Dichloromethane Degradation Pathway from Unsequenced Hyphomicrobium sp. MC8b Rapidly Explored by Pan-Proteomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Cultivation
2.2. Cell Lysis and Enzymatic Proteolysis
2.3. Mass Spectrometry and Data Interpretation
2.4. Genome Sequencing, Assembly and Annotation
2.5. Data
3. Results
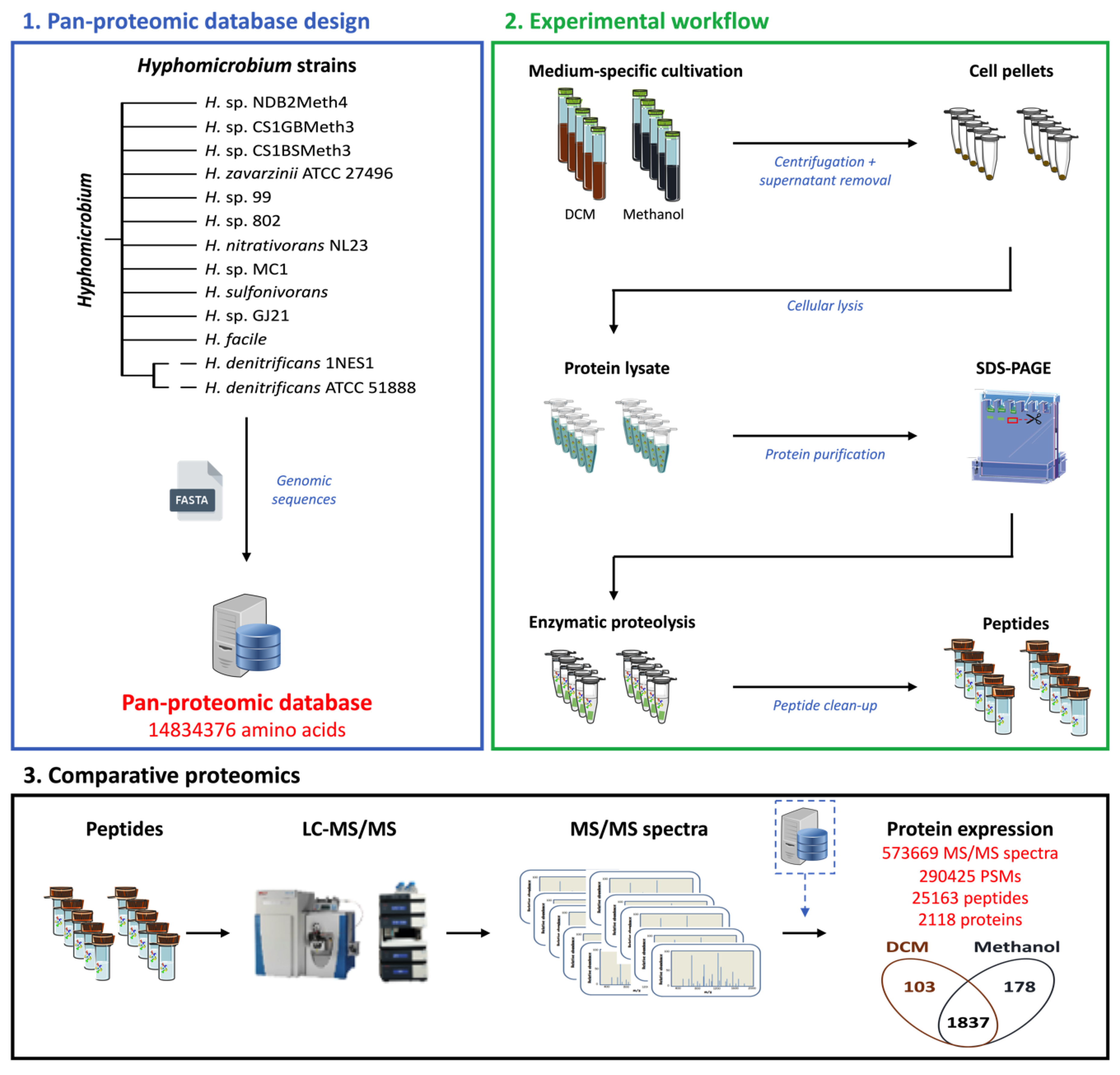
3.1. Pan-Proteomics Strategy for Characterizing DCM-Degrading Strain MC8b of Unknown Genome Sequence
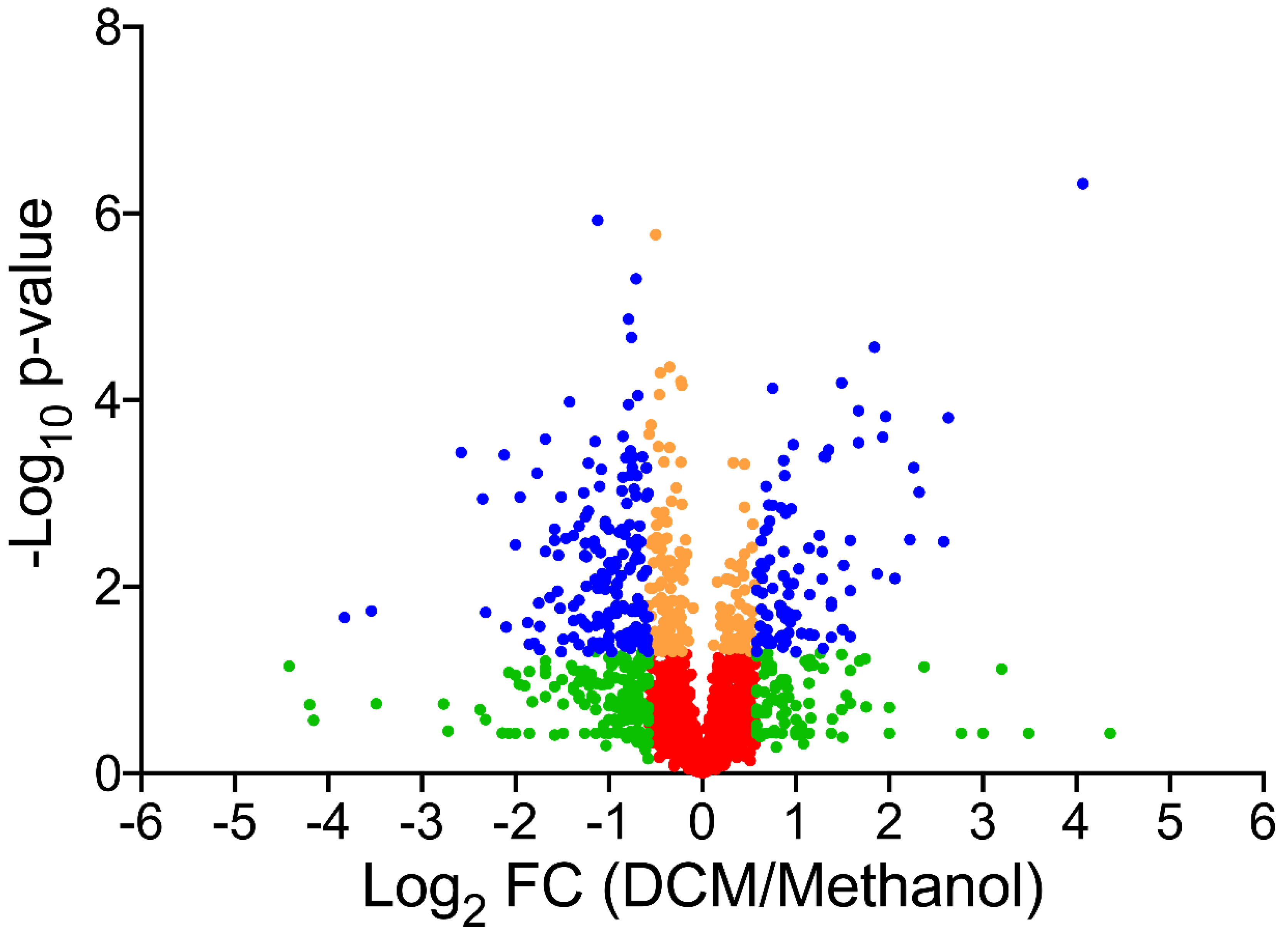
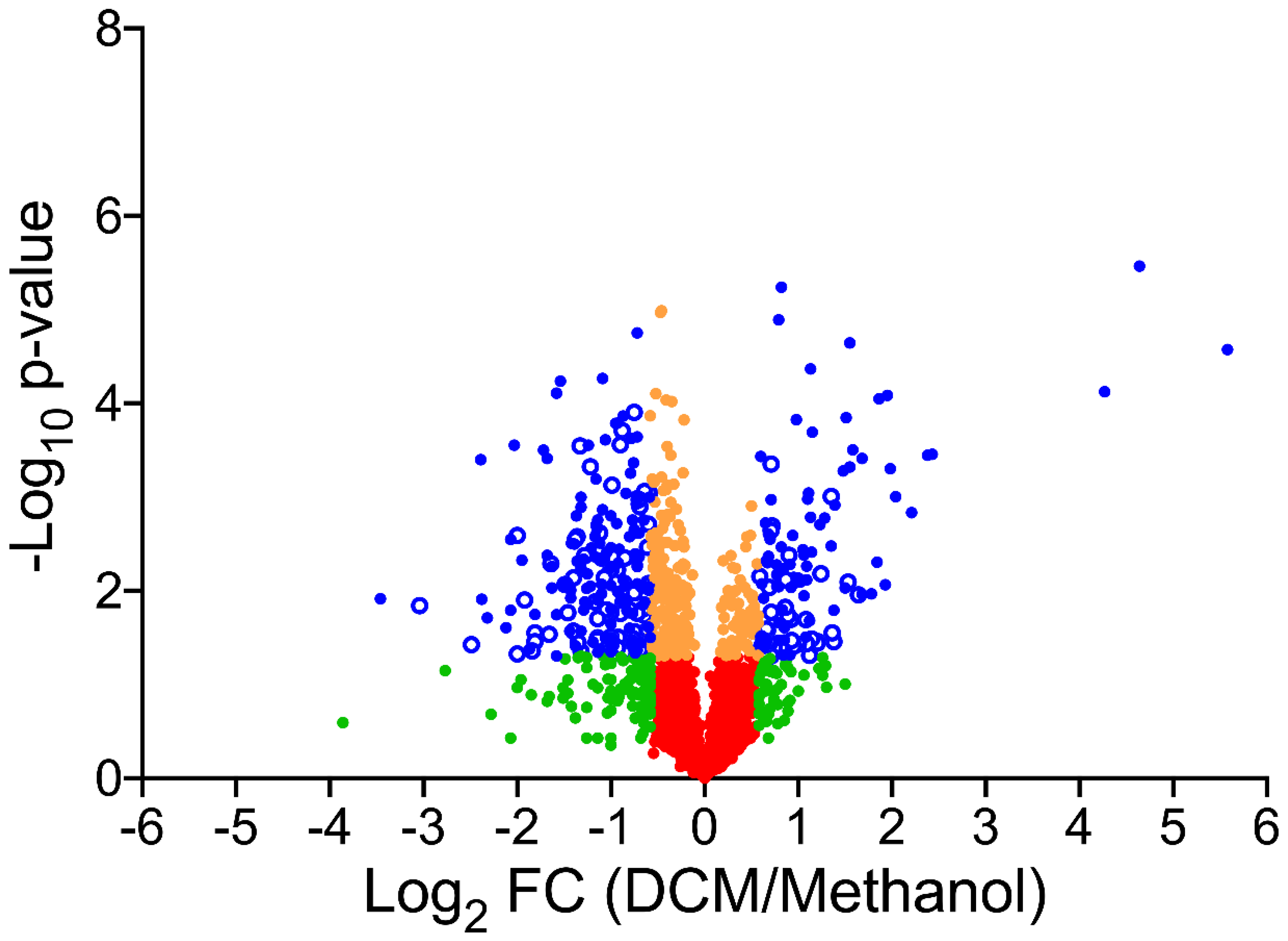
3.2. Global Changes in the Proteome Hyphomicrobium sp. MC8b upon Growth with Dichloromethane
3.3. Proteomics-Driven Identification and Sequence Prediction of Strain MC8b DCM Dehalogenase
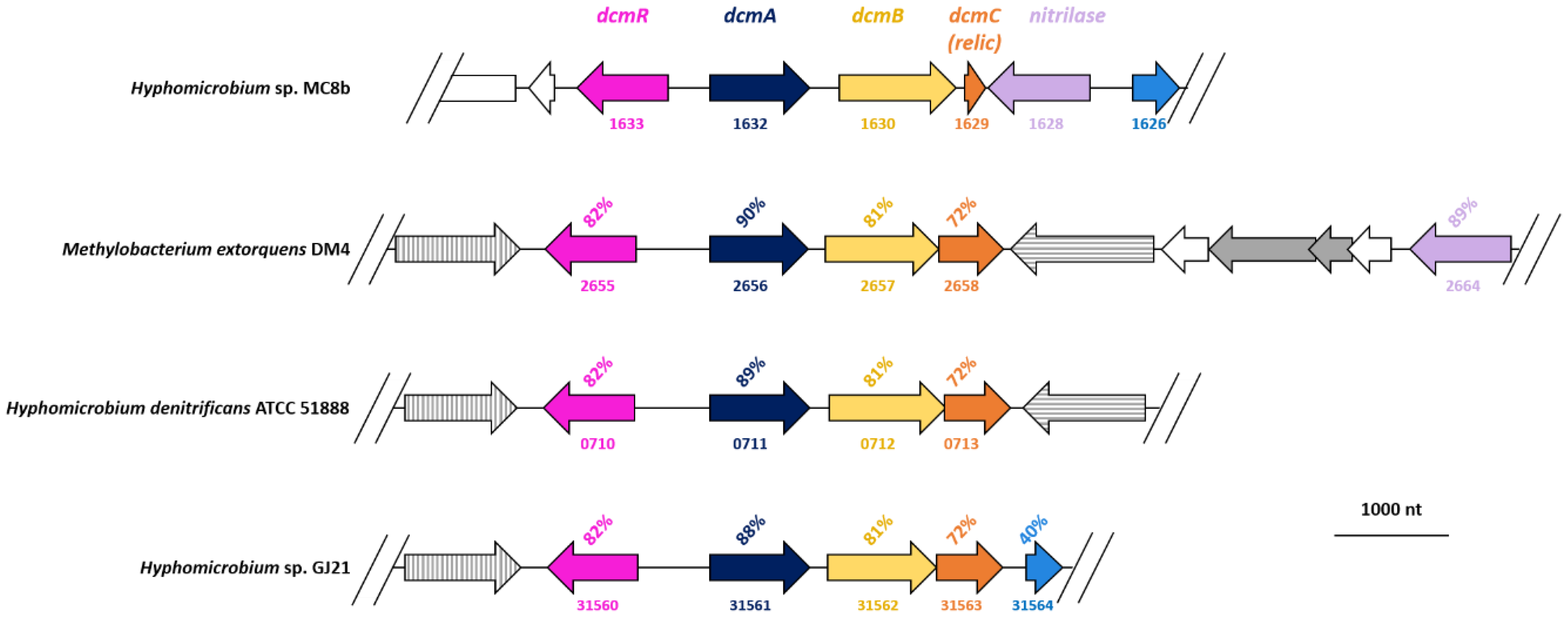
3.4. The Genome of Strain MC8b Features the Most Divergent Set of Dcm Genes Known So Far
3.5. Further Insights from Proteomic Analysis Underlines the Power of the Pan-Proteomics Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDS | coding DNA sequence |
| COG | cluster of orthologous groups |
| DCM | dichloromethane |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| PSM | peptide spectrum match |
References
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Bull, A.T. Microbial Diversity and Bioprospecting; American Society of Microbiology: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Gouveia, D.; Grenga, L.; Pible, O.; Armengaud, J. Quick microbial molecular phenotyping by differential shotgun proteomics. Environ. Microbiol. 2020, 22, 2996–3004. [Google Scholar] [CrossRef]
- Broadbent, J.A.; Broszczak, D.A.; Tennakoon, I.U.K.; Huygens, F. Pan-proteomics, a concept for unifying quantitative proteome measurements when comparing closely-related bacterial strains. Expert Rev. Proteom. 2016, 13, 355–365. [Google Scholar] [CrossRef]
