Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
2.1. Course of Infection of Ecoli in a DSS Mouse Model
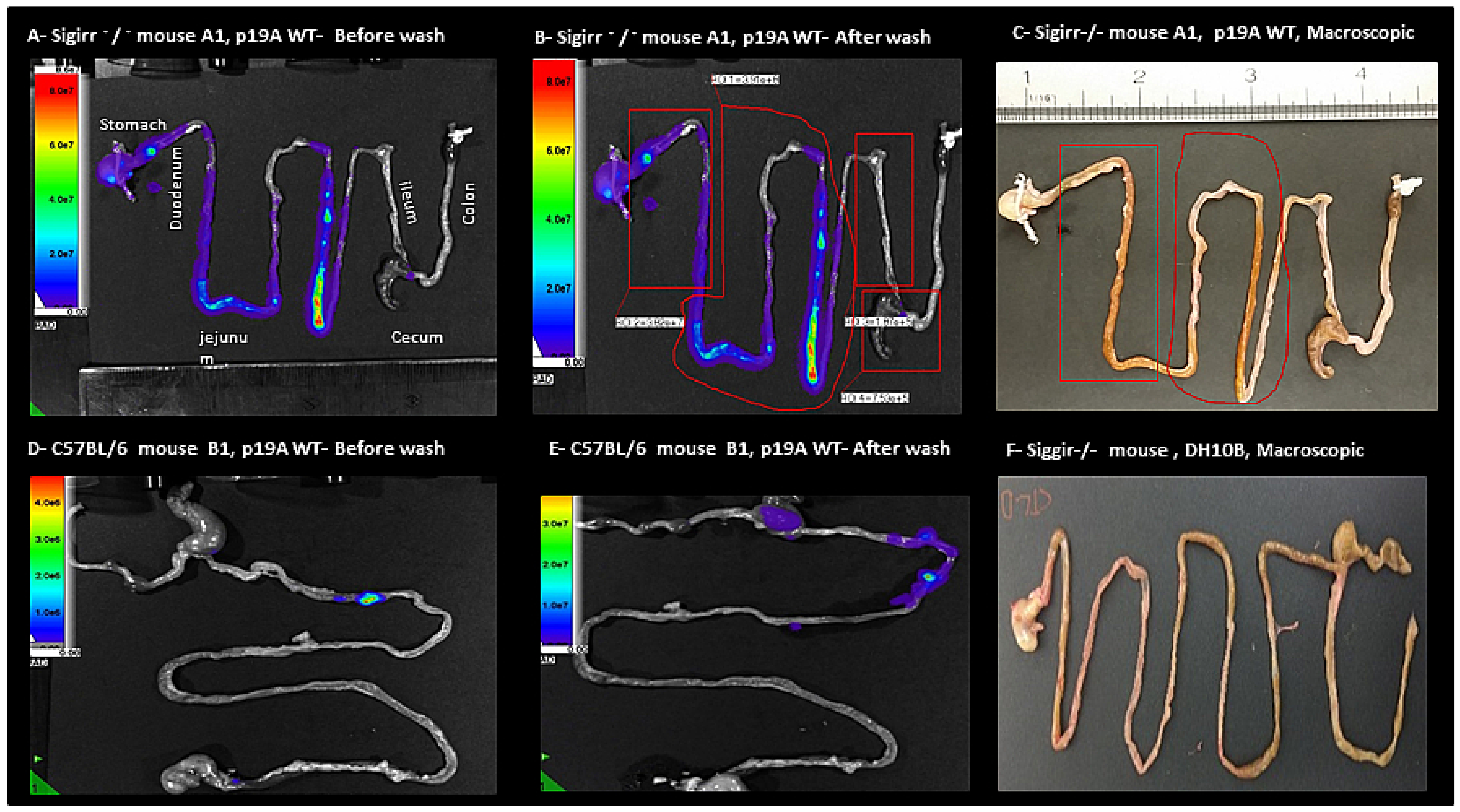
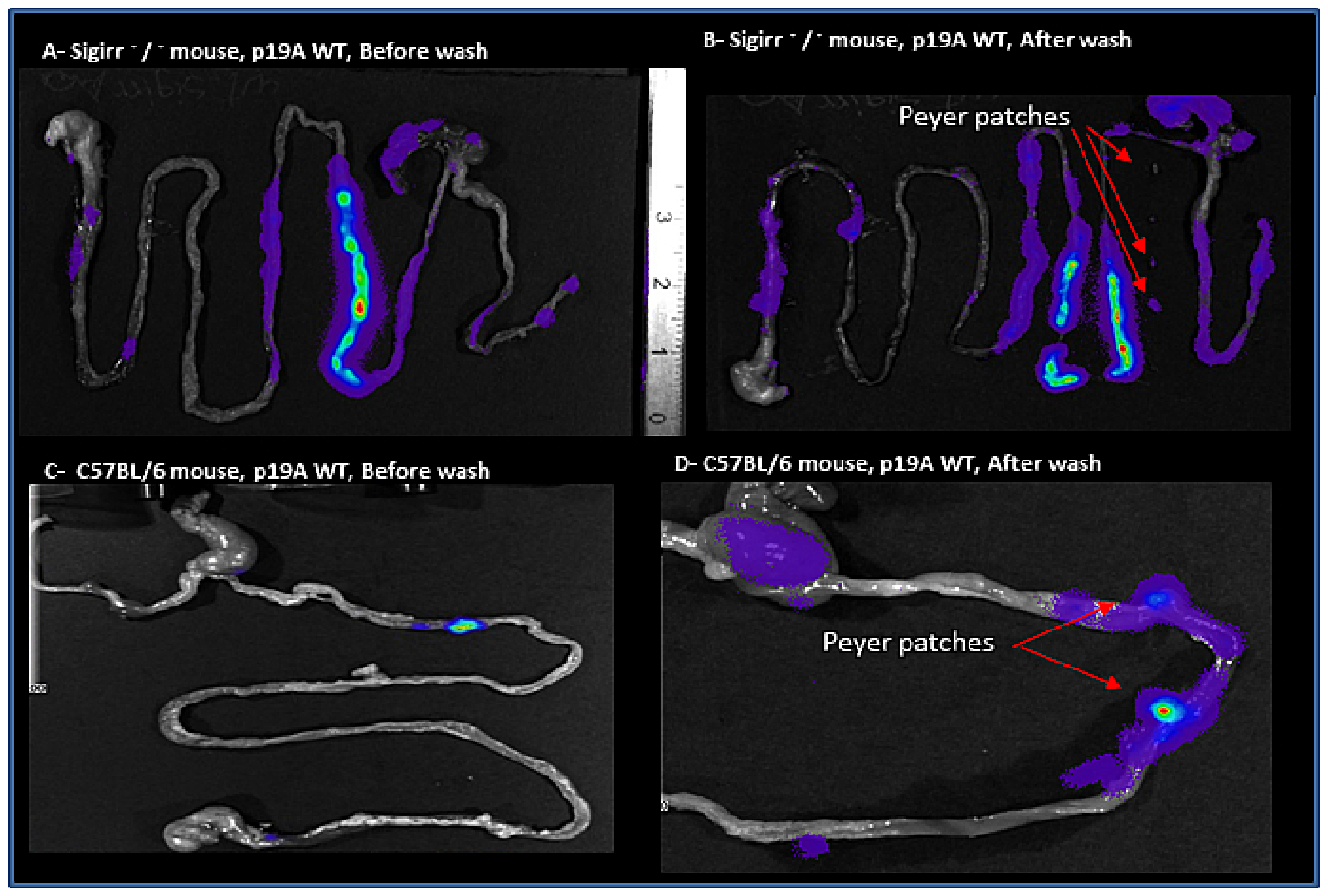
2.2. Macroscopic and In Vivo Imaging System
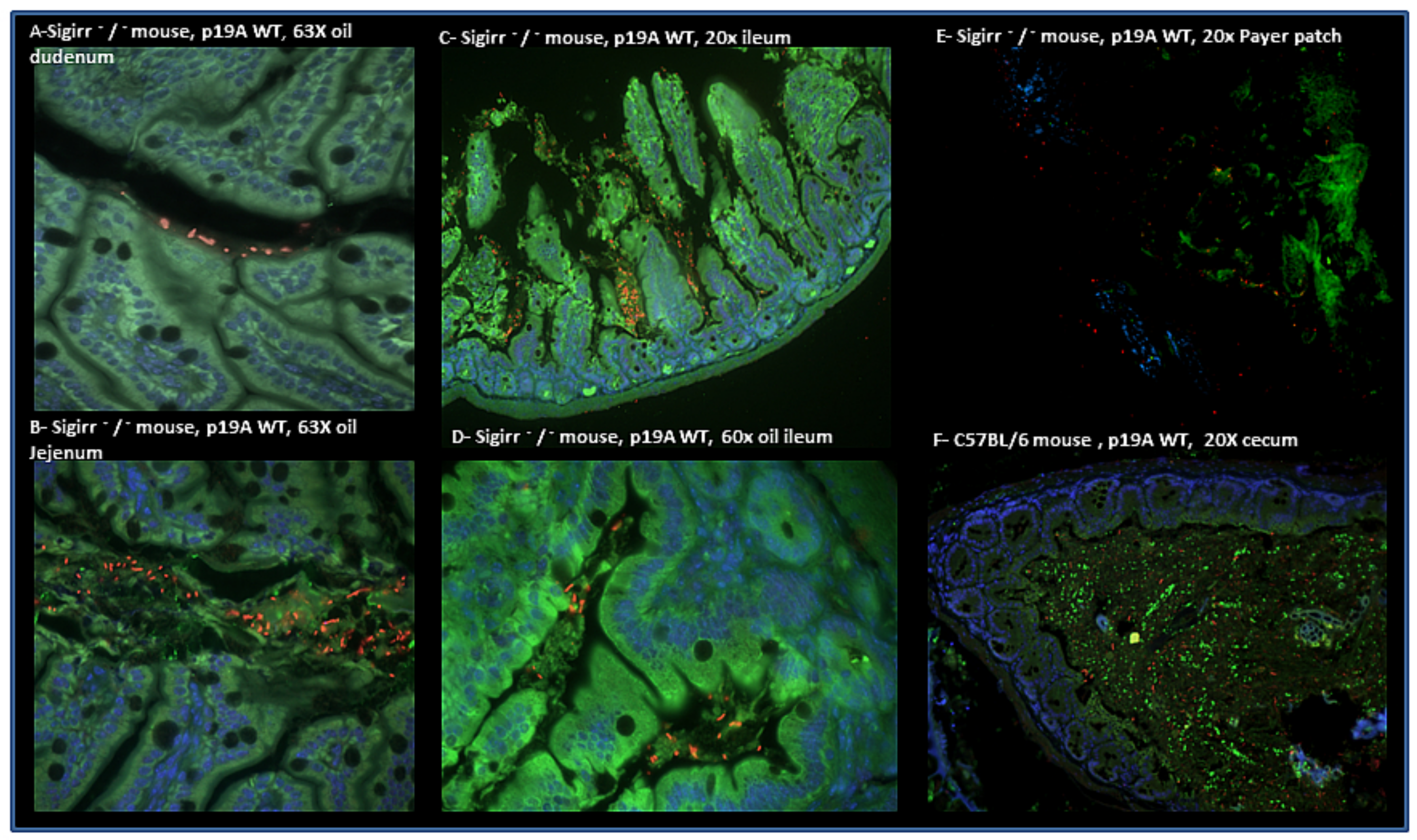
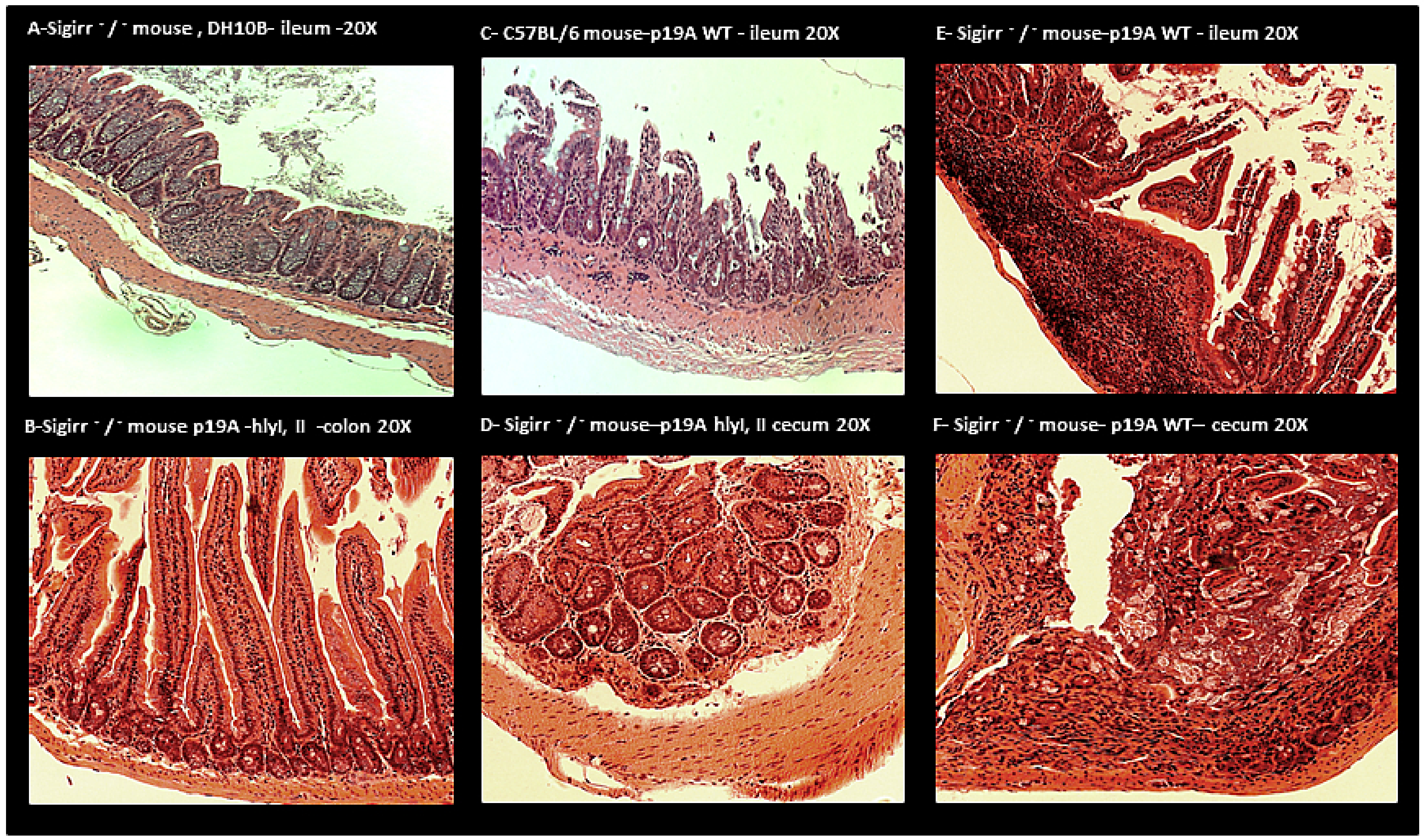
2.3. Histology
3. Discussion
4. Conclusions
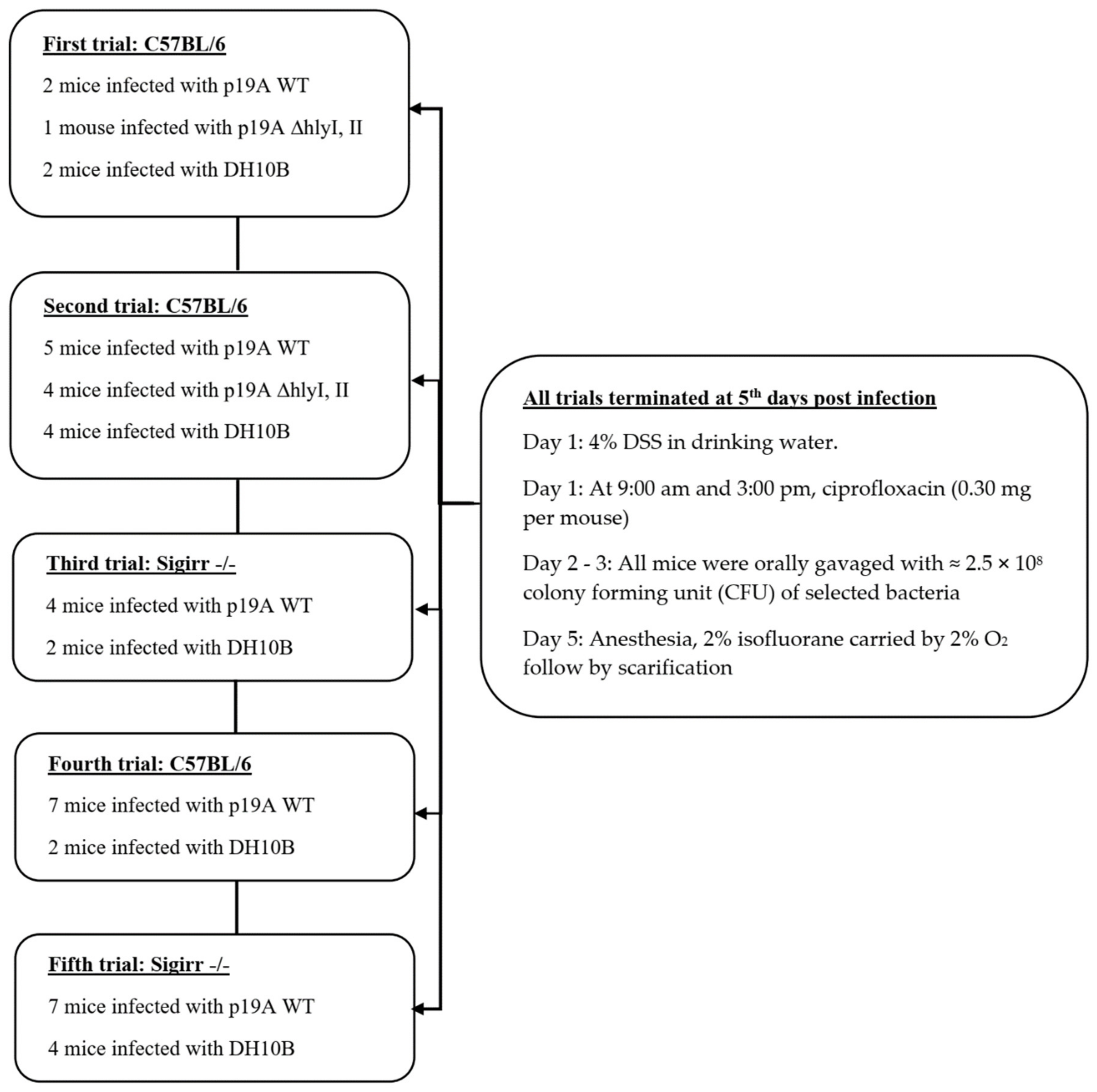
5. Materials and Methods
5.1. Clinical Isolate and Generated Mutants Used
5.2. Infection Course in DSS Mouse Model
5.3. Ethics Statement
5.4. Imaging
5.5. Tissue Collection
5.6. Histological and Immunofluorescence Staining
5.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| GIT | Gastrointestinal tract |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| ExPEC | Extra-intestinal pathogenic E. coli |
| AIEC | Adherent-invasive E. coli |
| DC | Dendritic cells |
| DSS | Dextran sodium sulfate |
| E. coli | Escherichia coli |
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic Bacteria with Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Kinnebrew, M.A.; Pamer, E.G. Innate immune signaling in defense against intestinal microbes. Immunol. Rev. 2012, 245, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Gulen, M.F.; Qin, J.; Yao, J.; Bulek, K.; Kish, D.; Altuntas, C.Z.; Wald, D.; Ma, C.; Zhou, H.; et al. The Toll-Interleukin-1 