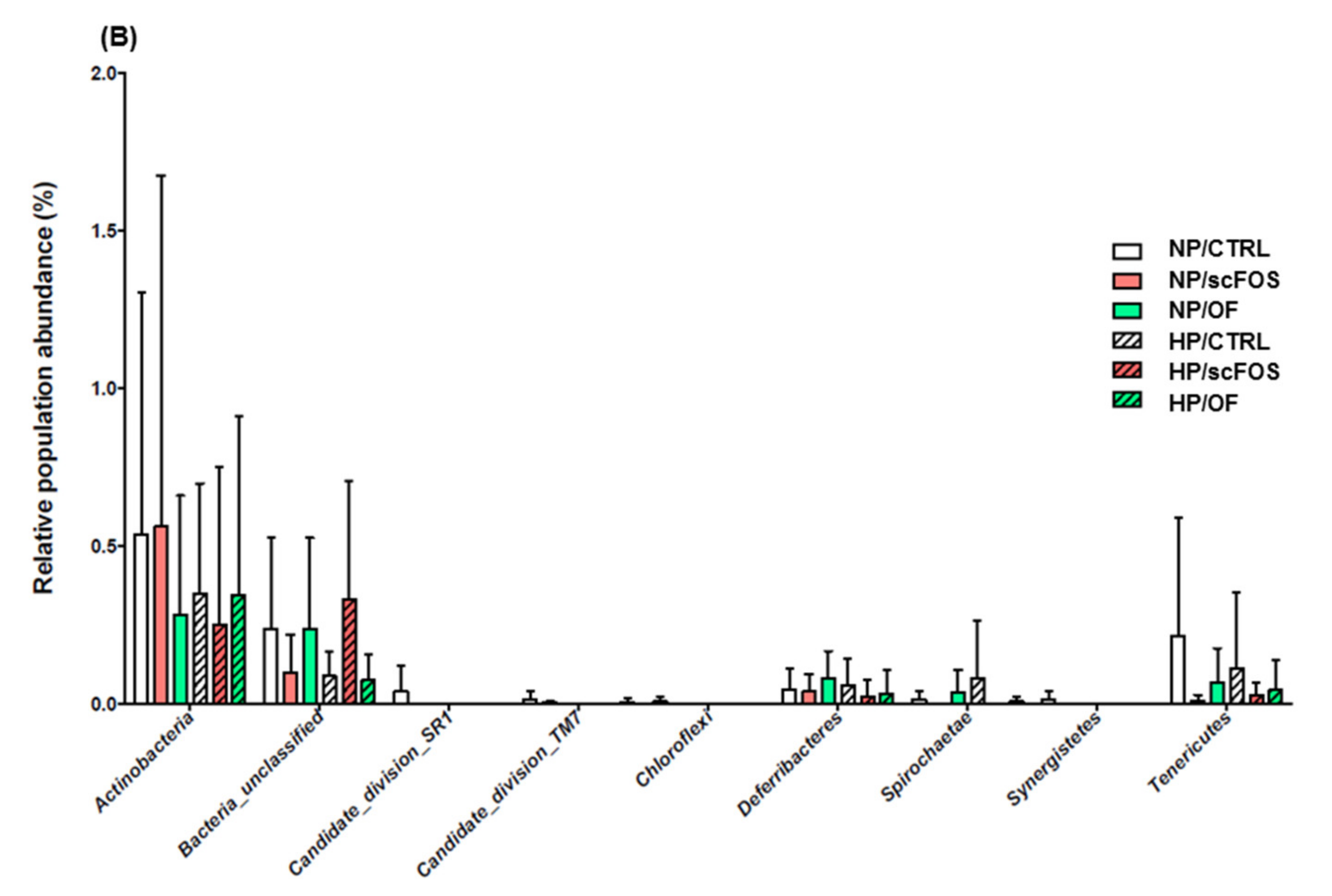
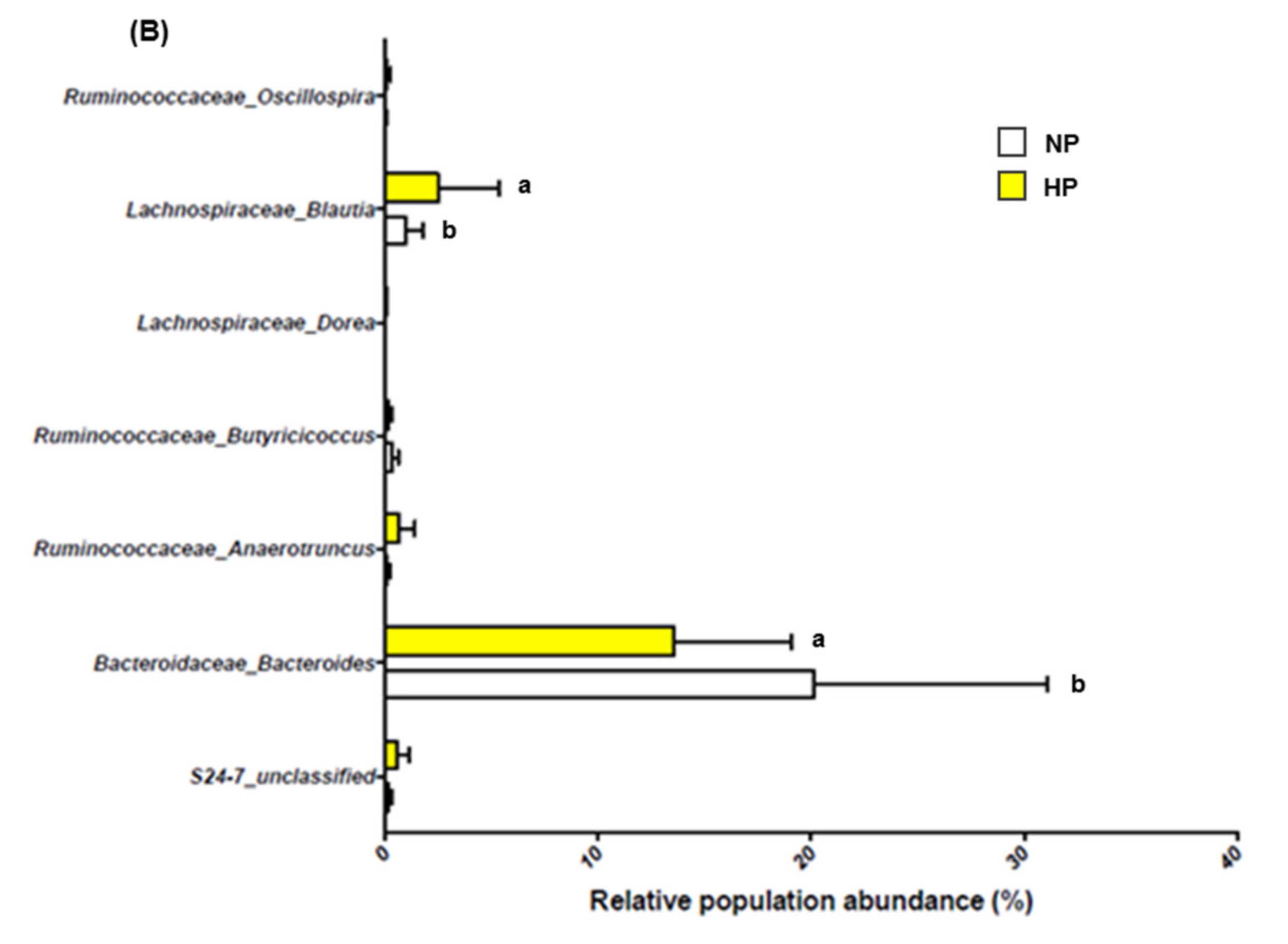
3.2. Effects of Dietary Protein Level and Prebiotic Supplementation on Fecal Microbiota
3.3. Relationships between Gut Microbiota and Host Metabolism Parameters
3.3.1. Metabolomic Metavariables
3.3.2. Metagenomic Metavariables
3.3.3. Global Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toll, P.; Yamka, R.M.; Schoenherr, W.D.; Hand, M.S. Obesity. In Small Animal Clinical Nutrition, 5th ed.; Thatcher, C.D., Remillard, R.L., Roudebush, P., Norvotny, B.J., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 502–542. [Google Scholar]
- Colliard, L.; Ancel, J.; Benet, J.J.; Paragon, B.M.; Blanchard, G. Risk factors for obesity in dogs in France. J. Nutr. 2006, 136, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.L.; Morris, P.J.; Abdulla, Z.; Hackett, R.; Rawlings, J.M. Risk factors associated with excess body weight in dogs in the UK. J. Anim. Physiol. Anim. Nutr. 2007, 91, 166–167. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Ceron, J.J.; Holden, S.L.; Cuthbertson, D.J.; Biourge, V.; Morris, P.J.; German, A.J. Obesity-related metabolic dysfunction in dogs: A comparison with human metabolic syndrome. BMC Vet. Res. 2012, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.W.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. (Lond.) 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.E.; Kim, H.B.; Isaacson, R.E.; Seo, K.W.; Song, K.H. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J. Vet. Intern. Med. 2015, 29, 43–50. [Google Scholar] [CrossRef]