- Silva, W.M.; Sousa, C.S.; Oliveira, L.C.; Soares, S.C.; Souza, G.F.M.H.; Tavares, G.C.; Resende, C.P.; Folador, E.L.; Pereira, F.L.; Figueiredo, H.; et al. Comparative proteomic analysis of four biotechnological strains Lactococcus lactis through label-free quantitative proteomics. Microb. Biotechnol. 2019, 12, 265–274. [Google Scholar] [CrossRef]
- Tavares, G.C.; Pereira, F.L.; Barony, G.M.; Rezende, C.P.; da Silva, W.M.; de Souza, G.H.M.F.; Verano-Braga, T.; de Carvalho Azevedo, V.A.; Leal, C.A.G.; Figueiredo, H.C.P. Delineation of the pan-proteome of fish-pathogenic Streptococcus agalactiae strains using a label-free shotgun approach. BMC Genom. 2019, 20, 11. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Eravci, M.; Weise, C.; Roesler, U.; Sprague, L.D.; Neubauer, H.; Wareth, G. Pan-proteomic analysis and elucidation of protein abundance among the closely related Brucella species, Brucella abortus and Brucella melitensis. Biomolecules 2020, 10, 836. [Google Scholar] [CrossRef]
- Atashgahi, S.; Liebensteiner, M.G.; Janssen, D.B.; Smidt, H.; Stams, A.J.M.; Sipkema, D. Microbial synthesis and transformation of inorganic and organic chlorine compounds. Front. Microbiol. 2018, 9, 3079. [Google Scholar] [CrossRef]
- Janssen, D.B. Biocatalysis by dehalogenating enzymes. Adv. Appl. Microbiol. 2007, 61, 233–252. [Google Scholar] [CrossRef]
- Gribble, G.W. Newly discovered naturally occurring organohalogens. Arkivoc 2018, 2018, 372–410. [Google Scholar] [CrossRef]
- Muller, E.E.L.; Bringel, F.; Vuilleumier, S. Dichloromethane-degrading bacteria in the genomic age. Res. Microbiol. 2011, 162, 869–876. [Google Scholar] [CrossRef]