Receptor Member SIGIRR Regulates Colonic Epithelial Homeostasis, Inflammation, and Tumorigenesis. Immunity 2007, 26, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Ries, J.; Vermeulen, J.; Yang, H.; Sham, H.P.; Crowley, S.M.; Badayeva, Y.; Turvey, S.E.; Gaynor, E.C.; Li, X.; et al. A Novel Mouse Model of Campylobacter jejuni Gastroenteritis Reveals Key Pro-inflammatory and Tissue Protective Roles for Toll-like Receptor Signaling during Infection. PLoS Pathog. 2014, 10, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin. Microbial. Rev. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Hoffmann, J.C.; Pawlowski, N.N.; Kühl, A.A.; Höhne, W.; Zeitz, M. Animal models of inflammatory bowel disease: An overview. Pathobiol. J. Immunopathol. Mol. Cell Biol. 2008, 70, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Schulz, E.; Günzel, D.; Bojarski, C.; Lee, I.F.M.; John, L.J.; Wiegand, S.; Janßen, T.; Wieler, L.H.; Dobrindt, U.; et al. α-Haemolysin of Escherichia coli in IBD: A potentiator of inflammatory activity in the colon. Gut 2019, 63, 1893–1901. [Google Scholar] [CrossRef]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Feller, M.; Huwiler, K.; Shang, A.; Schoepfer, A.; Furrer, H.; Egger, M. Long-term antibiotic treatment for Crohn’s disease: Systematic review and meta-analysis of placebo-controlled trials. Clin. Infect. Dis. 