Simultaneous Genome Sequencing of Prosthecochloris ethylica and Desulfuromonas acetoxidans within a Syntrophic Mixture Reveals Unique Pili and Protein Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultures and DNA Preparation
2.2. DNA Sequencing, Assembly, and Annotation
2.3. Metagenomic Binning
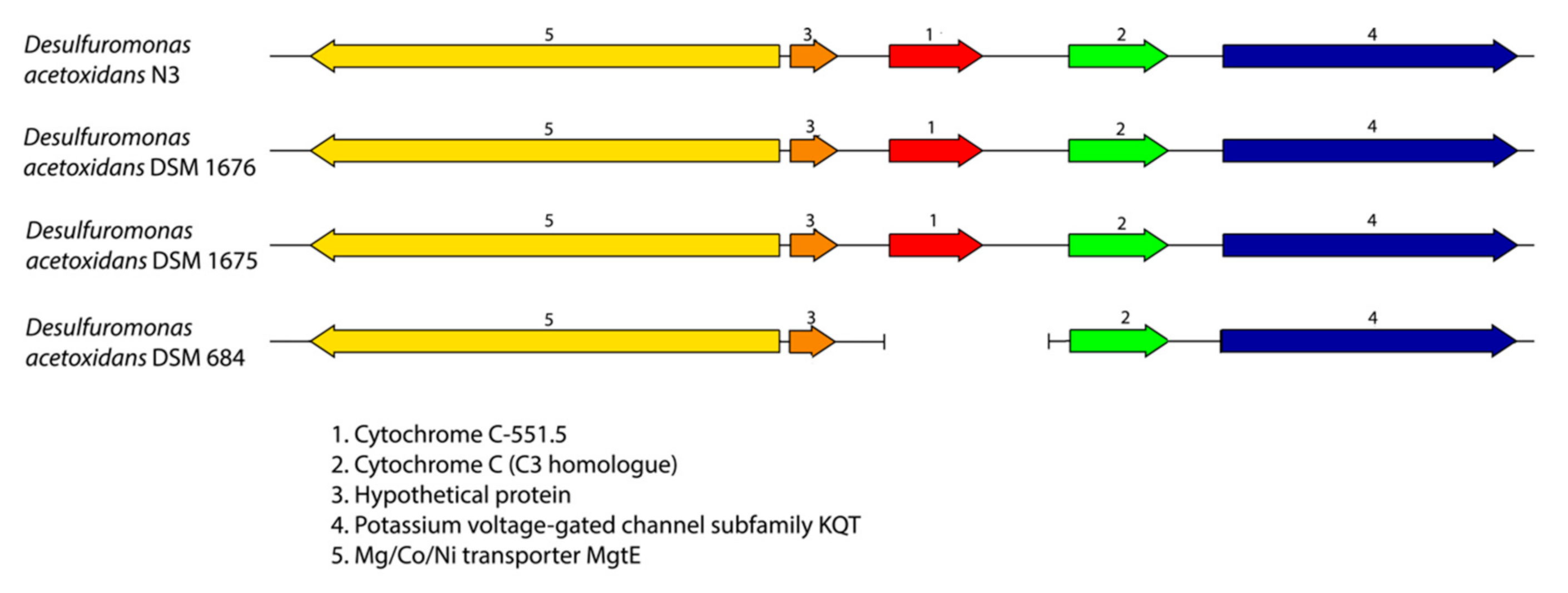
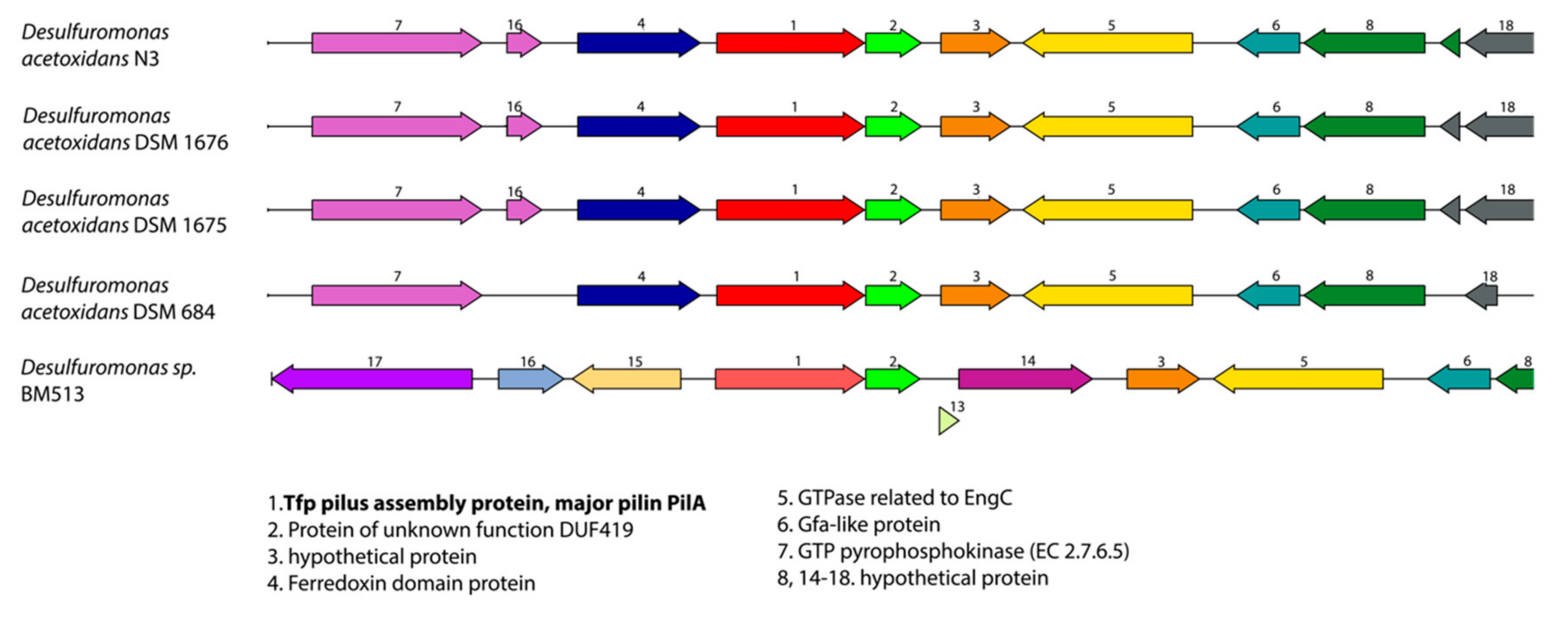
2.4. Synteny Analysis
3. Results
3.1. Prosthecochloris Genome from the Green Sulfur Component
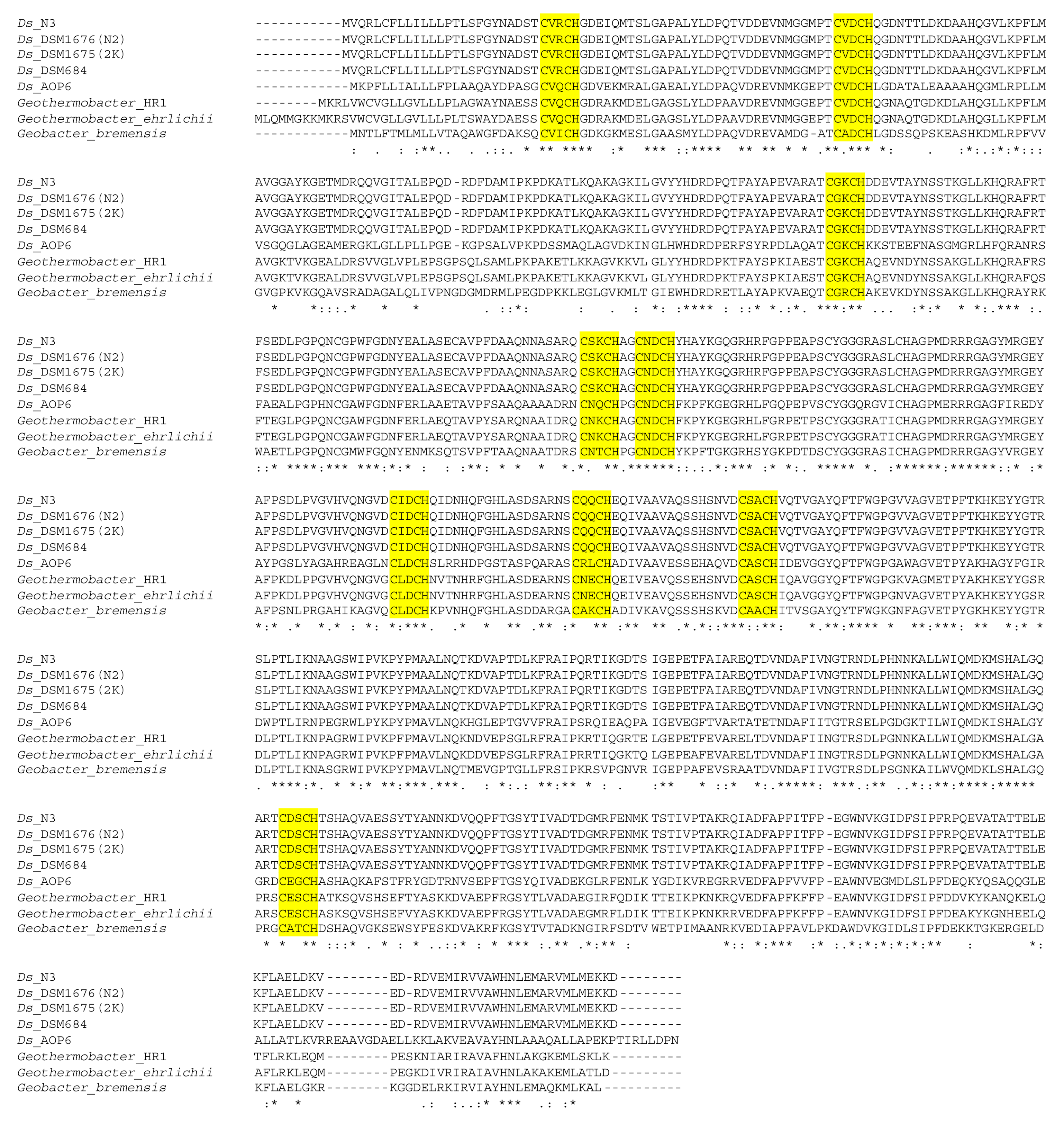
3.2. Desulfuromonas Genome from the Colorless Sulfur-Reducing Component
3.3. Comparison of the Geobacter sulfurreducens Genomes to the Desulfuromonas Genomes
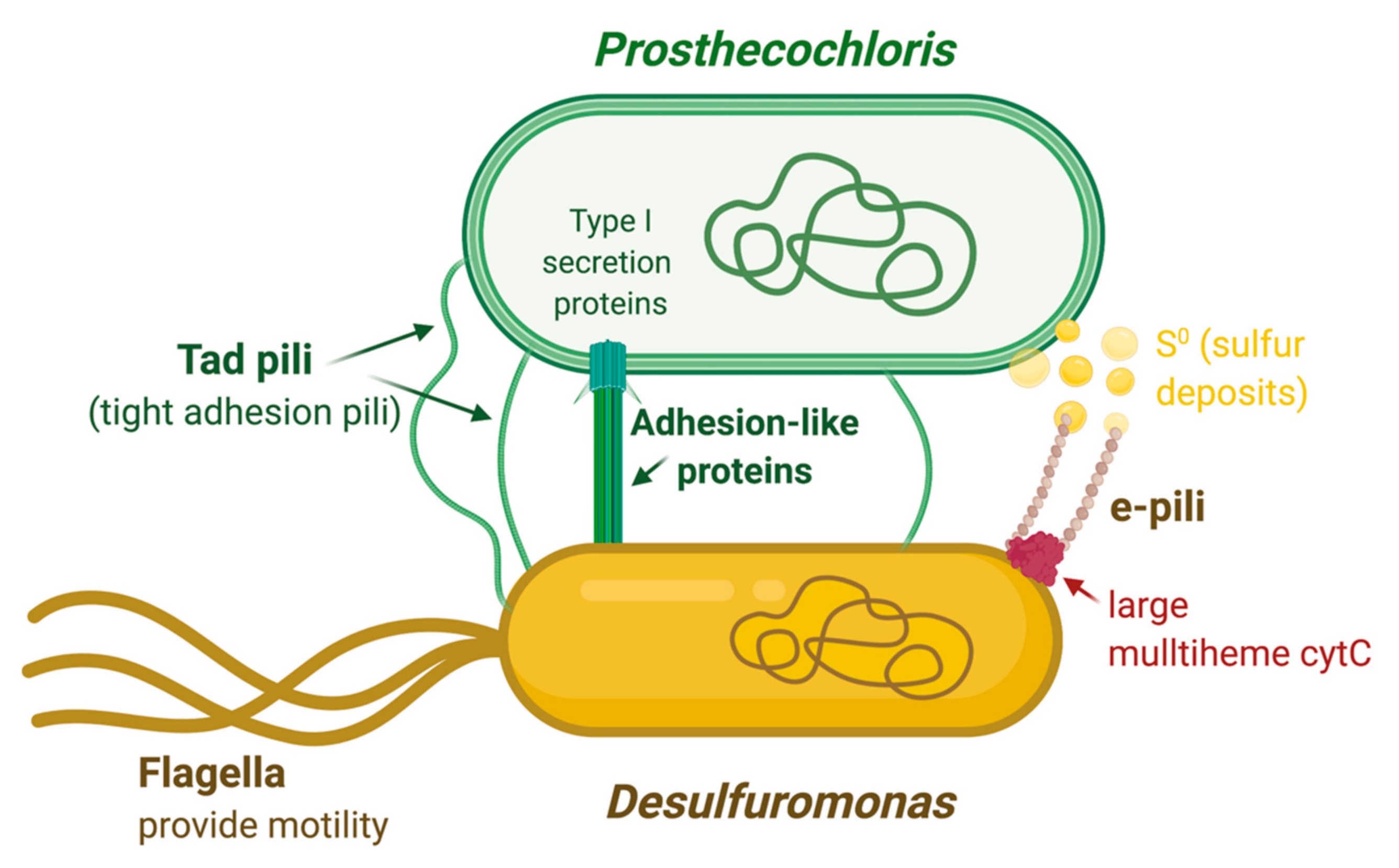
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Shaposhnikov, V.V.; Kondratieva, E.N.; Federov, V.D. A new species of green sulfur bacteria. Nature 1960, 187, 167–168. [Google Scholar] [CrossRef]
- Olson, J.M. Historical note on Chloropseudomonas ethylica strain 2-K. Int. J. Syst. Bacteriol. 1973, 23, 265–266. [Google Scholar] [CrossRef][Green Version]
- Olson, J.M. Confused history of Chloropseudomonas ethylica 2-K. Int. J. Syst. Bacteriol. 1978, 28, 128–129. [Google Scholar] [CrossRef]
- Pfennig, N.; Biebl, H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch. Microbiol. 1976, 110, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Van Beeumen, J.; Ambler, R.P.; Meyer, T.E.; Kamen, M.O.; Olson, J.M.; Shaw, E.K. The amino acid sequences of the cytochromes c-555 from two green sulphur bacteria of the genus Chlorobium. Biochem. J. 1976, 159, 757–769. [Google Scholar] [CrossRef]
- Ambler, R.P. The amino acid sequence of cytochrome c-551.5 (Cytochrome c(7)) from the green photosynthetic bacterium Chloropseudomonas ethylica. FEBS Lett. 1971, 18, 351–353. [Google Scholar] [CrossRef]
- Gray, B.H.; Fowler, C.F.; Nugent, N.A.; Fuller, R.C. A reevaluation of the presence of low midpoint potential cytochrome C-551.5 in the green photosynthetic bacterium Chloropseudomonas ethylica. Biochem. Biophys. Res. Commun. 1972, 47, 322–327. [Google Scholar] [CrossRef]