- Jergens, A.E.; Guard, B.C.; Redfern, A.; Rossi, G.; Mochel, J.P.; Pilla, R.; Chandra, L.; Seo, Y.J.; Steiner, J.M.; Lidbury, J.; et al. Microbiota-related changes in unconjugated fecal bile acids are associated with naturally occurring, insulin-dependent diabetes mellitus in dogs. Front. Vet. Sci. 2019, 6, 199–210. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, 167–177. [Google Scholar] [CrossRef]
- Diez, M.; Nguyen, P.; Jeusette, I.; Devois, C.; Istasse, L.; Biourge, V. Weight loss in obese dogs: Evaluation of a high-protein, low-carbohydrate diet. J. Nutr. 2002, 132, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Holden, S.L.; Bissot, T.; Morris, P.J.; Biourge, V. A high protein high fibre diet improves weight loss in obese dogs. Vet. J. 2012, 183, 294–297. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Leriche, I.; Chaix, G.; Thorin, C.; Burger, M.; Nguyen, P. Recovery of insulin sensitivity and optimal body composition after rapid weight loss in obese dogs fed a high-protein medium-carbohydrate diet. J. Anim. Physiol. Anim. Nutr. 2017, 101, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, G.; Mitchell, P.; Beaudoin, M.S.; Marette, A. Impact of dietary proteins on energy balance, insulin sensitivity and glucose homeostasis: From proteins to peptides to amino acids. In the Molecular Nutrition of Amino Acids and Proteins, 1st ed.; D. Daveret: Quebec, Canada, 2016; pp. 241–264. [Google Scholar]