2010, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Keighley, M.R.; Arabi, Y.; Dimock, F.; Burdon, D.W.; Allan, R.N.; Alexander-Williams, J. Influence of inflammatory bowel disease on intestinal microflora. Gut 1978, 19, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Giaffer, M.H.; Holdsworth, C.D.; Duerden, B.I. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut 1992, 33, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Ilnyckyj, A.; Greenberg, H.; Bernstein, C.N. Escherichia coli O157:H7 infection mimicking Crohn’s disease. Gastroenterology 1997, 112, 995–999. [Google Scholar] [CrossRef]
- Mirsepasi-Lauridsen, H.C.; Halkjaer, S.I.; Mortensen, E.M.; Lydolph, M.C.; Nordgaard-Lassen, I.; Krogfelt, K.A.; Petersen, A.M. Extraintestinal pathogenic Escherichia coli are associated with intestinal inflammation in patients with ulcerative colitis. Sci. Rep. 2016, 6, 31152. [Google Scholar] [CrossRef]
- Kotlowski, R.; Bernstein, C.N.; Sepehri, S.O.; Krause, D. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 2007, 56, 669–675. [Google Scholar] [CrossRef]
- Petersen, A.M.; Nielsen, E.M.; Litrup, E.; Brynskov, J.; Mirsepasi, H.; Krogfelt, K.A. A phylogenetic group of Escherichia coli associated with active left-sided inflammatory bowel disease. BMC Microbiol. 2009, 9, 171. [Google Scholar] [CrossRef]