- Gray, B.H.; Fowler, C.F.; Nugent, N.A.; Rigopoulos, N.; Fuller, R.C. Reevaluation of Chloropseudomonas ethylica strain 2-K. Int. J. Syst. Bacteriol. 1973, 23, 256–264. [Google Scholar] [CrossRef][Green Version]
- Overmann, J. Phototrophic Consortia: A Tight Cooperation Between Non-Related Eubacteria. In Symbiosis. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2001; Volume 4. [Google Scholar]
- Schink, B.; Stams, A.J.M. Syntrophism among Prokaryotes. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef]
- Klatt, C.; Wood, J.; Rusch, D.; Bateson, M.M.; Hamamura, N.; Heidelberg, J.F.; Grossman, A.R.; Bhaya, D.; Cohan, F.M.; Kühl, M.; et al. Community ecology of hot spring cyanobacterial mats: Predominant populations and their functional potential. ISME J. 2011, 5, 1262–1278. [Google Scholar] [CrossRef]
- Liu, Z.; Müller, J.; Li, T.; Alvey, R.M.; Vogl, K.; Frigaard, N.U.; Rockwell, N.C.; Boyd, E.S.; Tomsho, L.P.; Schuster, S.C.; et al. Genomic analysis reveals key aspects of prokaryotic symbiosis in the phototrophic consortium “Chlorochromatium aggregatum”. Genome Biol. 2013, 14, R127. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Overmann, J. Close interspecies interactions between prokaryotes from sulfureous environments. Front. Microbiol. 2011, 2, 146. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Kosaka, T.; Hori, K.; Hotta, Y.; Watanabe, K. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl. Environ. Microbiol. 2005, 71, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Hohn, M.J.; Stetter, K.O.; Rachel, R. The phylum Nanoarchaeota: Present knowledge and future perspectives of a unique form of life. Res. Microbiol. 2003, 154, 165–171. [Google Scholar] [CrossRef]
- Lauterborn, R. Zur Kenntnis der sapropelischen Flora. Allg. Bot. Z. 1906, 12, 196–197. [Google Scholar]