- Ergas, S.J.; Kinney, K.; Fuller, M.E.; Scow, K.M. Characterization of compost biofiltration system degrading dichloromethane. Biotechnol. Bioeng. 1994, 44, 1048–1054. [Google Scholar] [CrossRef]
- Heraty, L.J.; Fuller, M.E.; Huang, L.; Abrajano, T.; Sturchio, N.C. Isotopic fractionation of carbon and chlorine by microbial degradation of dichloromethane. Org. Geochem. 1999, 30, 793–799. [Google Scholar] [CrossRef]
- Nikolausz, M.; Kappelmeyer, U.; Nijenhuis, I.; Ziller, K.; Kästner, M. Molecular characterization of dichloromethane-degrading Hyphomicrobium strains using 16S rDNA and DCM dehalogenase gene sequences. Syst. Appl. Microbiol. 2005, 28, 582–587. [Google Scholar] [CrossRef]
- Roselli, S.; Nadalig, T.; Vuilleumier, S.; Bringel, F. The 380 kb pCMU01 plasmid encodes chloromethane utilization genes and redundant genes for vitamin B12- and tetrahydrofolate-dependent chloromethane metabolism in Methylobacterium extorquens CM4: A proteomic and bioinformatics study. PLoS ONE 2013, 8, e56598. [Google Scholar] [CrossRef]
- Mappa, C.; Pible, O.; Armengaud, J.; Alpha-Bazin, B. Assessing the ratio of Bacillus spores and vegetative cells by shotgun proteomics. Environ. Sci. Pollut. Res. 2018, 1–9. [Google Scholar] [CrossRef]
- Hartmann, E.M.; Allain, F.; Gaillard, J.C.; Pible, O.; Armengaud, J. Taking the shortcut for high-throughput shotgun proteomic analysis of bacteria. Methods Mol. Biol. 2014, 1197, 275–285. [Google Scholar] [CrossRef]
- Klein, G.; Mathé, C.; Biola-Clier, M.; Devineau, S.; Drouineau, E.; Hatem, E.; Marichal, L.; Alonso, B.; Gaillard, J.C.; Lagniel, G.; et al. RNA-binding proteins are a major target of silica nanoparticles in cell extracts. Nanotoxicology 2016, 10, 1555–1564. [Google Scholar] [CrossRef]