- Satterwhite, T.K.; Evans, D.G.; Dupont, H.L.; Evans, D.J., Jr. Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet 1978, 2, 181–184. [Google Scholar] [CrossRef]
- Meconi, S.; Vercellone, A.; Levillain, F.; Payré, B.; Al Saati, T.; Capilla, F.; Desreumaux, P.; Darfeuille-Michaud, A.; Altare, F. Adherent-invasive Escherichia coli isolated from Crohn’s disease patients induce granulomas in vitro. Cell Microbiol. 2007, 9, 1252–1261. [Google Scholar] [CrossRef]
- Jensen, S.R.; Mirsepasi-Lauridsen, H.C.; Thysen, A.H.; Brynskov, J.; Krogfelt, K.A.; Petersen, A.M.; Pedersen, A.E.; Brix, S. Distinct inflammatory and cytopathic characteristics of Escherichia coli isolates from inflammatory bowel disease patients. Int. J. Med. Microbiol. 2015, 305, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Du, Z.; Struve, C.; Charbon, G.; Karczewski, J.; Krogfelt, K.A.; Petersen, A.M.; Wells, J.M. Secretion of Alpha-Hemolysin by Escherichia coli Disrupts Tight Junctions in Ulcerative Colitis Patients. Clin. Transl. Gastroenterol. 2016, 7, e149. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.V.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar]
- Sham, H.P.; Yu, E.Y.S.; Gulen, M.F.; Bhinder, G.; Stahl, M.; Chan, J.M.; Brewster, L.; Morampudi, V.; Gibson, D.L.; Hughes, M.R.; et al. SIGIRR, a Negative Regulator of TLR/IL-1R Signalling Promotes Microbiota Dependent Resistance to Colonization by Enteric Bacterial Pathogens. PLoS Pathog. 