- Biebl, H.; Pfennig, N. Growth yields of green sulfur bacteria in mixed cultures with sulfur and sulfate reducing bacteria. Arch. Microbiol. 1978, 117, 9–16. [Google Scholar] [CrossRef]
- Warthmann, R.; Cypionka, H.; Pfennig, N. Photoproduction of H2 from acetate by syntrophic cocultures of green sulfur bacteria and sulfur-reducing bacteria. Arch. Microbiol. 1992, 157, 343–348. [Google Scholar] [CrossRef]
- Wanner, G.; Vogl, K.; Overmann, J. Ultrastructural characterization of the prokaryotic symbiosis in “Chlorochromatium aggregatum”. J. Bacteriol. 2008, 190, 3721–3730. [Google Scholar] [CrossRef]
- Copeland, A.; Lucas, S.; Lapidus, A.; Barry, K.; Detter, J.C.; Glavina del Rio, T.; Hammon, N.; Israni, S.; Dalin, E.; Tice, H.; et al. Sequencing of the draft genome and assembly of Desulfuromonas acetoxidans DSM 684. Jt. Genome Inst. 2005, unpublished. [Google Scholar]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Fromsma, K.; Gerdes, S.; Glass, E.M.; Kabul, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J.J.S.B. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Stamatakis, A.J.B. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2014, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Freed, S.; Robertson, S.; Meyer, T.; Kyndt, J. Draft Whole-Genome Sequence of the Green Sulfur Photosynthetic Bacterium Chlorobaculum sp. Strain 24CR, Isolated from the Carmel River. Microbiol. Resour. Announc. 2019, 8, e00116-19. [Google Scholar] [CrossRef] [PubMed]
- Overmann, J.; Pfennig, N. Pelodictyon phaeoclathiforme sp. nov., a new brown-colored member of the Chlorobiaceae forming net-like colonies. Arch. Microbiol. 1989, 152, 401–406. [Google Scholar] [CrossRef]
- Tomich, M.; Planet, P.J.; Figurski, D.H. The tad locus: Postcards from the widespread colonization island. Nat. Rev. Microbiol. 2007, 5, 363–375. [Google Scholar] [CrossRef]
- Bodenmiller, D.; Toh, E.; Brun, Y.V. Development of surface adhesion in Caulobacter crescentus. J. Bacteriol. 2004, 186, 1438–1447. [Google Scholar] [CrossRef]
