Longitudinal Study of Viral and Bacterial Contamination of Hospital Pediatricians’ Mobile Phones
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Sampling of MPs
2.3. Microbiological Analyses
2.4. Hygiene Habits and Behavior
2.5. Statistics
2.6. Ethics
3. Results
3.1. Detection of Viral Genomes and Bacterial Strains on MPs
3.2. Contamination with Bacteria
3.3. Detection of Viral Genomes
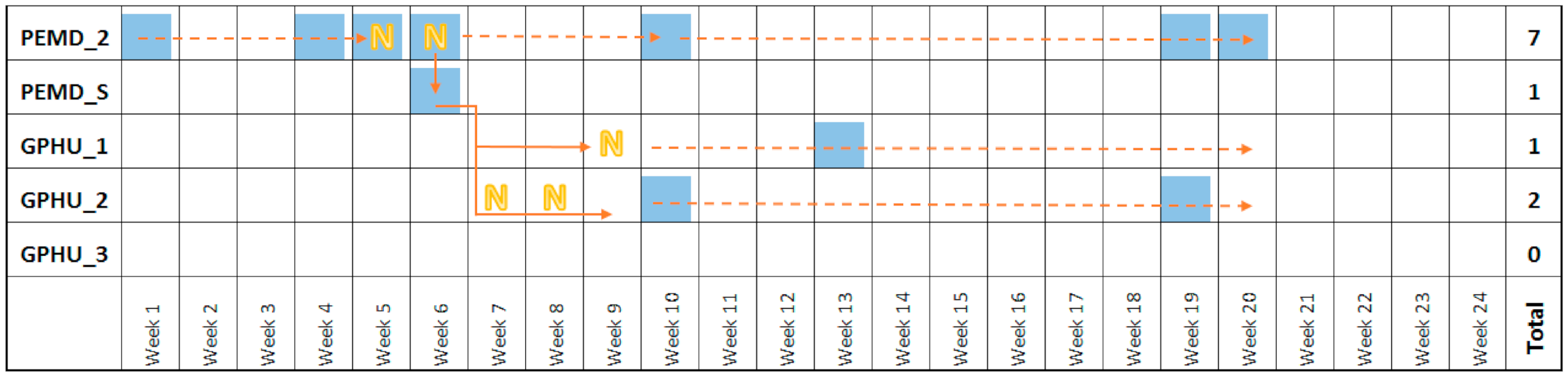
3.4. Transfer of Contamination
3.5. Hygiene Behaviours
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soto, R.G.; Chu, L.F.; Goldman, J.M.; Rampil, I.J.; Ruskin, K.J. Communication in critical care environments: Mobile telephones improve patient care. Anesth. Analg. 2006, 102, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.G.; Neves, S.E.; Papadakos, P.J.; Shapiro, F.E. Personal electronic device use in the operating room: A survey of usage patterns, risks and benefits. Eur. J. Anaesthesiol. 2017, 34, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, S.; Biggs, C. Cell Phones in the Neonatal Intensive Care Unit: How to Eliminate Unwanted Germs. Adv. Neonatal Care 2016, 16, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Juyal, D.; Adekhandi, S.; Sharma, M.; Prakash, R.; Sharma, N.; Rana, A.; Parihar, A. Mobile phones: Reservoirs for the transmission of nosocomial pathogens. Adv. Biomed. Res. 2015, 4, 144. [Google Scholar] [CrossRef]
- Ulger, F.; Dilek, A.; Esen, S.; Sunbul, M.; Leblebicioglu, H. Are healthcare workers’ mobile phones a potential source of nosocomial infections? Review of the literature. J. Infect. Dev. Ctries 2015, 9, 1046–1053. [Google Scholar] [CrossRef]
- Ustun, C.; Cihangiroglu, M. Health care workers’ mobile phones: A potential cause of microbial cross-contamination between hospitals and community. J. Occup. Environ. Hyg. 2012, 9, 538–542. [Google Scholar] [CrossRef]
- Brady, R.R.W.; Verran, J.; Damani, N.N.; Gibb, A.P. Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J. Hosp. Infect. 2009, 71, 295–300. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Khatri, M.; Bhardwaj, S.K.; Sonne, C.; Deep, A.; Kim, K.-H. A review on mobile phones as bacterial reservoirs in healthcare environments and potential device decontamination approaches. Environ. Res. 2020, 186, 109569. [Google Scholar] [CrossRef]
- Olsen, M.; Campos, M.; Lohning, A.; Jones, P.; Legget, J.; Bannach-Brown, A.; McKirdy, S.; Alghafri, R.; Tajouri, L. Mobile phones represent a pathway for microbial transmission: A scoping review. Travel Med. Infect. Dis. 2020, 35, 101704. [Google Scholar] [CrossRef]
- Brady, R.R.W.; Chitnis, S.; Stewart, R.W.; Graham, C.; Yalamarthi, S.; Morris, K. NHS connecting for health: Healthcare professionals, mobile technology, and infection control. Telemed. J. E Health 2012, 18, 289–291. [Google Scholar] [CrossRef]
- Pillet, S.; Berthelot, P.; Gagneux-Brunon, A.; Mory, O.; Gay, C.; Viallon, A.; Lucht, F.; Pozzetto, B.; Botelho-Nevers, E. Contamination of healthcare workers’ mobile phones by epidemic viruses. Clin. Microbiol. Infect. 2016, 22, 456.e1–456.e6. [Google Scholar] [CrossRef]