- Pais, R.; Gribble, F.M.; Reimann, F. Stimulation of incretin secreting cells. Ther. Adv. Endocrinol. Metab. 2016, 7, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Lubbs, D.C.; Vester, B.M.; Fastinger, N.D.; Swanson, K.S. Dietary protein concentration affects intestinal microbiota of adult cats: A study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J. Anim. Physiol. Anim. Nutr. 2009, 93, 113–121. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Swanson, K.; Grieshop, C.; Flickinger, E.; Bauer, L.; Chow, J.; Wolf, B.; Garleb, K.; Fahey, G. Fructo-oligosaccharides and Lactobacillus acidophilus modify gut microbial populations, total tract digestibilities and fecal protein catabolite in healthy adult dogs. J. Nutr. 2002, 132, 3721–3731. [Google Scholar] [CrossRef]
- Respondek, F.; Swanson, K.S.; Belsito, K.R.; Vester, B.M.; Wagner, A.; Istasse, L.; Diez, M. Short-chain fructo-oligosaccharides influence insulin sensitivity and gene expression of fat tissue in obese dogs. J. Nutr. 2008, 138, 1712–1718. [Google Scholar] [CrossRef]
- Massimino, S.P.; McBurney, M.I.; Field, C.J.; Thomson, A.B.; Keelan, M.; Hayek, M.G.; Sunvold, G.D. Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J. Nutr. 1998, 128, 1786–1793. [Google Scholar] [CrossRef]
- Daumas, C.; Lhoest, E.; Hornick, J.L.; Istasse, L.; Diez, M. Development of a practical test of insulin resistance in obese Beagle dogs and effects of scFOS. In Proceedings of the 13th Congress of the European Society of Veterinary and Comparative Nutrition, Oristano, Italy, 15–17 October 2009; p. 89. [Google Scholar]
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- National Research Council. Nutrient Requirements of Dogs; National Academies Press: Washington, DC, USA, 1985; Volume 8. [Google Scholar]
- WALTHAM. 2016. Available online: https://www.waltham.com (accessed on 21 January 2016).