- Entcheva-Dimitrov, P.; Spormann, A.M. Dynamics and control of biofilms of the oligotrophic bacterium Caulobacter crescentus. J. Bacteriol. 2004, 186, 8254–8266. [Google Scholar] [CrossRef] [PubMed]
- Sangermani, M.; Hug, I.; Sauter, N.; Pfohl, T.; Jenal, U. Tad Pili play a dynamic role in Caulobacter crescentus surface colonization. mBio 2019, 10, e01237-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fives-Taylor, P.M. Molecular strategies for fimbrial expression and assembly. Crit. Rev. Oral Biol. Med. 2001, 12, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.A.; Berenguer, J. Secretion and assembly of regular surface structures in Gram-negative bacteria. FEMS Microbiol. Rev. 2000, 24, 21–44. [Google Scholar] [CrossRef]
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef]
- Satchell, K.J. Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu. Rev. Microbiol. 2011, 65, 71–90. [Google Scholar] [CrossRef]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Lasa, I.; Penades, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef]
- Yousef, F.; Espinosa-Urgel, M. In silico analysis of large microbial surface proteins. Res. Microbiol. 2007, 158, 545–550. [Google Scholar] [CrossRef]
- Treangen, T.J.; Salzberg, S.L. Repetitive DNA and next-generation sequencing: Computational challenges and solutions. Nat. Rev. Genet. 2011, 13, 36–46. [Google Scholar] [CrossRef]
- Nagarajan, H.; Butler, J.E.; Klimes, A.; Qiu, Y.; Zengler, K.; Ward, J.; Young, N.D.; Methé, B.A.; Palsson, B.Ø.; Lovley, D.R.; et al. De novo assembly of the complete genome of an enhanced electricity-producing variant of Geobacter sulfurreducens using only short reads. PLoS ONE 2010, 5, e10922. [Google Scholar] [CrossRef] [PubMed]