- Dupierris, V.; Masselon, C.; Court, M.; Kieffer-Jaquinod, S.; Bruley, C. A toolbox for validation of mass spectrometry peptides identification and generation of database: IRMa. Bioinformatics 2009, 25, 1980–1981. [Google Scholar] [CrossRef]
- Cogne, Y.; Almunia, C.; Gouveia, D.; Pible, O.; François, A.; Degli-Esposti, D.; Geffard, O.; Armengaud, J.; Chaumot, A. Comparative proteomics in the wild: Accounting for intrapopulation variability improves describing proteome response in a Gammarus pulex field population exposed to cadmium. Aquat. Toxicol. 2019, 214, 105244. [Google Scholar] [CrossRef]
- Carvalho, P.C.; Yates, J.R., III; Barbosa, V.C. Improving the TFold test for differential shotgun proteomics. Bioinformatics 2012, 28, 1652–1654. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Matsen, F.A.; Kodner, R.B.; Armbrust, E.V. pplacer: Linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinform. 2010, 11, 538. [Google Scholar] [CrossRef]
- Vallenet, D.; Calteau, A.; Dubois, M.; Amours, P.; Bazin, A.; Beuvin, M.; Burlot, L.; Bussell, X.; Fouteau, S.; Gautreau, G.; et al. MicroScope: An integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res. 2020, 48, D579–D589. [Google Scholar] [CrossRef]
- Gliesche, C.; Fesefeldt, A.; Hirsch, P. Hyphomicrobium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Maucourt, B.; Vuilleumier, S.; Bringel, F. Transcriptional regulation of organohalide pollutant utilisation in bacteria. FEMS Microbiol. Rev. 2020, 44, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.B.; Kysela, D.T.; Buechlein, A.; Hemmerich, C.; Brun, Y.V. Genome sequences of eight morphologically diverse Alphaproteobacteria. J. Bacteriol. 