- Verkest, K.R.; Fleeman, L.M.; Rand, J.S.; Morton, J.M. Basal measures of insulin sensitivity and insulin secretion simplified glucose tolerance tests in dogs. Domest. Anim. Endocrinol. 2010, 39, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Humblet, M.; Coghe, J.; Lekeux, P.; Godeau, J.M. Acute phase proteins assessment for an early selection of treatments in growing calves suffering from bronchopneumonia under field conditions. Res. Vet. Sci. 2004, 77, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, T.; Peplies, P.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Martin, A.P. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl. Environ. Microbiol. 2002, 68, 3673–3682. [Google Scholar] [CrossRef]
- Parks, D.H.; Beiko, R. GIdentifying biologically relevant differences between metagenomic communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef]
- Opgen-Rhein, R.; Strimmer, K. Inferring gene dependency networks from genomic longitudinal data: A functional data approach. Rev. Stat. 2006, 4, 53–65. [Google Scholar]
- Opgen-Rhein, R.; Strimmer, K. Using regularized dynamic correlation to infer gene dependency networks from time-series microarray data. In Proceedings of the 4th International Workshop on Computational Systems Biology, Tampere, Finland, 12–13 June 2006; pp. 73–76. [Google Scholar]
- Schäfer, J.; Strimmer, K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat. Appl. Genet. Mol. Biol. 2005, 4, 1175–1189. [Google Scholar] [CrossRef]
- Jeusette, I.; Grauwels, M.; Cuvelier, C.; Tonglet, C.; Istasse, L.; Diez, M. Hypercholesterolaemia in a family of rough collie dogs. J. Small Anim. Pract. 2004, 45, 319–324. [Google Scholar] [CrossRef]
- Ricci, R.; Jeusette, I.; Godeau, J.M.; Contiero, B.; Diez, M. Effect of short-chain fructo-oligosaccharide-enriched energy-restricted diet on weight loss and serum haptoglobin concentration in Beagle dogs. Br. J. Nutr. 2011, 106, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Verbrugghe, A.; Hesta, M.; Gommeren, K.; Daminet, S.; Wuyts, B.; Buyse, J.; Janssens, G.P. Oligofructose and inulin modulate glucose and amino acid metabolism through propionate production in normal-weight and obese cats. Br. J. Nutr. 2009, 102, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Liu, T.W.; Devendran, S.; Theis, S.; Ridlon, J.M.; Suchodolski, J.S.; de Godoy, M.R.C.; Swanson, K.S. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and fecal microbiota and bile acids in overweight dogs. FASEB J. 2017, 31, 965. [Google Scholar]
- Le Bourgot, C.; Apper, E.; Blat, S.; Respondek, F. Fructo-oligosaccharides and glucose homeostasis: A systematic review and meta-analysis in animal models. Nutr. Metabol. 2018, 15, 9. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Ader, M.; Stefanovski, D.; Richey, J.M.; Kim, S.P.; Kolka, C.M.; Ionut, K.J.; Catalano, K.; Hucking, M.; Ellmerer, G.; Van Citters, I.R.; et al. Failure of homeostatic model assessment of insulin resistance to detect marked diet-induced insulin resistance in dogs. Diabetes 2014, 63, 1914–1919. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Goryca, K.; Karczmarski, J.; Rubel, T.; Wojtowicz, W.; Mlynarz, P.; Tomecki, R. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes 2016, 7, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Dhotre, D.P.; Chavan, S.G.; Sultan, A.; Jain, D.S.; Lanjekar, V.B.; Gangawani, J.; Shah, P.S.; Todkar, J.S.; Shah, S.; et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J. Biosci. 2012, 37, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.; Lorca, G.L.; Casella, G.; Giongo, A.; Naranjo, A.; Pionzio, A.M.; Li, N.; Mai, V.; Wasserfall, C.H.; Schatz, D.; et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. 2009, 3, 536–548. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monoplayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Wang, J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Ajami, N.; Michalek, R.D.; Tian, X.; Wong, M.; Losee-Olson, S.H.; Petrosino, J.F.; Yoo, S.H.; Chen, Z. Transmissible microbial and metabolomic remodeling by soluble dietary fiber improves metabolic homeostasis. Sci. Rep. UK 2015, 5, 10604. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Hardt, M.; Hartman, M.; Schulte, F.; Tavares, M.; Mutawa, A.; Ariga, J.; Soparkar, P.; Behbehani, J.; Behbehani, K.; et al. Identification of salivary and plasma biomarkers for obesity in children by non-targeted metabolomic analysis. BioRxiv 2018. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, F.; Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 2011, 78, 511–518. [Google Scholar] [CrossRef] [PubMed]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep.UK 2017, 7, 46337. [Google Scholar] [CrossRef] [PubMed]
- Bennion, L.J.; Grundy, S.M. Effects of diabetes mellitus on cholesterol metabolism in man. N. Engl. J. Med. 1977, 296, 1365–1371. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, M.A.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Capuani, G.; Tomassini Miccheli, A.; Puccetti, C.; Delfini, M.; Iaconelli, A.; Nanni, G.; Mingrone, G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int. J. Obes. 2005, 34, 1095–1098. [Google Scholar] [CrossRef]
- Shearer, J.; Duggan, G.; Weljie, A.; Hittel, D.S.; Wasserman, D.H.; Vogel, H.J. Metabolomic profiling of dietary-induced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes. Metab. 2008, 10, 950–958. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Spector, T.D.; Menni, C. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. UK 2017, 7, 13670. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 4309. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Vogt, S.; Stuckler, F.; Krumsiek, J.; Bartel, J.; Kacprowski, T.; Schramm, K.; Carstensen, M.; Rathmann, W.; Roden, M.; et al. Multi-omic signature of body weight change: Results from a population-based cohort study. BMC Med. 2015, 13, 48–65. [Google Scholar] [CrossRef]
- Martin, J.C.; Berton, A.; Ginies, C.; Bott, R.; Scheercousse, P.; Saddi, A.; Gripois, D.; Landrier, J.F.; Dalemans, D.; Alessi, M.C.; et al. Multi-level systems biology modeling characterized the atheroprotective efficiencies of modified dairy fats in a hamster model. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, 935–945. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | Normal Protein | High Protein |
|---|---|---|
| g/kg | g/kg | |
| Cereals and derivatives | 595.6 | 522.0 |