- Parge, H.E.; Forest, K.T.; Hickey, M.J.; Christensen, D.A.; Getzoff, E.D.; Tainer, J.A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 1995, 378, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Xu, A.; Zhang, L.; Liu, H.; Ma, L.Z. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Walker, D.J.F. Geobacter Protein Nanowires. Front. Microbiol. 2019, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires: A new paradigm for biological electron transfer and bioelectronics. ChemSusChem 2012, 5, 1039–1046. [Google Scholar] [CrossRef]
- Aklujkar, M.; Haveman, S.A.; DiDonato, R.; Chertov, O.; Han, C.S.; Land, M.L.; Brown, P.; Lovley, D.R. The genome of Pelobacter carbinolicus reveals surprising metabolic capabilities and physiological features. BMC Genom. 2012, 13, 690. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef]
- Zanetti, G.; Pandini, V. Ferredoxin. In Encyclopedia of Biological Chemistry, 2nd ed.; William, J., Lennarz, M., Lane, D., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 296–298. ISBN 9780123786319. [Google Scholar]
- Marsh, J.W.; Taylor, R.K. Genetic and Transcriptional Analyses of the Vibrio cholerae Mannose-Sensitive Hemagglutinin Type 4 Pilus Gene Locus. J. Bacteriol. 1999, 181, 1110–1117. [Google Scholar] [CrossRef]
- Ha, P.T.; Lindemann, S.R.; Shi, L.; Dohnalkova, A.C.; Fredrickson, J.K.; Madigan, M.T.; Beyenal, H. Syntrophic Anaerobic Photosynthesis via Direct Interspecies Electron Transfer. Nat. Commun. 2017, 8, 13924. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Aoyagi, T.; Inaba, T.; Sato, Y.; Habe, H.; Hori, T. Complete Genome Sequence of Desulfuromonas sp. Strain AOP6, an Iron(III) Reducer Isolated from Subseafloor Sediment. Microbiol. Res. Announc. 2020, 9, e01325-19. [Google Scholar] [CrossRef] [PubMed]
- Childers, S.E.; Ciufo, S.; Lovley, D.R. Geobacter metallireducens accesses insoluble Fe(III)oxide by chemotaxis. Nature 2002, 416, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Pokkuluri, P.R.; Londer, Y.Y.; Duke, N.E.; Long, W.C.; Schiffer, M. Family of cytochrome c7-type proteins from Geobacter sulfurreducens: Structure of one cytochrome c7 at 1.45 Å resolution. Biochemistry 2004, 43, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Probst, I.; Bruschi, M.; Pfennig, N.; Le Gall, J. Cytochrome c-551.5 (c7) from Desulfuromonas acetoxidans. Biochim. Biophys. Acta 1977, 460, 58–64. [Google Scholar] [CrossRef]