2011, 193, 4567–4568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bringel, F.; Postema, C.P.; Mangenot, S.; Bibi-Triki, S.; Chaignaud, P.; Haque, M.F.U.; Gruffaz, C.; Hermon, L.; Louhichi, Y.; Maucourt, B.; et al. Genome sequence of the dichloromethane-degrading bacterium Hyphomicrobium sp. strain GJ21. Genome Announc. 2017, 5, 622. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, S.; Ivoš, N.; Dean, M.; Leisinger, T. Sequence variation in dichloromethane dehalogenases/glutathione S-transferases. Microbiology 2001, 147, 611–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coque, J.J.R.; Enguita, F.J.; Martín, J.F.; Liras, P. A 2-protein component 7-alpha-cephem-methoxylase encoded by 2 genes of the cephamycin-C cluster converts cephalosporin-C to 7-methoxycephalosporin-C. J. Bacteriol. 1995, 177, 2230–2235. [Google Scholar] [CrossRef]
- Mottram, J.C.; North, M.J.; Barry, J.D.; Coombs, G.H. A cysteine proteinase cDNA from Trypanosoma brucei predicts an enzyme with an unusual C-terminal extension. FEBS Lett. 1989, 258, 211–215. [Google Scholar] [CrossRef]
- Bibi-Triki, S.; Husson, G.; Maucourt, B.; Vuilleumier, S.; Carapito, C.; Bringel, F. N-terminome and proteogenomic analysis of the Methylobacterium extorquens DM4 reference strain for dichloromethane utilization. J. Proteom. 2018, 179, 131–139. [Google Scholar] [CrossRef]
- Vuilleumier, S.; Nadalig, T.; Haque, M.F.U.; Magdelenat, G.; Lajus, A.; Roselli, S.; Muller, E.E.L.; Gruffaz, C.; Barbe, V.; Médigue, C.; et al. Complete genome sequence of the chloromethane-degrading Hyphomicrobium sp. strain MC1. J. Bacteriol. 2011, 193, 5035–5036. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Kristensen, D.M.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Microbial genome analysis: The COG approach. Brief. Bioinform. 2019, 20, 1063–1070. [Google Scholar] [CrossRef]
