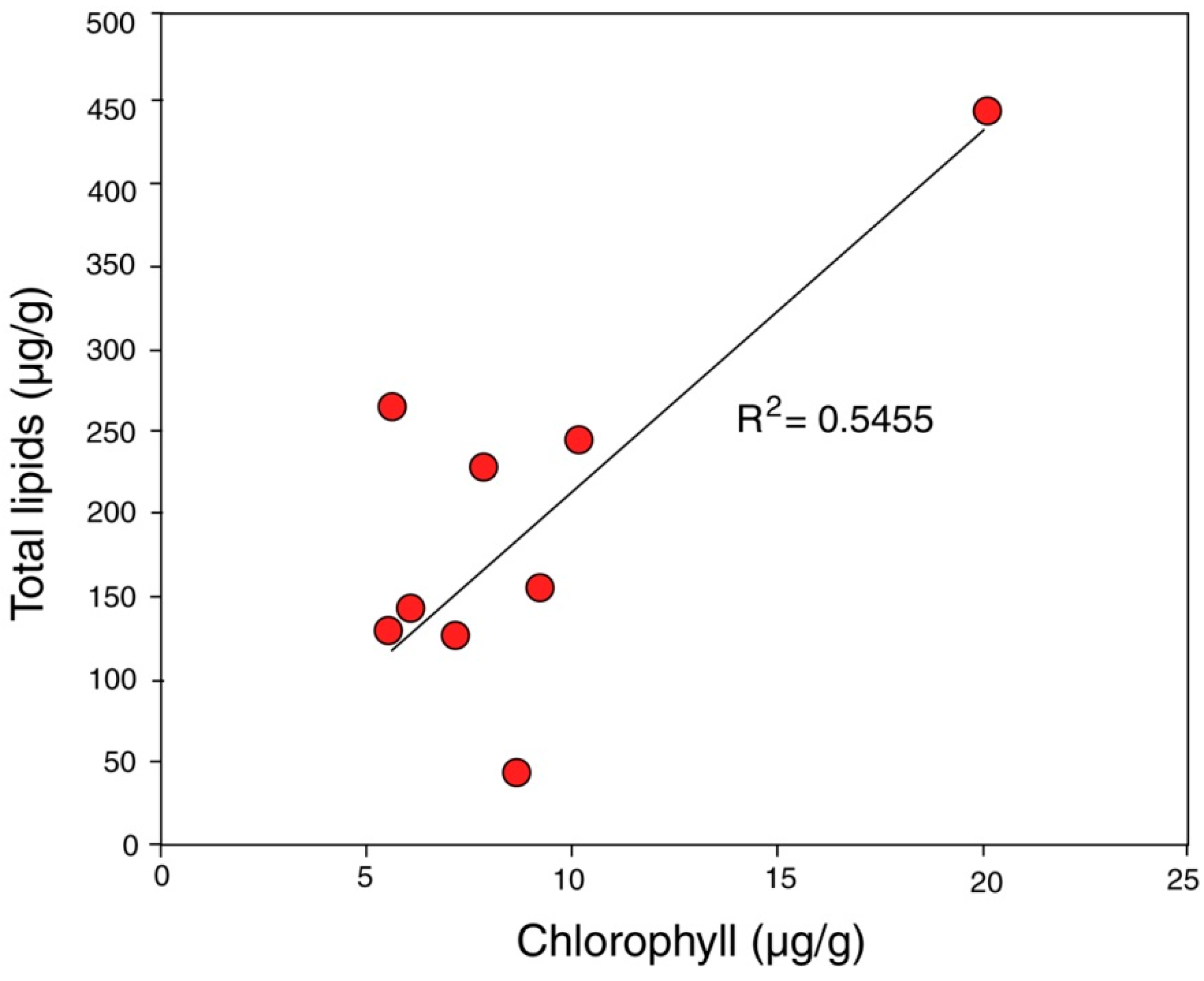
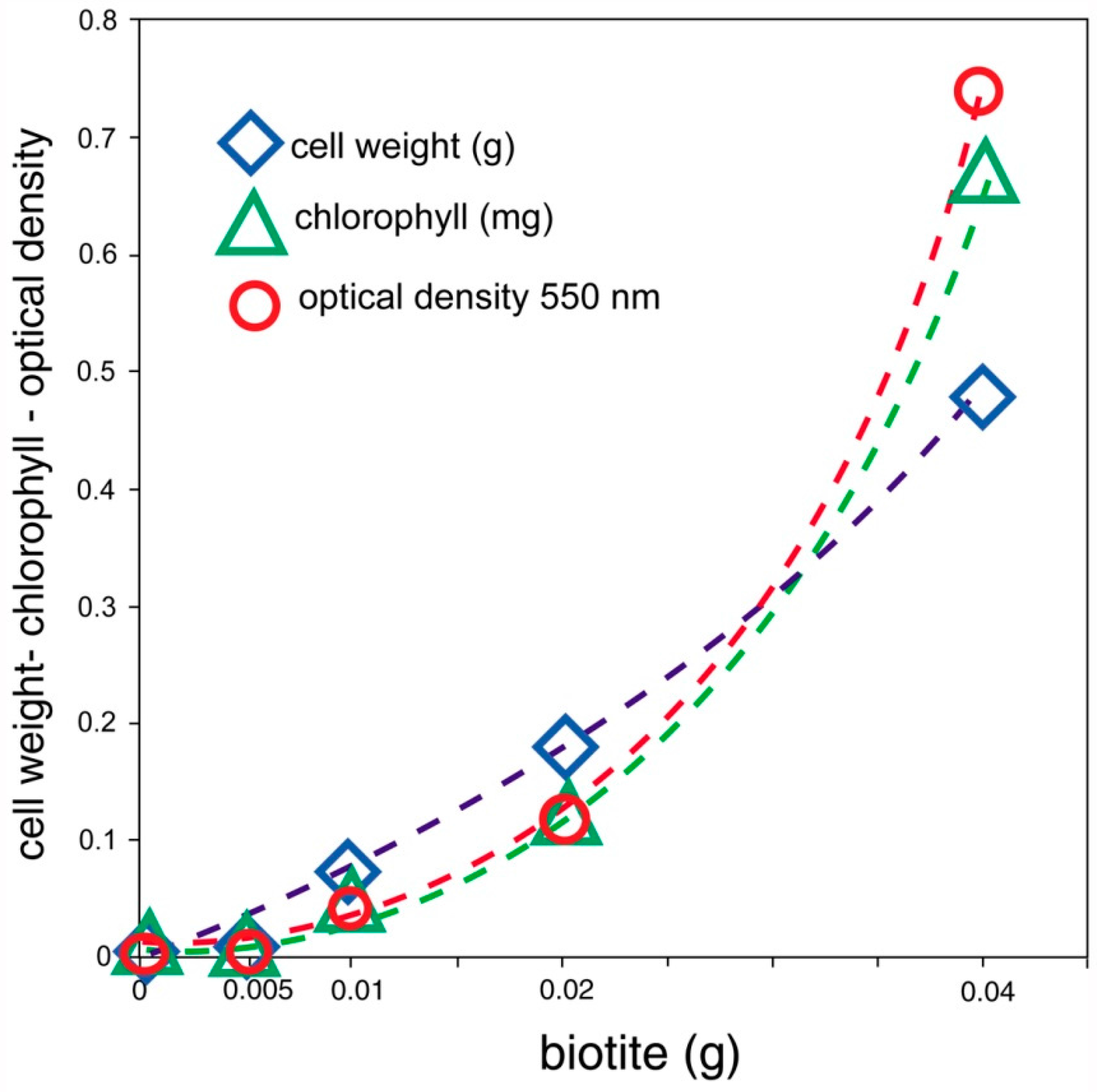
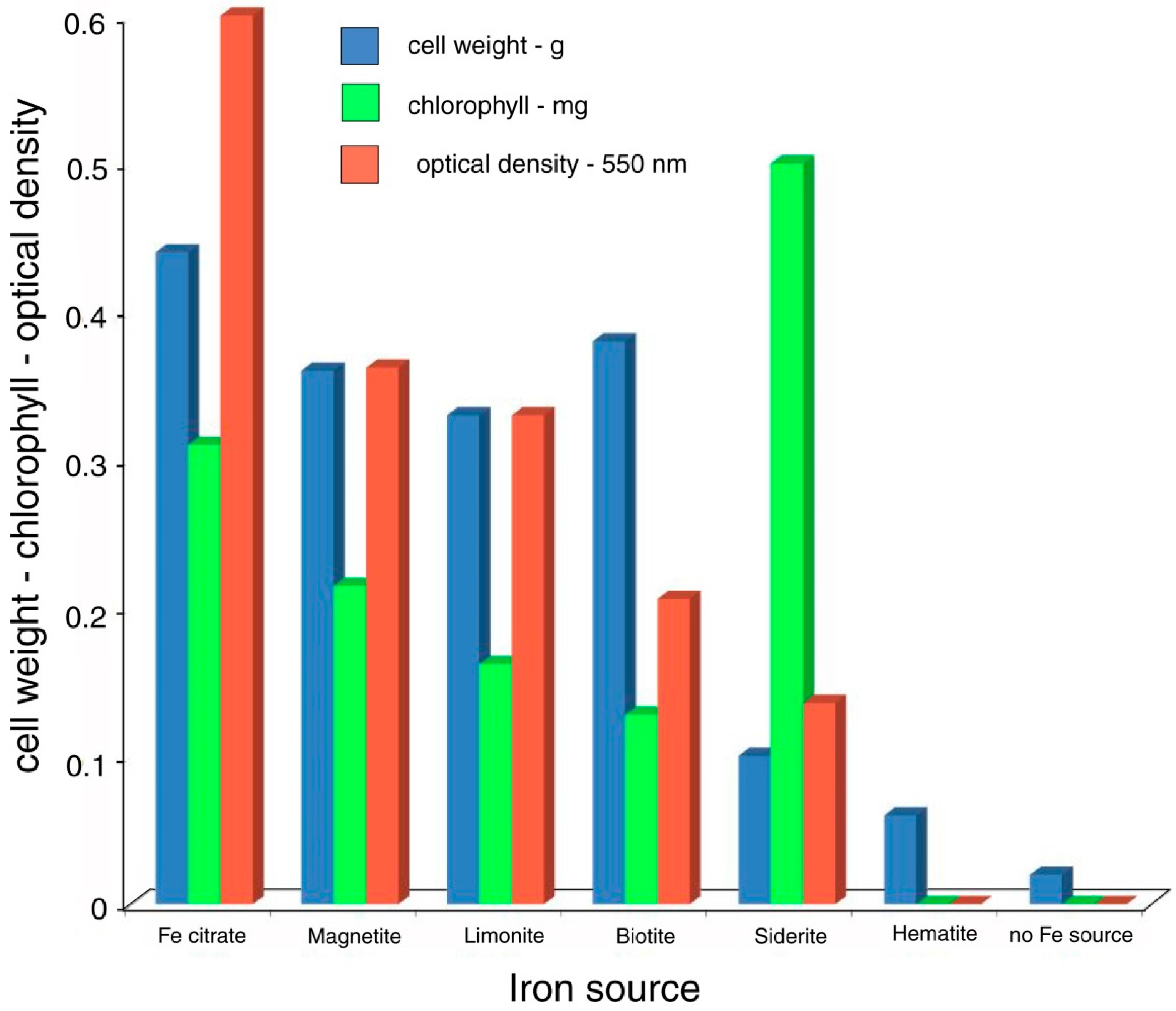
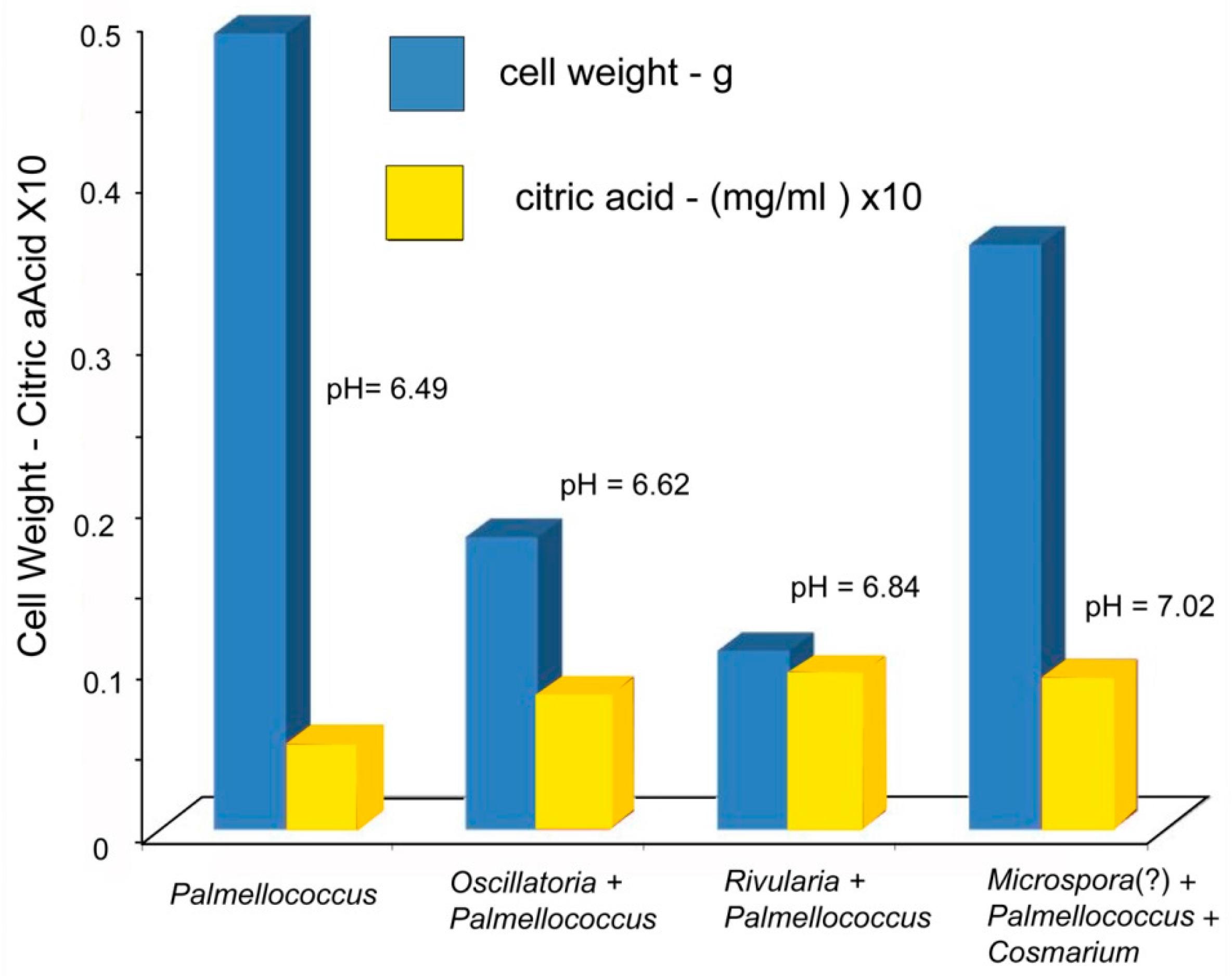
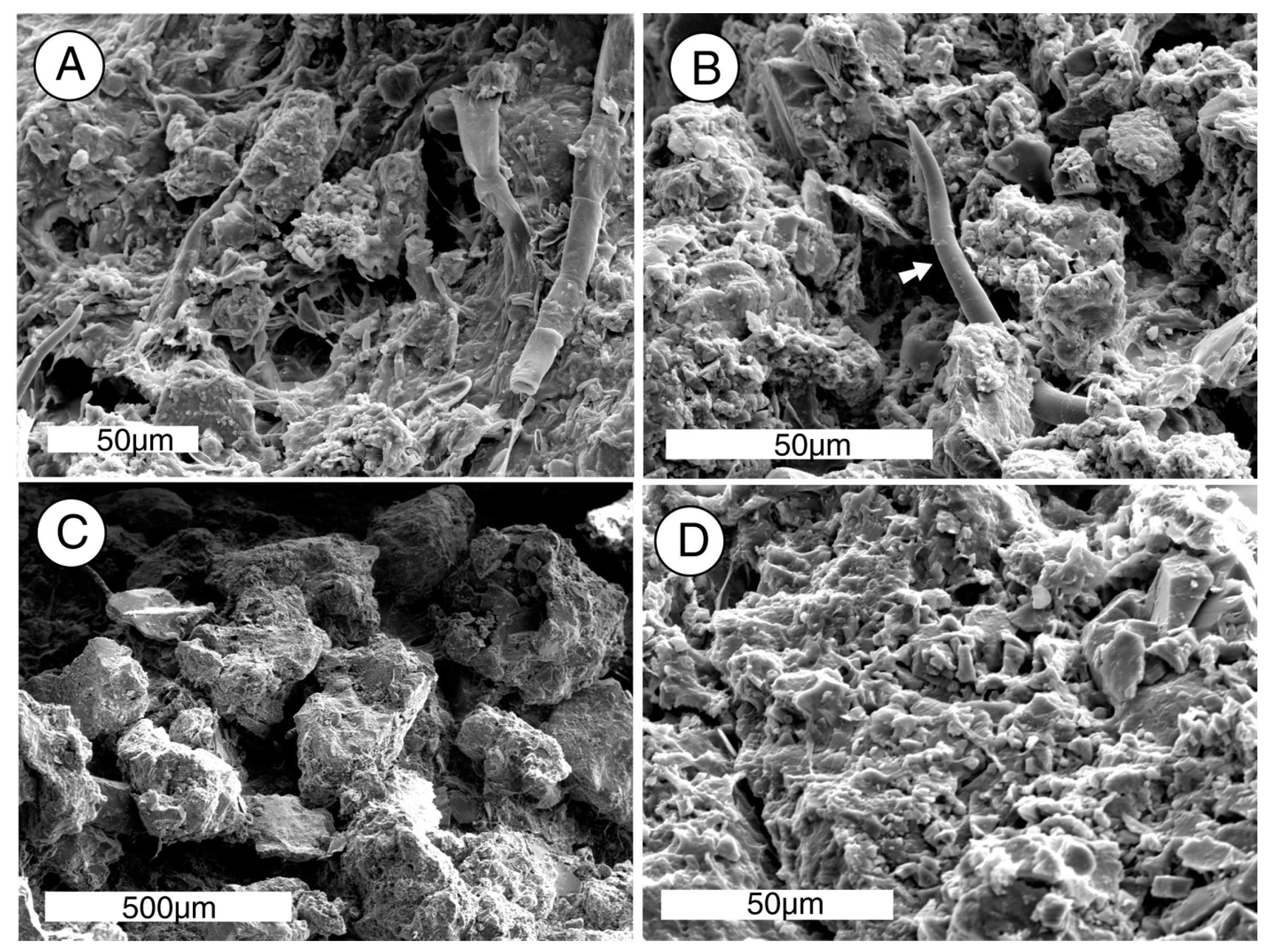
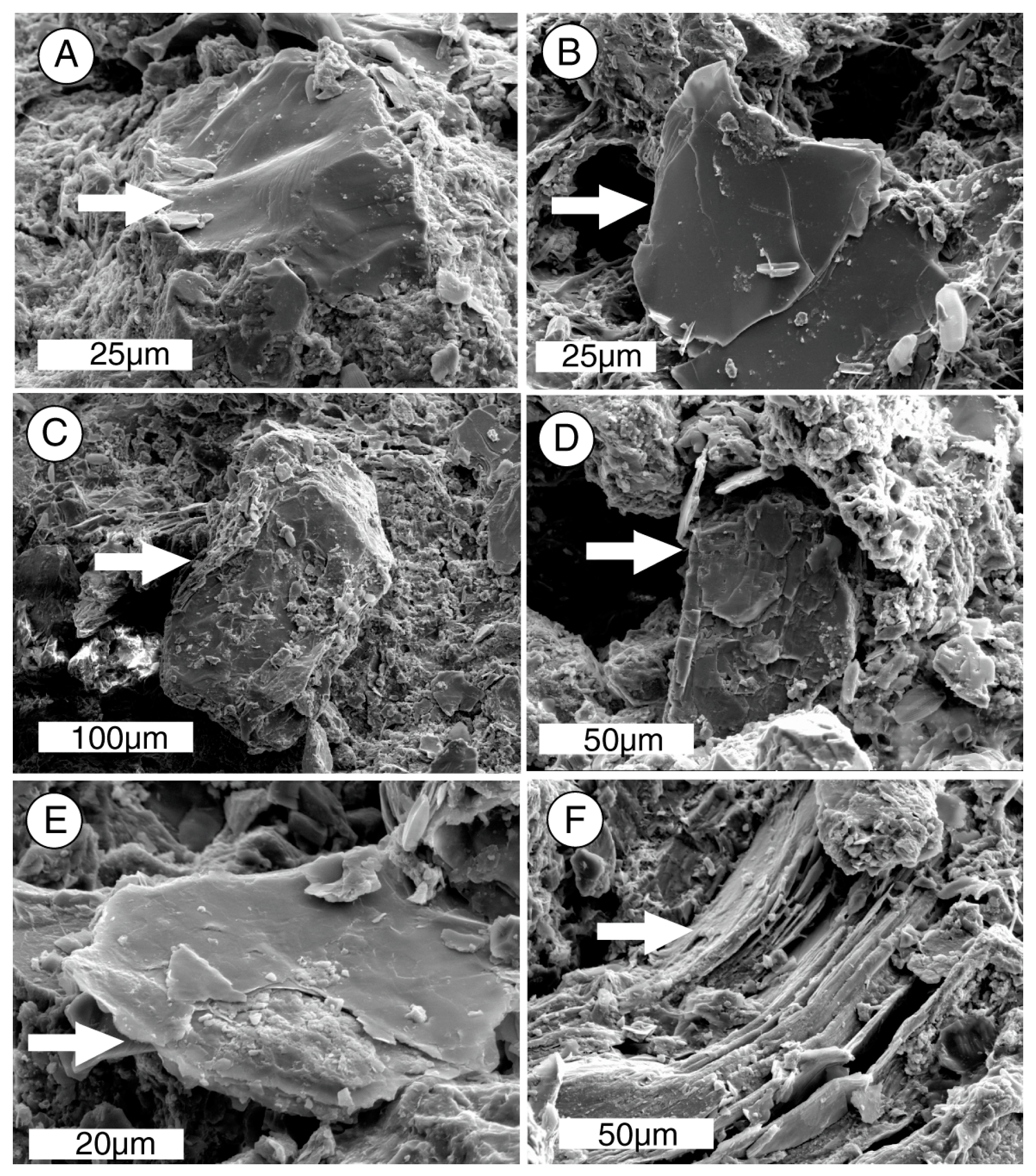
The ability of biologically produced compounds to