- Pereira, I.A.; Pacheco, I.; Liu, M.Y.; Legall, J.; Xavier, A.V.; Teixeira, M. Multiheme cytochromes from the sulfur-reducing bacterium Desulfuromonas acetoxidans. Eur. J. Biochem. 1997, 248, 323–328. [Google Scholar] [CrossRef]
- Mathews, F.S. The structure, function and evolution of cytochromes. Prog. Biophys. Mol. Biol. 1985, 45, 1–56. [Google Scholar] [CrossRef]
- Edwards, M.J.; Richardson, D.J.; Paquete, C.M.; Clarke, T.A. Role of multiheme cytochromes involved in extracellular anaerobic respiration in bacteria. Protein Sci. 2020, 29, 830–842. [Google Scholar] [CrossRef]
- Leang, C.; Qian, X.; Mester, T.; Lovley, D.R. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 4080–4084. [Google Scholar] [CrossRef]
- Imhoff, J.F. Biology of Green Sulfur Bacteria. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]

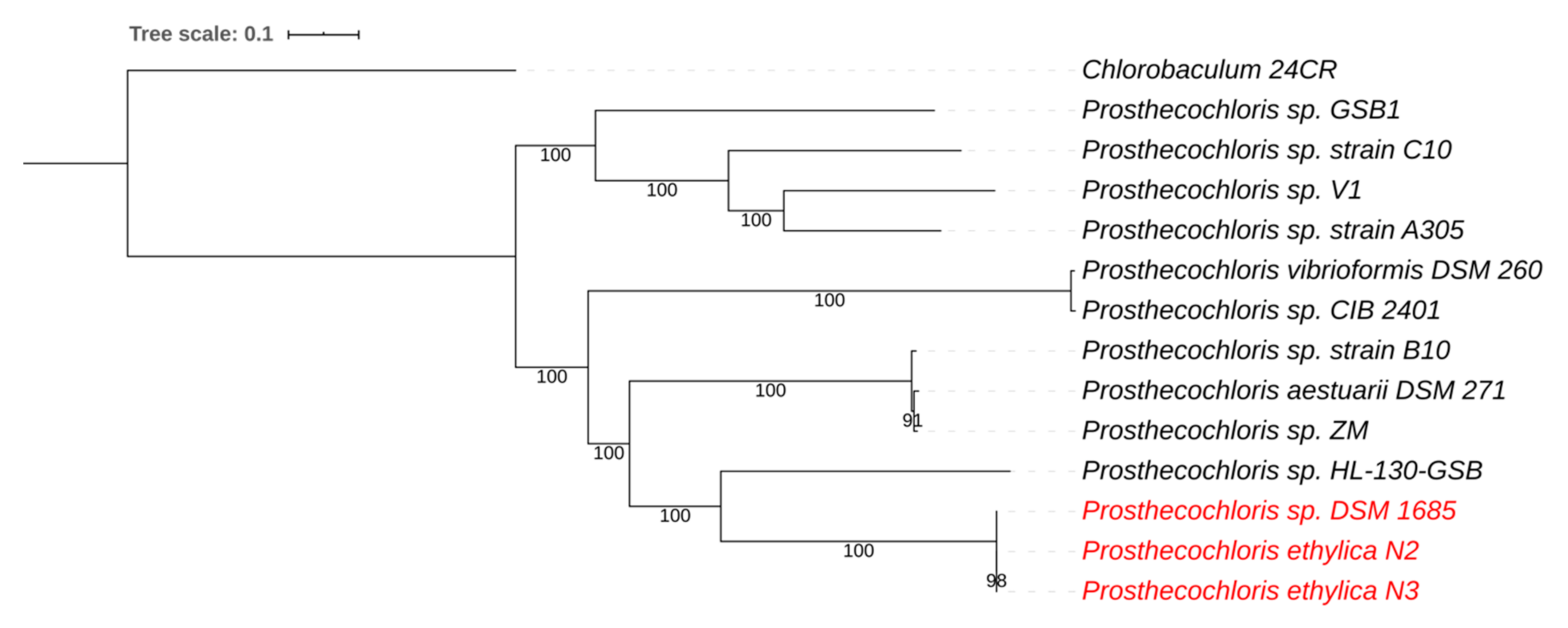
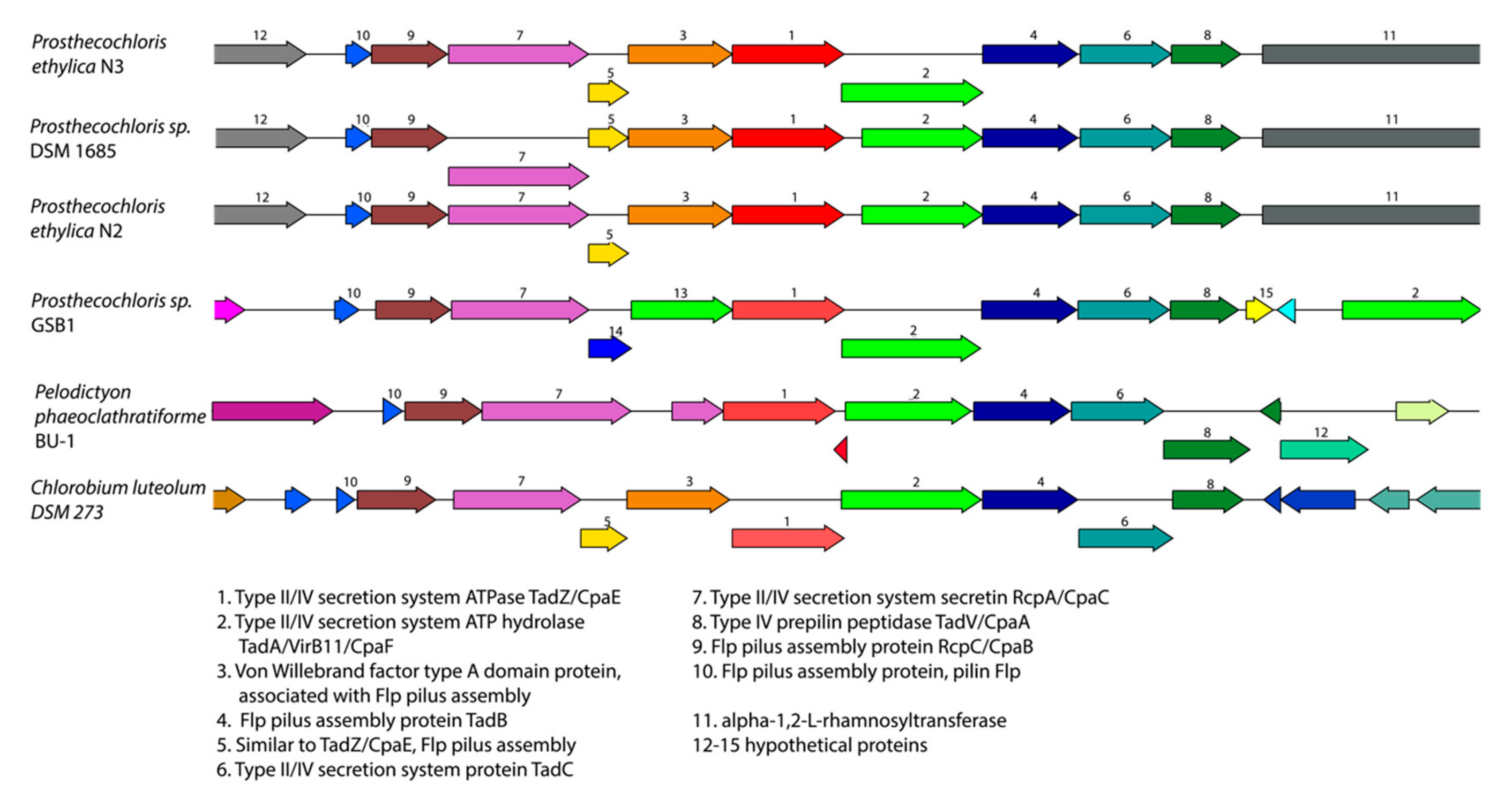
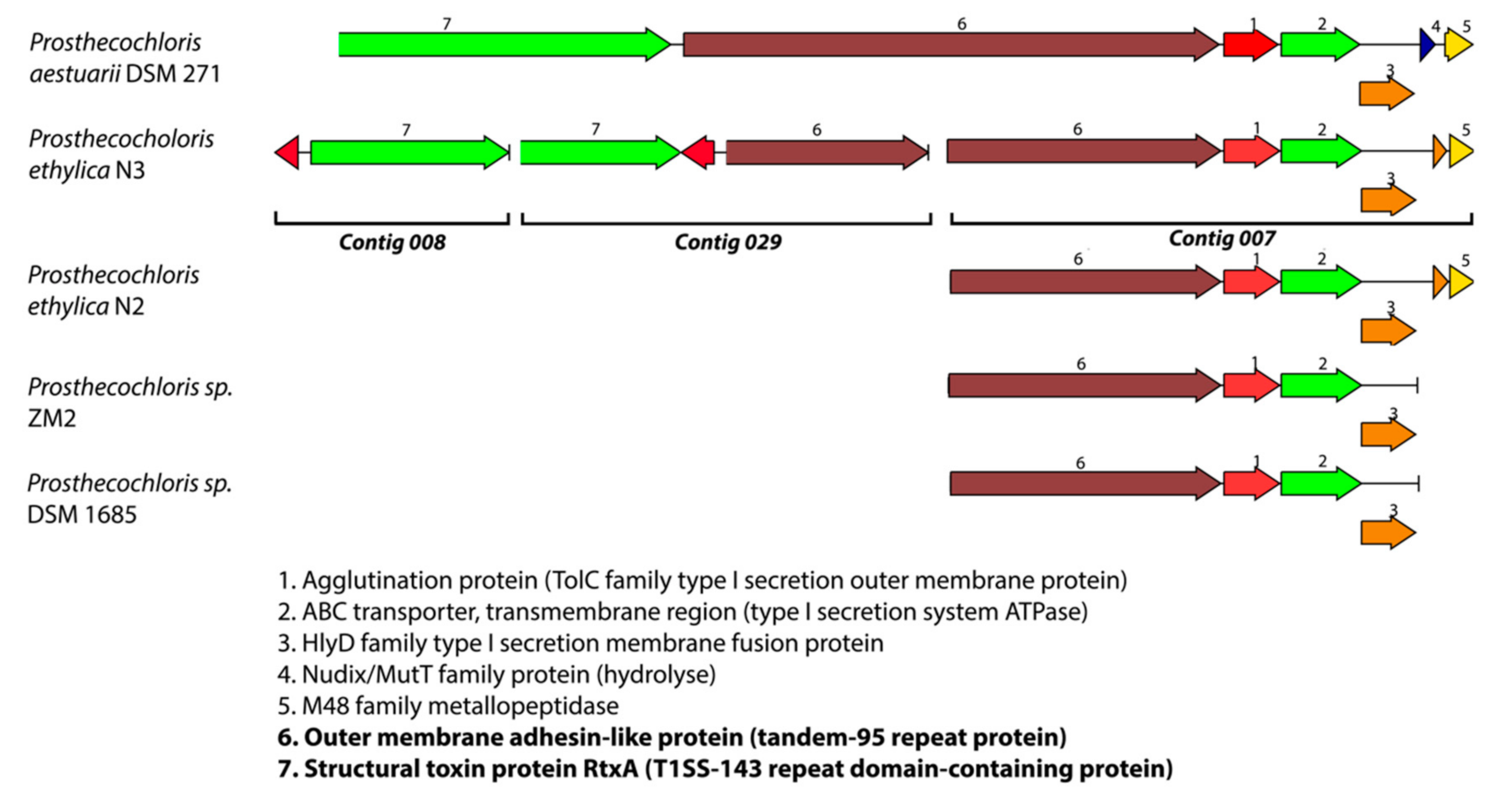
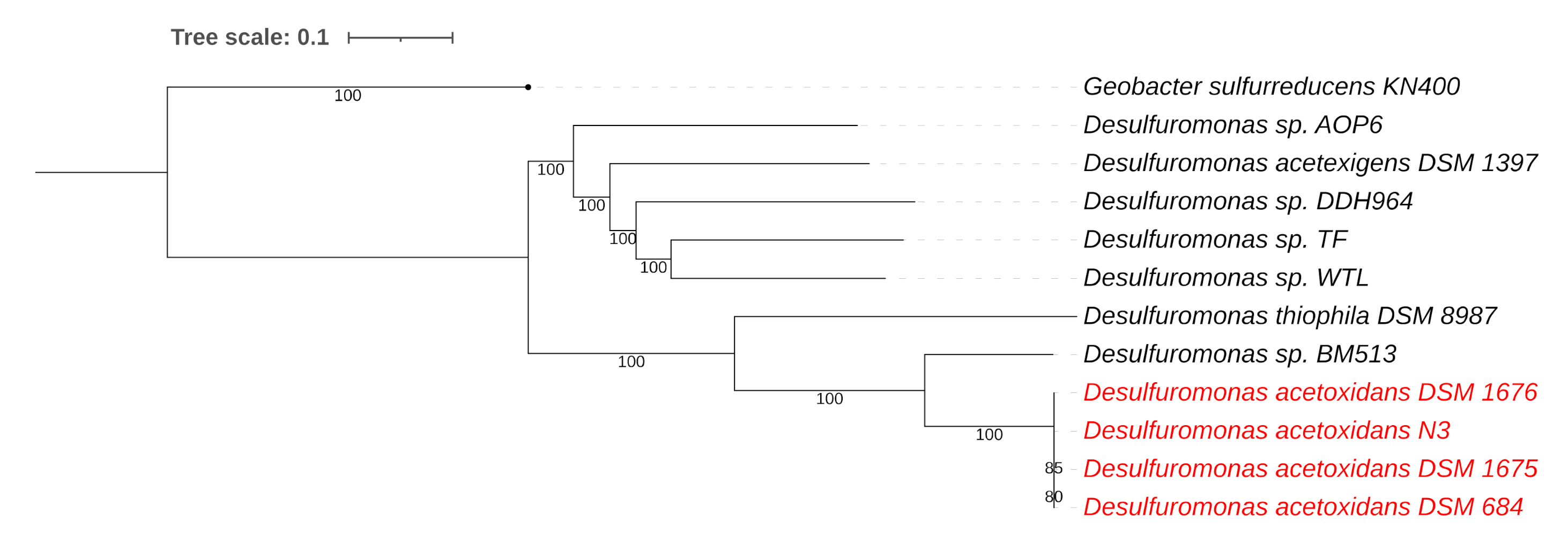
| Species | Genome Size | GC Content | Contigs | Coverage | CDS | tRNAs | Reference | Genbank Accession # |
|---|---|---|---|---|---|---|---|---|
| Prosthecochloris (N3 mix) | 2.45 Mb | 55.1 | 72 | 223x | 2480 | 45 | this study | JADGII000000000 |
| Prosthecochloris (N2 mix) | 2.47 Mb | 55.1 | 60 | 187x | 2340 | 46 | this study | JADGIH000000000 |
| Prosthecochloris (2K, DSM 1685) | 2.44 Mb | 55.1 | 66 | 123x | 2348 | 45 | this study | JABVZQ010000000 |
| Desulfuromonas (N3 mix) | 3.78 Mb | 51.9 | 121 | 84x | 3557 | 51 | this study | JADGIJ000000000 |
| Desulfuromonas (N2, DSM 1676) | 3.81 Mb | 51.9 | 52 | 38x | 3567 | 55 | this study | JABWTG010000000 |
| Desulfuromonas (2K, DSM 1675) | 3.80 Mb | 51.9 | 59 | 39x | 3550 | 55 | this study | JABWTF010000000 |