- Junqueira, M.; Spirin, V.; Balbuena, T.S.; Thomas, H.; Adzhubei, I.; Sunyaev, S.; Shevchenko, A. Protein identification pipeline for the homology-driven proteomics. J. Proteom. 2008, 71, 346–356. [Google Scholar] [CrossRef]
- Waridel, P.; Frank, A.; Thomas, H.; Surendranath, V.; Sunyaev, S.; Pevzner, P.; Shevchenko, A. Sequence similarity-driven proteomics in organisms with unknown genomes by LC-MS/MS and automated de novo sequencing. Proteomics 2007, 7, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Liska, A.J.; Popov, A.V.; Sunyaev, S.; Coughlin, P.; Habermann, B.; Shevchenko, A.; Bork, P.; Karsenti, E.; Shevchenko, A. Homology-based functional proteomics by mass spectrometry: Application to the Xenopus microtubule-associated proteome. Proteomics 2004, 4, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bhattacharya, S.G. Charting novel allergens from date palm pollen (Phoenix sylvestris) using homology driven proteomics. J. Proteom. 2017, 165, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, S.; Chourey, K.; Chen, G.; Murdoch, R.W.; Higgins, S.A.; Iyer, R.; Campagna, S.R.; Mack, E.E.; Seger, E.S.; Hettich, R.L.; et al. Proteogenomics reveals novel reductive dehalogenases and methyltransferases expressed during anaerobic dichloromethane metabolism. Appl. Environ. Microbiol. 2019, 85, 2768. [Google Scholar] [CrossRef]
- Muller, E.E.L.; Hourcade, E.; Louhichi-Jelail, Y.; Hammann, P.; Vuilleumier, S.; Bringel, F. Functional genomics of dichloromethane utilization in Methylobacterium extorquens DM4. Environ. Microbiol. 2011, 13, 2518–2535. [Google Scholar] [CrossRef]