2013, 9, e1003539. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Seaver, L.C.; Imlay, J.A. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J. Biol. Chem. 2004, 279, 48742–48750. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Nuding, S.; Fellermann, K.; Wehkamp, J.; Stange, E.F. Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut 2007, 56, 1240–1247. [Google Scholar] [CrossRef]
- Newman, B.; Siminovitch, K. Inflammatory bowel disease: Crohn’s disease and the success of NODern genetics. Clin. Investig. Med. 2003, 26, 303–314. [Google Scholar]
- Chassaing, B.; Rolhion, N.; De Vallée, A.; Salim, S.Y.; Prorok-Hamon, M.; Neut, C.; Campbell, B.J.; Söderholm, J.D.; Hugot, J.-P.; Colombel, J.-F.; et al. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J. Clin. Investig. 2011, 121, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Vejborg, R.M.; Hancock, V.; Schembri, M.A.; Klemm, P. Comparative genomics of Escherichia coli strains causing urinary tract infections. Appl. Environ. Microbiol. 2011, 77, 3268–3278. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 2010, 1, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, R.; Ferrieri, P.; Wannamaker, L.W. Dynamics of Escherichia coli Infection and Meningitis in Infant Rats. Infect. Immun. 1978, 22, 480–485. [Google Scholar] [CrossRef]