- Cavari, Y.; Kaplan, O.; Zander, A.; Hazan, G.; Shemer-Avni, Y.; Borer, A. Healthcare workers mobile phone usage: A potential risk for viral contamination. Surveillance pilot study. Infect. Dis. Lond. Engl. 2016, 48, 432–435. [Google Scholar] [CrossRef]
- Ganime, A.C.; Carvalho-Costa, F.A.; Santos, M.; Costa Filho, R.; Leite, J.P.G.; Miagostovich, M.P. Viability of human adenovirus from hospital fomites. J. Med. Virol. 2014, 86, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- L’Huillier, A.G.; Tapparel, C.; Turin, L.; Boquete-Suter, P.; Thomas, Y.; Kaiser, L. Survival of rhinoviruses on human fingers. Clin. Microbiol. Infect. 2015, 21, 381–385. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, D.V.; Cohen, B.; Bovino, M.E.; Desai, S.; Whittier, S.; Larson, E.L. Survival of influenza virus on hands and fomites in community and laboratory settings. Am. J. Infect. Control 2012, 40, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.U.; Gerba, C.P.; Tamimi, A.H.; Kitajima, M.; Maxwell, S.L.; Rose, J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 2013, 79, 5728–5734. [Google Scholar] [CrossRef]
- Tuladhar, E.; Hazeleger, W.C.; Koopmans, M.; Zwietering, M.H.; Duizer, E.; Beumer, R.R. Transfer of noroviruses between fingers and fomites and food products. Int. J. Food Microbiol. 2013, 167, 346–352. [Google Scholar] [CrossRef]
- Ganime, A.C.; Leite, J.P.G.; da Silva Figueiredo, C.E.; Carvalho-Costa, F.A.; Melgaço, F.G.; Malta, F.C.; Fumian, T.M.; Miagostovich, M.P. Dissemination of human adenoviruses and rotavirus species A on fomites of hospital pediatric units. Am. J. Infect. Control 2016, 44, 1411–1413. [Google Scholar] [CrossRef]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Cantais, A.; Mory, O.; Plat, A.; Bourmaud, A.; Giraud, A.; Costille, M.; Pozzetto, B.; Pillet, S. Impact of bedside diagnosis of influenza in the paediatric emergency ward. Clin. Microbiol. Infect. 2019, 25, 898–903. [Google Scholar] [CrossRef] [PubMed]
- de Rougemont, A.; Kaplon, J.; Fremy, C.; Legrand-Guillien, M.-C.; Minoui-Tran, A.; Payan, C.; Vabret, A.; Mendes-Martins, L.; Chouchane, M.; Maudinas, R.; et al. Clinical severity and molecular characteristics of circulating and emerging rotaviruses in young children attending hospital emergency departments in France. Clin. Microbiol. Infect. 2016, 22, 737.e9–737.e15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Legeay, C.; Bourigault, C.; Lepelletier, D.; Zahar, J.R. Prevention of healthcare-associated infections in neonates: Room for improvement. J. Hosp. Infect. 2015, 89, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, P.; Grattard, F.; Fascia, P.; Fichtner, C.; Moulin, M.; Lavocat, M.P.; Teyssier, G.; Lucht, F.; Pozzetto, B. Implication of a healthcare worker with chronic skin disease in the transmission of an epidemic strain of methicillin-resistant Staphylococcus aureus in a pediatric intensive care unit. Infect. Control. Hosp. Epidemiol. 2003, 24, 299–300. [Google Scholar] [CrossRef][Green Version]