- Bradley, A.S.; Swanson, P.K.; Muller, E.E.L.; Bringel, F.; Caroll, S.M.; Pearson, A.; Vuilleumier, S.; Marx, C.J. Hopanoid-free Methylobacterium extorquens DM4 overproduces carotenoids and has widespread growth impairment. PLoS ONE 2017, 12, e0173323. [Google Scholar] [CrossRef]
- Michener, J.K.; Neves, A.A.C.; Vuilleumier, S.; Bringel, F.; Marx, C.J. Effective use of a horizontally-transferred pathway for dichloromethane catabolism requires post-transfer refinement. eLife 2014, 3, 04279. [Google Scholar] [CrossRef]
- Michener, J.K.; Vuilleumier, S.; Bringel, F.; Marx, C.J. Transfer of a catabolic pathway for chloromethane in Methylobacterium strains highlights different limitations for growth with chloromethane or with dichloromethane. Front. Microbiol. 2016, 7, 1116. [Google Scholar] [CrossRef]
- Chaignaud, P.; Maucourt, B.; Weiman, M.; Alberti, A.; Kolb, S.; Cruveiller, S.; Vuilleumier, S.; Bringel, F. Genomic and transcriptomic analysis of growth-supporting dehalogenation of chlorinated methanes in Methylobacterium. Front. Microbiol. 2017, 8, 1600. [Google Scholar] [CrossRef]
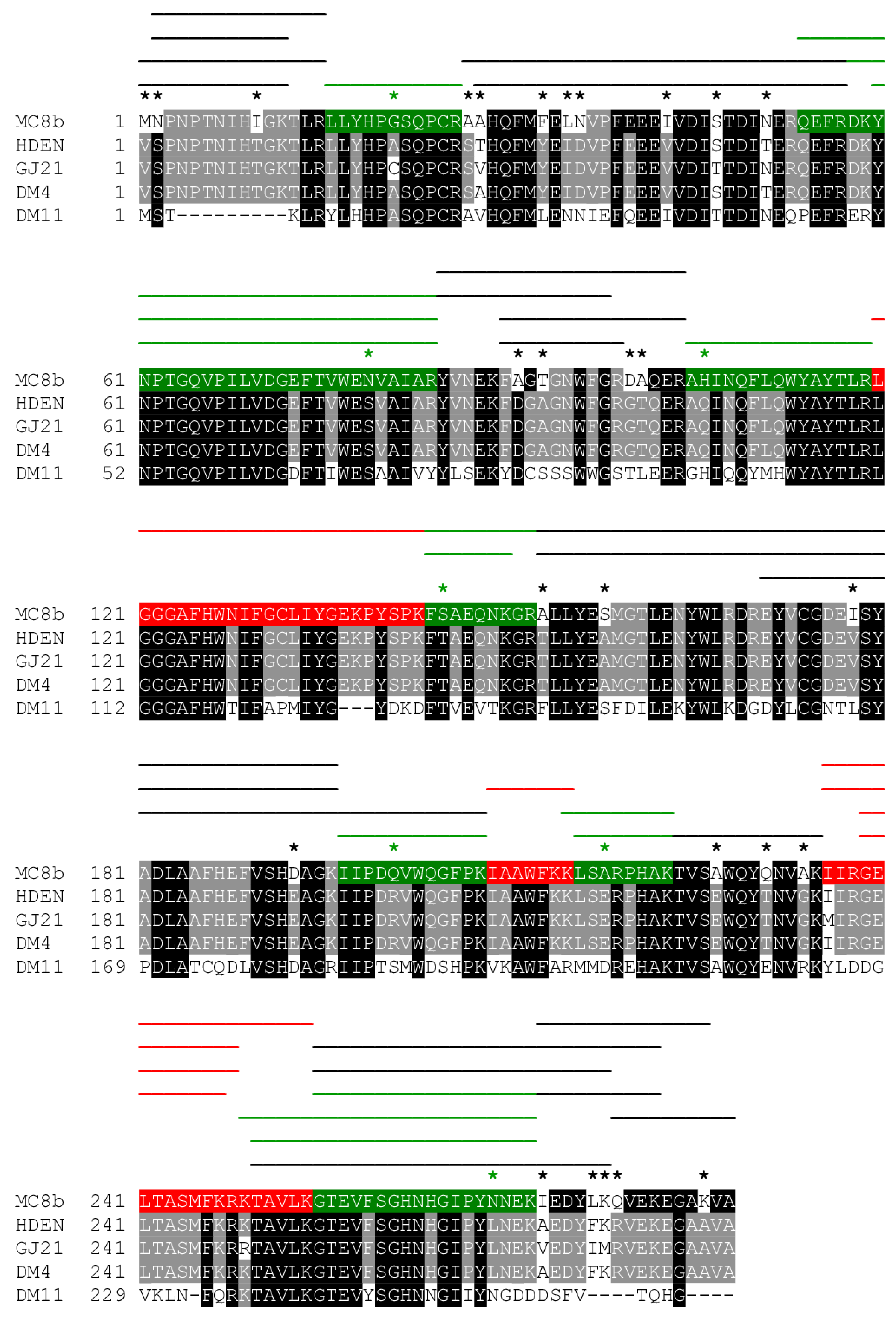
| First | Last | Sequence 1 | #Peptides 2 | Analysis |
|---|---|---|---|---|
| 16 | 26 | LLYHPGSQPCR | 1 | error-tolerant mode |
| 54 | 84 | QEFRDKYNPTGQVPILVDGEFTVWENVAIAR | 3 | error-tolerant mode |
| 85 | 99 | YVNEKFTGAGNWFGR | 2 | error-tolerant mode |
| 105 | 119 | AHINQFLQWYAYTLR | 1 | error-tolerant mode |
| 120 | 143 | LGGGAFHWNIFGCLIYGEKPYSPK | 1 | no mismatch |
| 144 | 152 | FSAEQNKGR | 2 | error-tolerant mode |
| 153 | 168 | ALLYEAMGTLENYWLR | 3 | error-tolerant mode |
| 197 | 208 | IIPDQVWQGFPK | 2 | error-tolerant mode |