- Ferrières, L.; Hémery, G.; Nham, T.; Guérout, A.-M.; Mazel, D.; Beloin, C.; Ghigo, J.-M. Silent Mischief: Bacteriophage Mu Insertions Contaminate Products of Escherichia coli Random Mutagenesis Performed Using Suicidal Transposon Delivery Plasmids Mobilized by Broad-Host-Range. J. Bacteriol. 2010, 192, 6418–6427. [Google Scholar]
- Sham, H.P.; Shames, S.R.; Croxen, M.A.; Ma, C.; Chan, J.M.; Khan, M.A.; Wickham, M.E.; Deng, W.; Finlay, B.B.; Vallance, B.A. Attaching and Effacing Bacterial Effector NleC Suppresses Epithelial Inflammatory Responses by Inhibiting NF-κB and p38 Mitogen-Activated Protein Kinase Activation. Infect. Immun. 2011, 79, 3552–3562. [Google Scholar] [CrossRef][Green Version]
- Choi, K.H.; Gaynor, J.B.; White, K.G.; Lopez, C.; Bosio, C.M.; Karkhoff-Schweizer, R.R.; Schweizer, H.P. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2005, 2, 443–448. [Google Scholar] [CrossRef]
- Robinson, G.M.; Tonks, K.M.; Thorn, R.M.; Reynolds, D.M. Application of Bacterial Bioluminescence to Assess the Efficacy of Fast-Acting Biocides. Antimicrob. Agents Chemother. 2011, 55, 5214–5219. [Google Scholar] [CrossRef]
- Lutz, R.; Bujard, H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I 1 -I 2 regulatory elements. Nucleic Acids Res. 1997, 25, 1203–1210. [Google Scholar] [CrossRef]