- Berthelot, P.; Grattard, F.; Patural, H.; Ros, A.; Jelassi-Saoudin, H.; Pozzetto, B.; Teyssier, G.; Lucht, F. Nosocomial colonization of premature babies with Klebsiella oxytoca: Probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect. Control. Hosp. Epidemiol. 2001, 22, 148–151. [Google Scholar] [CrossRef]
- Laurent, F.; Butin, M. Staphylococcus capitis and NRCS-A clone: The story of an unrecognized pathogen in neonatal intensive care units. Clin. Microbiol. Infect. 2019, 25, 1081–1085. [Google Scholar] [CrossRef]
- Walsh, T.R.; Bolmström, A.; Qwärnström, A.; Ho, P.; Wootton, M.; Howe, R.A.; MacGowan, A.P.; Diekema, D. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 2001, 39, 2439–2444. [Google Scholar] [CrossRef]
- van Belkum, A.; Kluytmans, J.; van Leeuwen, W.; Bax, R.; Quint, W.; Peters, E.; Fluit, A.; Vandenbroucke-Grauls, C.; van den Brule, A.; Koeleman, H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1995, 33, 1537–1547. [Google Scholar] [CrossRef]
- Kim, H.-K.; Oh, S.-H.; Yun, K.A.; Sung, H.; Kim, M.-N. Comparison of Anyplex II RV16 with the xTAG respiratory viral panel and Seeplex RV15 for detection of respiratory viruses. J. Clin. Microbiol. 2013, 51, 1137–1141. [Google Scholar] [CrossRef][Green Version]
- Sciandra, I.; Piccioni, L.; Coltella, L.; Ranno, S.; Giannelli, G.; Falasca, F.; Antonelli, G.; Concato, C.; Turriziani, O. Comparative analysis of 2 commercial molecular tests for the detection of gastroenteric viruses on stool samples. Diagn. Microbiol. Infect. Dis. 2020, 96, 114893. [Google Scholar] [CrossRef]
- Bourgeois, F.T.; Valim, C.; Wei, J.C.; McAdam, A.J.; Mandl, K.D. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics 2006, 118, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Singh, T.; Agarwal, H.; Mehta, G.; Dutta, R. Bacterial colonization of rings and cell phones carried by health-care providers: Are these mobile bacterial zoos in the hospital? Trop. Doct. 2011, 41, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Banawas, S.; Abdel-Hadi, A.; Alaidarous, M.; Alshehri, B.; Bin Dukhyil, A.A.; Alsaweed, M.; Aboamer, M. Multidrug-Resistant Bacteria Associated with Cell Phones of Healthcare Professionals in Selected Hospitals in Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 6598918. [Google Scholar] [CrossRef] [PubMed]
- Lasek, R.; Szuplewska, M.; Mitura, M.; Decewicz, P.; Chmielowska, C.; Pawłot, A.; Sentkowska, D.; Czarnecki, J.; Bartosik, D. Genome Structure of the Opportunistic Pathogen Paracoccus yeei (Alphaproteobacteria) and Identification of Putative Virulence Factors. Front. Microbiol. 2018, 9, 2553. [Google Scholar] [CrossRef] [PubMed]

| PICU_1 | PICU_2 | PICU_3 | PICU_S | PEMD_1 | PEMD_2 | PEMD_S | GPHU_1 | GPHU_2 | GPHU_3 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory viruses * | |||||||||||
| Adenovirus | 9 | 5 | 4 | 4 | 15 | 8 | 16 | 5 | 7 | 2 | 75 |