| 209 | 215 | IAAWFKK | 1 | no mismatch |
| 215 | 223 | KLSARPHAK | 2 | error-tolerant mode |
| 236 | 248 | IIRGELTASMFKR | 4 | no mismatch |
| 248 | 254 | RKTAVLK | 1 | no mismatch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayoun, K.; Geersens, E.; Laczny, C.C.; Halder, R.; Lázaro Sánchez, C.; Manna, A.; Bringel, F.; Ryckelynck, M.; Wilmes, P.; Muller, E.E.L.; et al. Dichloromethane Degradation Pathway from Unsequenced Hyphomicrobium sp. MC8b Rapidly Explored by Pan-Proteomics. Microorganisms 2020, 8, 1876. https://doi.org/10.3390/microorganisms8121876
Hayoun K, Geersens E, Laczny CC, Halder R, Lázaro Sánchez C, Manna A, Bringel F, Ryckelynck M, Wilmes P, Muller EEL, et al. Dichloromethane Degradation Pathway from Unsequenced Hyphomicrobium sp. MC8b Rapidly Explored by Pan-Proteomics. Microorganisms. 2020; 8(12):1876. https://doi.org/10.3390/microorganisms8121876
Chicago/Turabian StyleHayoun, Karim, Emilie Geersens, Cédric C. Laczny, Rashi Halder, Carmen Lázaro Sánchez, Abhijit Manna, Françoise Bringel, Michaël Ryckelynck, Paul Wilmes, Emilie E. L. Muller, and et al. 2020. "Dichloromethane Degradation Pathway from Unsequenced Hyphomicrobium sp. MC8b Rapidly Explored by Pan-Proteomics" Microorganisms 8, no. 12: 1876. https://doi.org/10.3390/microorganisms8121876
APA StyleHayoun, K., Geersens, E., Laczny, C. C., Halder, R., Lázaro Sánchez, C., Manna, A., Bringel, F., Ryckelynck, M., Wilmes, P., Muller, E. E. L., Alpha-Bazin, B., Armengaud, J., & Vuilleumier, S. (2020). Dichloromethane Degradation Pathway from Unsequenced Hyphomicrobium sp. MC8b Rapidly Explored by Pan-Proteomics. Microorganisms, 8(12), 1876. https://doi.org/10.3390/microorganisms8121876