| Desulfuromonas DSM 684 | 3.83 Mb | 51.8 | 51 | N/A | 3573 | 49 | unpublished | NZ_AAEW00000000 |
| Pr. ethylica DSM 1685, isolated in pure culture from the Cp. ethylica 2K mixture | |||||||||||
| 99.91 | Pr. ethylica N2 in the Cp. ethylica N2 mixture | ||||||||||
| 99.95 | 99.93 | Pr. ethylica N3 in the Cp. ethylica N3 mixture | |||||||||
| 74.5 | 74.7 | 74.6 | Prosthecochloris sp HL130 | ||||||||
| 71 | 70.9 | 70.9 | 70.3 | Prosthecochloris sp Ty Vent | |||||||
| 72.8 | 73 | 72.9 | 72.2 | 70.8 | Pr. aestuarii DSM 271 | ||||||
| 69.5 | 69.5 | 69.5 | 69.5 | 70.5 | 70.2 | Pr. marina VI | |||||
| 69.8 | 69.9 | 69.8 | 69.6 | 71.1 | 70.5 | 77.2 | Prosthecochloris sp A305 | ||||
| 70.3 | 70.3 | 70.3 | 70.5 | 71.5 | 71.4 | 74.6 | 76.3 | Pr. phaeobacteroides BS1 | |||
| 72.2 | 72.3 | 72.3 | 72 | 70 | 71.2 | 68.9 | 69 | 70.4 | Pr. vibrioforme DSM 260 | ||
| 72 | 72.2 | 72.1 | 71.8 | 69.9 | 71 | 68.9 | 69 | 70.3 | 98.3 | Pr. phaeum 2401 | |
| Ds. acetoxidans DSM 684T pure culture | ||||||||
| 99.92 | Ds. acetoxidans 2K DSM 1675 pure culture | |||||||
| 99.91 | 99.99 | Ds. acetoxidans N2 DSM 1676 pure culture | ||||||
| 99.86 | 99.92 | 99.93 | Ds. acetoxidans in the Cp. ethylica N3 mixture | |||||
| 69.9 | 69.9 | 70 | 70 | Ds. thiophila DSM 8987 | ||||
| 67.2 | 67.4 | 67.4 | 67.4 | 68.9 | Ds. acetexigens null | |||
| 67.4 | 67.5 | 67.5 | 67.4 | 68.7 | 72.1 | Ds. sp. WTL | ||
| 67.2 | 67.3 | 67.3 | 67.2 | 69.5 | 71.5 | 72.3 | Ds. sp. DDH 964 | |
| Gene | PGFam | Protein Description | Size (aa.) | |
|---|---|---|---|---|
| PilA | PGF_00056426 | Tfp pilus major pilin assembly protein | 314 | |
| PilZ | PGF_00414431 | Type IV pilus assembly protein | 121 | |
| PilX | PGF_03889320 | Type IV fimbrial biogenesis protein | 174 | |
| PGF_04686021 | 204 | |||
| PilV | PGF_04940117 | Type IV fimbrial biogenesis protein | 141 | |
| PGF_05800309 | 126 | |||
| PilP | PGF_06326322 | Type IV fimbrial biogenesis protein | 171 | |
| PilW | PGF_10370792 | Type IV fimbrial biogenesis protein | 330 | |