dissolve minerals is known from laboratory studies and field observation.
Living organisms are an important force during rock weathering. The solubilization of minerals by biologically-derived compounds has been recognized for lichen, fungi, bacteria, cyanobacteria, and degradation products from higher plants. The ability of lichens to secrete compounds that attack minerals is well documented [
4,
5,
6,
7,
8,
9,
10,
11,
12]. Lichens may also cause rock deterioration as a result of expansion and contraction during wetting and drying cycles [
13,
14,
15]. Bacteria are also agents of biogenic weathering [
16,
17,
18,
19,
20,
21,
22,
23], but fungi are perhaps the most important organism [
24,
25,
26,
27,
28,
29]. Research interest has been stimulated by the realization that fungi and other microbes can be useful for mitigating sites polluted with heavy metals, radionuclides, asbestos, and other hazardous materials [
26,
30,
31,
32]. Higher plants can be a cause of biogenic weathering for several reasons. Roots growing along fractures can cause structural damage to bedrock, and rootlets are commonly associated with mycorrhizal fungi that release compounds that solubilize nutrient minerals from adjacent soil [
33,
34,
35]. Perhaps the greatest factor comes after death, when the degradation of plant tissues produces soil organic matter that contains powerful chelating agents. The chemical mechanisms involved in these biogenic weathering processes are described below.