- Gibson, D.L.; Ma, C.; Rosenberger, C.M.; Bergstrom, K.S.B.; Valdez, Y.; Huang, J.T.; Khan, M.A.; Vallance, B.A. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008, 10, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ma, C.; Knodler, L.A.; Valdez, Y.; Rosenberger, C.M.; Deng, W.; Finlay, B.B.; Vallance, B.A. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 2006, 74, 2522–2536. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirsepasi-Lauridsen, H.C.; Struve, C.; Petersen, A.M.; Krogfelt, K.A. Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease. Microorganisms 2020, 8, 1971. https://doi.org/10.3390/microorganisms8121971
Mirsepasi-Lauridsen HC, Struve C, Petersen AM, Krogfelt KA. Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease. Microorganisms. 2020; 8(12):1971. https://doi.org/10.3390/microorganisms8121971
Chicago/Turabian StyleMirsepasi-Lauridsen, Hengameh Chloé, Carsten Struve, Andreas Munk Petersen, and Karen Angeliki Krogfelt. 2020. "Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease" Microorganisms 8, no. 12: 1971. https://doi.org/10.3390/microorganisms8121971
APA StyleMirsepasi-Lauridsen, H. C., Struve, C., Petersen, A. M., & Krogfelt, K. A. (2020). Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease. Microorganisms, 8(12), 1971. https://doi.org/10.3390/microorganisms8121971