| Meat and derivatives | 350.0 | 423.6 |
| Beet pulp | 20 | 20 |
| Hydrolysates | 20 | 20 |
| Linseed | 10 | 10 |
| Mineral Premix | 3.75 | 3.74 |
| Calcium propionate | 0.5 | 0.5 |
| Vitamin premix | 0.15 | 0.15 |
| Dry matter | 884 | 894 |
| g/100 g DM | g/100 g DM | |
| Protein | 25.6 | 36.9 |
| Fat | 11.6 | 11.7 |
| Ash | 9.4 | 9.5 |
| NFE | 47.8 | 36.8 |
| Starch | 34.1 | 26.1 |
| Crude fiber (ADF) | 5.6 | 5.1 |
| Calcium | 2.5 | 2.5 |
| Phosphorus | 1.5 | 1.6 |
| Protein | Prebiotic | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Normal | High | CTRL | OF | scFOS | Protein | Prebiotic | |
| Body weight, kg | 13.5 ± 0.31 | 13.4 ± 0.31 | 13.5 ± 0.47 | 13.3 ± 0.43 | 13.4 ± 0.34 | 0.664 | 0.917 |
| Excess body weight, % | 51.6 ± 3.40 | 52.9 ± 6.13 | 54.4 ± 7.08 | 51.8 ± 4.98 | 50.1 ± 5.50 | 0.875 | 0.149 |
| Fasting glucose, mmol/L | 5.29 ± 0.07 | 5.17 ± 0.06 | 5.21 ± 0.09 | 5.32 ± 0.08 | 5.16 ± 0.06 | 0.292 | 0.418 |
| Fasting insulin, mU/L | 17.1 ± 2.07 | 15.7 ± 1.99 | 17.0 ± 2.85 | 17.6 ± 2.42 | 14.6 ± 2.15 | 0.628 | 0.613 |
| Haptoglobin, g/L | 2.01 ± 0.18 | 2.10 ± 0.41 | 1.99 ± 0.25 | 2.44 ± 0.54 | 1.70 ± 0.21 | 0.832 | 0.499 |
| Cholesterol, mmol/L | 7.4 ± 0.27 | 7.1 ± 0.33 | 7.5 ± 0.39 | 7.2 ± 0.37 | 7.0 ± 0.33 | 0.292 | 0.727 |
| Protein | Prebiotic | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Normal | High | CTRL | OF | scFOS | Protein | Prebiotic | |
| Total fecal output, g/d | 573 ± 31.6 | 503 ± 25.7 | 544 ± 35.4 | 516 ± 31 | 563 ± 46.8 | 0.548 | 0.841 |
| Fecal dry matter, % | 40.3 ± 0.98 | 41.7 ± 1.08 | 41.4 ± 1.03 | 41.3 ± 1.58 | 40.2 ± 1.15 | 0.319 | 0.894 |
| Stool frequency/d | 2.58 ± 0.156 | 2.17 ± 0.103 | 2.36 ± 0.176 | 2.33 ± 0.167 | 2.48 ± 0.198 | 0.380 | 0.933 |
| Volatile fatty acids (VFA), µg/mL fecal water | |||||||
| Butyrate | 717 ± 84.5 | 841 ± 101.4 | 713 a ± 74.1 | 728 a ± 106.2 | 895 b ± 155.2 | 0.636 | 0.039 |
| Acetate | 3383 ± 322 | 3963 ± 337 | 4096 ± 253 | 3265 ± 338 | 3598 ± 589 | 0.546 | 0.646 |
| Propionate | 1736 ± 251 | 2352 ± 334 | 2059 ± 119 | 1857 ± 323 | 2172 ± 578 | 0.244 | 0.880 |
| Total VFA | 5836 ± 610 | 7155 ± 740 | 6868 ± 406 | 5849 ± 725 | 6665 ± 1281 | 0.479 | 0.109 |
| Branched-chain fatty acids (BCFA), µg/mL fecal water | |||||||
| Isobutyrate | 139 ± 14.3 | 176 ± 15.9 | 190 ± 13.7 | 135 ± 18.2 | 142 ± 21.9 | 0.311 | 0.306 |
| Isovalerate | 190 ± 20.0 | 237 ± 19.1 | 260 ± 19.9 | 181 ± 22.0 | 194 ± 27.4 | 0.307 | 0.249 |
| Valerate | 19.0 ± 4.01 | 20.1 ± 1.94 | 17.8 ± 1.86 | 21.3 ± 4.60 | 19.5 ± 5.17 | 0.952 | 0.875 |
| Total BCFA | 348 ± 35.9 | 433 ± 35.8 | 468 ± 34.3 | 337 ± 41.5 | 356 ± 52.7 | 0.340 | 0.328 |
| Item | n | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|
| Body weight, kg | 36 | 13.56 | 13.75 | 10.55 | 15.70 | 1.36 |
| Excess body weight, % | 36 | 53.51 | 53.00 | 19.90 | 90.00 | 18.45 |
| Fasting glucose, mmol/L | 36 | 5.24 | 5.30 | 4.60 | 5.90 | 0.262 |
| Fasting insulin, mU/L | 36 | 16.64 | 13.00 | 7.00 | 40.00 | 8.60 |
| HOMA-IR | 36 | 2.12 | 1.63 | 0.92 | 4.98 | 1.16 |
| Haptoglobin, mg/L | 36 | 2140 | 1743 | 949 | 7268 | 1335 |
| Cholesterol, mmol/L | 36 | 7.35 | 7.47 | 4.87 | 9.59 | 1.19 |
| Total fecal output, g/d | 36 | 540.8 | 536.1 | 311.5 | 786.0 | 121.3 |