| FimT | PGF_10544072 | Type IV fimbrial biogenesis protein | 143 | |
| PglB | PGF_05008343 | Pilin glycosylation protein | 206 | |
| Ferro | PGF_00004340 | Ferrodoxin domain protein | 260 | |
| MshA | PGF_06702067 | MSHA pilin protein | 169 | |
| PGF_12682451 | 178 | |||
| MshD | PGF_01602851 | MSHA pilin protein | 152 | |
| MshI | PGF_05907321 | MSHA biogenesis protein | 198 | |
| MshJ | PGF_02203893 | MSHA biogenesis protein | 230 | |
| MshK | PGF_00018410 | MSHA biogenesis protein | 119 | |
| MshN | PGF_00938251 | MSHA biogenesis protein | 430 | |
| MshP | PGF_01166820 | MSHA biogenesis protein | 130 | |
| CytC | PGF_12883110 | Multiheme Cytochrome C family protein | 624 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyndt, J.A.; Van Beeumen, J.J.; Meyer, T.E. Simultaneous Genome Sequencing of Prosthecochloris ethylica and Desulfuromonas acetoxidans within a Syntrophic Mixture Reveals Unique Pili and Protein Interactions. Microorganisms 2020, 8, 1939. https://doi.org/10.3390/microorganisms8121939
Kyndt JA, Van Beeumen JJ, Meyer TE. Simultaneous Genome Sequencing of Prosthecochloris ethylica and Desulfuromonas acetoxidans within a Syntrophic Mixture Reveals Unique Pili and Protein Interactions. Microorganisms. 2020; 8(12):1939. https://doi.org/10.3390/microorganisms8121939
Chicago/Turabian StyleKyndt, John A., Jozef J. Van Beeumen, and Terry E. Meyer. 2020. "Simultaneous Genome Sequencing of Prosthecochloris ethylica and Desulfuromonas acetoxidans within a Syntrophic Mixture Reveals Unique Pili and Protein Interactions" Microorganisms 8, no. 12: 1939. https://doi.org/10.3390/microorganisms8121939
APA StyleKyndt, J. A., Van Beeumen, J. J., & Meyer, T. E. (2020). Simultaneous Genome Sequencing of Prosthecochloris ethylica and Desulfuromonas acetoxidans within a Syntrophic Mixture Reveals Unique Pili and Protein Interactions. Microorganisms, 8(12), 1939. https://doi.org/10.3390/microorganisms8121939