| Bocavirus | 7 | 6 | 6 | 13 | 14 | 8 | 6 | 3 | 63 | ||
| Respiratory syncytial virus | 3 | 1 | 1 | 5 | |||||||
| Rhinovirus | 2 | 1 | 1 | 1 | 5 | ||||||
| Enterovirus | 1 | 1 | 1 | 3 | |||||||
| Coronavirus | 1 | 2 | 3 | ||||||||
| Total | 19 | 5 | 7 | 11 | 22 | 24 | 31 | 15 | 13 | 7 | 154 |
| Enteric viruses * | |||||||||||
| Rotavirus | 3 | 9 | 4 | 8 | 3 | 1 | 28 | ||||
| Adenovirus | 1 | 1 | 4 | 6 | |||||||
| Total | 4 | 10 | 8 | 8 | 3 | 1 | 34 | ||||
| Bacteria | |||||||||||
| Staphylococcus aureus | 2 | 2 | 2 | 1 | 6 | 13 | |||||
| Staphylococcus capitis | 1 | 7 | 2 | 4 | 2 | 3 | 3 | 5 | 1 | 28 | |
| Pseudomonas aeruginosa | 1 | 5 | 1 | 7 | |||||||
| Paracoccus yeei | 7 | 1 | 1 | 2 | 11 | ||||||
| Total | 3 | 8 | 2 | 6 | 2 | 15 | 6 | 3 | 7 | 7 | 59 |
| Total | 22 | 13 | 9 | 17 | 28 | 49 | 45 | 26 | 23 | 15 | 247 |
| Before the Study (n = 8) | After the Study (n = 6) | p value Comparing the Percentage before/after the Study | 4 Years after the End of the Study (n = 6) | |
|---|---|---|---|---|
| HCWs who wash their hands before using the MP (%) * | 11.1 | 16.6 | 0.68 | 11.2 |
| HCWs who wash their hands after using the MP (%) * | 16.6 | 43.7 | 0.03 | 75 |
| HCWs who stop clinical examination to answer a phone call (%) * | 72.2 | 41.2 | 0.04 | 33 |
| HCWs who wash their hands before pursuing the examination (%) * | 42.5 | 68.7 | 0.07 | 75 |
| Number of calls received every day (mean (SD)) † | 12 (3.8) | 12.5 (4.1) | 0.97 | 11.6 (4.0) |
| Number of times the MP is cleaned every month (mean (SD)) † | 2.3 (4.1) | 7.6 (6.3) | 0.02 | 8.1 (10.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantais, A.; Grattard, F.; Gagnaire, J.; Mory, O.; Plat, A.; Lleres-Vadeboin, M.; Berthelot, P.; Bourlet, T.; Botelho-Nevers, E.; Pozzetto, B.; et al. Longitudinal Study of Viral and Bacterial Contamination of Hospital Pediatricians’ Mobile Phones. Microorganisms 2020, 8, 2011. https://doi.org/10.3390/microorganisms8122011
Cantais A, Grattard F, Gagnaire J, Mory O, Plat A, Lleres-Vadeboin M, Berthelot P, Bourlet T, Botelho-Nevers E, Pozzetto B, et al. Longitudinal Study of Viral and Bacterial Contamination of Hospital Pediatricians’ Mobile Phones. Microorganisms. 2020; 8(12):2011. https://doi.org/10.3390/microorganisms8122011
Chicago/Turabian StyleCantais, Aymeric, Florence Grattard, Julie Gagnaire, Olivier Mory, Aurélie Plat, Manon Lleres-Vadeboin, Philippe Berthelot, Thomas Bourlet, Elisabeth Botelho-Nevers, Bruno Pozzetto, and et al. 2020. "Longitudinal Study of Viral and Bacterial Contamination of Hospital Pediatricians’ Mobile Phones" Microorganisms 8, no. 12: 2011. https://doi.org/10.3390/microorganisms8122011
APA StyleCantais, A., Grattard, F., Gagnaire, J., Mory, O., Plat, A., Lleres-Vadeboin, M., Berthelot, P., Bourlet, T., Botelho-Nevers, E., Pozzetto, B., & Pillet, S. (2020). Longitudinal Study of Viral and Bacterial Contamination of Hospital Pediatricians’ Mobile Phones. Microorganisms, 8(12), 2011. https://doi.org/10.3390/microorganisms8122011