| Fecal dry matter, % | 36 | 41.1 | 41.5 | 34.2 | 50.1 | 4.00 |
| Stool frequency, /day | 36 | 2.35 | 2.40 | 1.40 | 3.80 | 0.59 |
| Metabolite | Metabolic Pathways | Correlation Coefficient |
|---|---|---|
| Fecal water LD1 | ||
| Cholic acids | Primary bile acids | 0.89 0.88 0.87 0.86 |
| Deoxycholic acids | Secondary bile acids | 0.87 0.78 |
| Taurocholic acids | Primary bile acids | 0.80 0.76 |
| L-Phenylalanine | Amino acid | 0.85 |
| Norleucine | Derived from lysine, involved in lipid metabolism | 0.82 |
| Tryptophan | Amino acid | 0.82 |
| Fecal water GD1 | ||
| L-Alanine | Amino acid | 0.91 |
| L-Valine | Amino acid | 0.90 |
| Cadaverine | Lysine metabolism | 0.89 |
| L-Proline | Amino acid | 0.87 |
| L-Threonine | Amino acid | 0.86 |
| L-Phenylalanine | Amino acid | 0.86 |
| Fecal water LD2 | ||
| Hexanoylcarnitine | Lipid and amino-acid metabolism | 0.67 |
| Limonene | Lipid metabolism connected with primary bile acid metabolism | 0.65 |
| Hippurate | Phenylalanine metabolism | −0.37 |
| Aminoadipate | Lysine metabolism | −0.52 |
| Acetylcholine | Neurotransmitter involved in many functions including insulin, bile and pancreatic secretion | −0.63 |
| Fecal water GD2 | ||
| Phenylpropanoate | Aromatic compounds, phenylalanine degradation, polyphenol metabolism | 0.62 |
| D-Fructose | Amino sugar and nucleotide sugar metabolism | 0.55 |
| PlasmaLD2 | ||
| Leucine Aspargine | Dipeptide – Amino-acid/protein metabolism | 0.74 |
| Cis or trans-4- Hydroxy-D-proline | Proline and derivatives – Marker of bone resorption, muscle degradation, depression and stress | 0.72 |
| Diaminoheptanedioate | Derived from lysine, specific to certain cell walls of gram-negative bacteria | 0.69 |
| Acylcarnitine | Produced from lysine and methionine, involved in fatty acid catabolism | −0.46 |
| Protein | Prebiotic | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Normal | High | CTRL | OF | scFOS | Protein | Prebiotic | |
| Plasma metabolome | |||||||
| PlasmaLD2 | 0.29 ± 41 | −0.33 ± 0.47 | −0.19 ± 0.55 | −0.16 ± 0.57 | 0.38 | 0.099 | 0.892 |
| Fecal metabolome | |||||||
| FaecalGD1 | −0.11 ± 0.89 | 0.13 ± 0.83 | −0.36 ± 0.82 | −0.17 ± 1.10 | 0.57 ± 1.28 | 0.910 | 0.937 |
| FaecalGD2* | −0.22 ± 0.62 | 0.24 ± 0.55 | 0.98 ± 0.80 | 0.92 ± 0.61 | 2.88 ± 0.67 | 0.609 | 0.013 |
| FaecalLD1 | 0.72 ± 0.91 | −0.82 ± 0.96 | −1.73 ± 0.50 | 0.49 ± 1.33 | 1.37 ± 1.35 | 0.121 | 0.372 |
| FaecalLD2 | 0.51 ± 0.46 | −0.58 ± 0.58 | −0.04 ± 0.56 | 0.20 ± 0.71 | −0.18 ± 0.70 | 0.420 | 0.741 |
| Metagenome | |||||||
| GenusD1 | −0.71 ± 0.55 | 0.81 ± 0.46 | 0.80 ± 0.72 | −0.11 ± 0.76 | −0.77 ± 0.37 | 0.367 | 0.429 |
| GenusD2 | 0.17 ± 0.49 | −0.19 ± 0.48 | 0.17 ± 0.68 | 0.13 ± 0.60 | −0.33 ± 0.48 | 0.598 | 0.935 |
| GenusD3 | −0.02 ± 0.51 | 0.02 ± 0.38 | −0.56 ± 0.45 | 0.66 ± 0.74 | −0.11 ± 0.14 | 0.921 | 0.601 |
| Bacterial Genus | Correlation Coefficient with the Metavariables |
|---|---|
| GenusD1 | |
| Lactobacillus | 0.75 |
| Bacteroides unclassified | 0.61 |
| Allobaculum | 0.60 |
| Lactobacillales unclassified | 0.54 |
| Escherichia Shigella | 0.52 |
| Parabacteroides | 0.48 |
| Campylobacter | −0.49 |
| GenusD2 | |
| Parabacteroides | 0.61 |
| Phascolarctobacterium | 0.57 |
| Campylobacter | 0.51 |
| Gammaproteobacteria unclassified | 0.49 |
| Fusobacterium | −0.48 |
| Wolinella | −0.51 |
| Brachyspira | −0.59 |
| GenusD3 | |
| Enterococcus | 0.67 |
| Escherichia Shigella | 0.59 |
| Allobaculum | 0.48 |
| Allisonella | 0.45 |
| Campylobacter | 0.44 |
| Clostridium sensu stricto1 | −0.43 |
| Anaeroplasma | −0.55 |
| Node 1 | Node 2 | Partial Correlation Coefficient | p-Value | Q-value |
|---|---|---|---|---|
| HOMA IR | Fasting insulin | 0.415 | 4.44 × 10−16 | 3.03 × 10−14 |
| FaecalLD1 | FaecalGD1 | 0.316 | 1.17 × 10−9 | 4.32 × 10−8 |
| FaecalLD2 | FaecalGD2 | 0.315 | 1.37 × 10−9 | 4.68 × 10−8 |
| Body weight | Excess body weight % | 0.298 | 1.06 × 10−8 | 2.90 × 10−7 |
| Haptoglobin | Cholesterol | −0.237 | 6.42 × 10−6 | 0.0001 |
| GenusD2 | Body weight | 0.219 | 3.27 × 10−5 | 0.0006 |
| PlasmaLD2 | FaecalLD1 | 0.206 | 9.00 × 10−5 | 0.0014 |
| GenusD1 | Excess body weight % | 9.05 × 10−5 | 0.206 | 0.0014 |
| Total fecal output | Stool frequency | 0.198 | 0.0002 | 0.0024 |
| Haptoglobin | Fecal dry matter % | 0.194 | 0.0002 | 0.0030 |
| FaecalLD2 | FaecalGD1 | −0.179 | 0.0007 | 0.0082 |
| GenusD1 | Cholesterol | 0.160 | 0.0025 | 0.0222 |
| Fecal dry matter % | Stool frequency | −0.155 | 0.0034 | 0.0273 |
| Total fecal output | Fecal dry matter % | −0.154 | 0.0036 | 0.0287 |
| GenusD1 | Fecal dry matter % | 0.152 | 0.0040 | 0.0305 |
| FaecalLD2 | Cholesterol | 0.152 | 0.0040 | 0.0305 |
| GenusD1 | Haptoglobin | 0.148 | 0.0053 | 0.0371 |
| GenusD2 | Stool frequency | −0.147 | 0.0057 | 0.0389 |
| PlasmaLD2 | Haptoglobin | −0.136 | 0.0104 | 0.0618 |
| FaecalLD1 | Fasting glucose | 0.136 | 0.0105 | 0.0618 |
| PlasmaLD2 | HOMA IR | 0.133 | 0.0123 | 0.0702 |
| PlasmaLD2 | Fecal dry matter % | 0.125 | 0.0190 | 0.0956 |
| PlasmaLD2 | Fasting insulin | 0.123 | 0.0207 | 0.1013 |
| HOMA IR | Excess body weight % | 0.122 | 0.0214 | 0.1035 |
| FaecalGD1 | Stool frequency | 0.116 | 0.0290 | 0.1247 |
| GenusD3 | HOMA IR | 0.115 | 0.0301 | 0.1272 |
| FaecalGD2 | Cholesterol | −0.115 | 0.0310 | 0.1296 |
| FaecalLD2 | GenusD1 | −0.113 | 0.0337 | 0.1356 |
| GenusD3 | Fasting insulin | 0.111 | 0.0359 | 0.1402 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apper, E.; Privet, L.; Taminiau, B.; Le Bourgot, C.; Svilar, L.; Martin, J.-C.; Diez, M. Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content. Microorganisms 2020, 8, 513. https://doi.org/10.3390/microorganisms8040513
Apper E, Privet L, Taminiau B, Le Bourgot C, Svilar L, Martin J-C, Diez M. Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content. Microorganisms. 2020; 8(4):513. https://doi.org/10.3390/microorganisms8040513
Chicago/Turabian StyleApper, Emmanuelle, Lisa Privet, Bernard Taminiau, Cindy Le Bourgot, Ljubica Svilar, Jean-Charles Martin, and Marianne Diez. 2020. "Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content" Microorganisms 8, no. 4: 513. https://doi.org/10.3390/microorganisms8040513
APA StyleApper, E., Privet, L., Taminiau, B., Le Bourgot, C., Svilar, L., Martin, J.-C., & Diez, M. (2020). Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content. Microorganisms, 8(4), 513. https://doi.org/10.3390/microorganisms8040513