Chemical Mechanisms
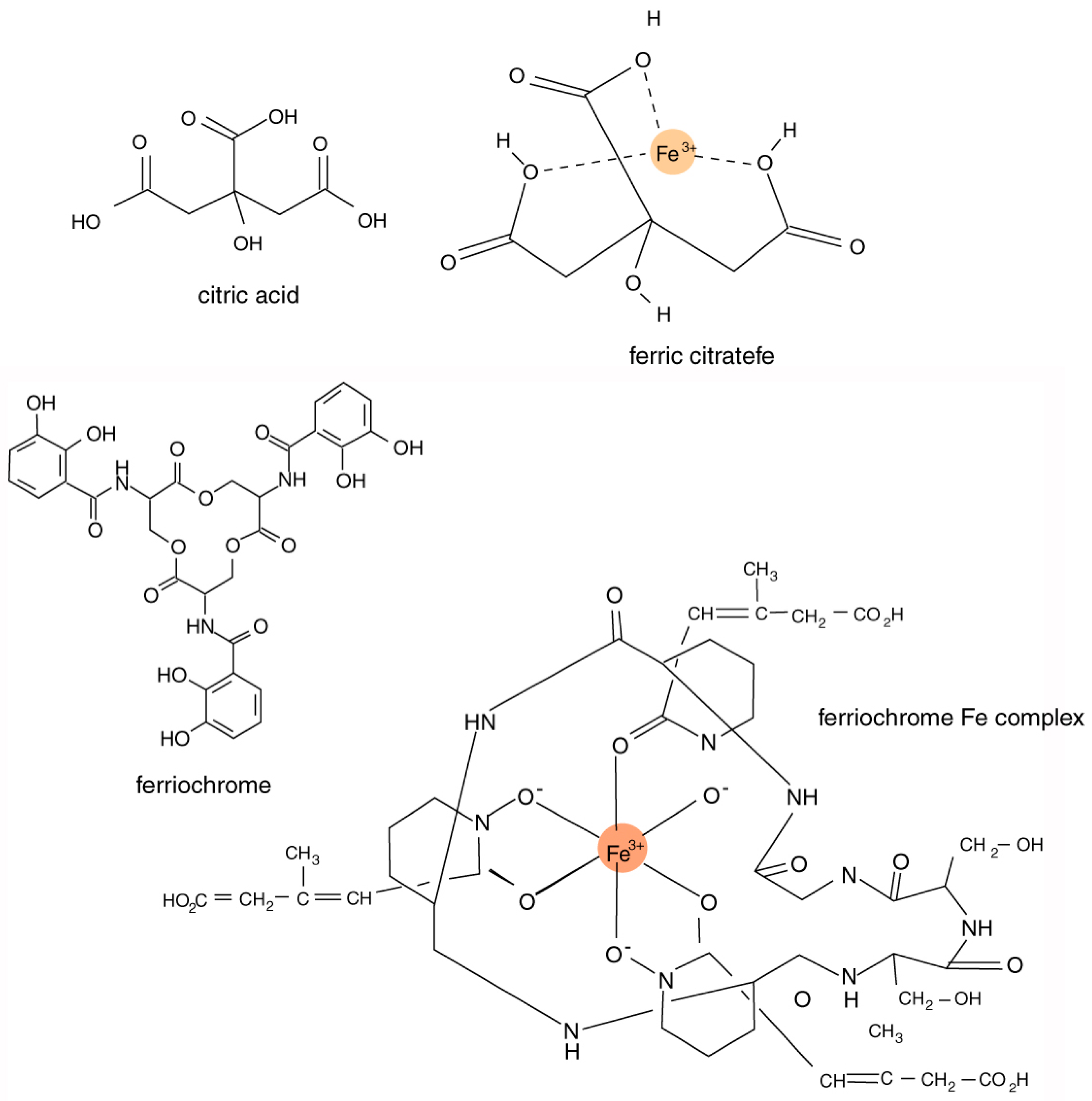
Iron-chelating compounds secreted by organisms are sometimes referred to as siderophores [
36,
37,
38,
39]. Although iron is abundant in many natural environments, the element is commonly present as oxides that have a very low solubility, and it is thus unavailable for metabolic use. The secretion of siderophores allows microorganisms to obtain iron from ferric minerals by the formation of soluble Fe
3+ organometal complexes.
The chemical composition of siderophores is highly variable, including peptides, chatecolates (phenolates), hydroxamates, and carboxylates. These compounds typically have an ability to form stable octahedral complexes with Fe
3+ ions. The most important structural characteristic of these complexing agents is their tendency to dissociate in aqueous solution to donate two protons (diprotic acids) or three protons (triprotic acids), allowing the formation of complexes with multivalent metals (
Figure 3). Bacteria and fungi produce a diverse variety of catecholate and hydroxamate siderophores [
40,
41,
42]. In algae and higher plants, carboxylic acid siderophores predominate [
43].
For organic acids, three principal reaction pathways may be responsible for mineral decomposition: these involve attack by hydronium ions, low molecular weight organic acids (LMWOR), and high molecular weight organic acids (HMWOR).
Hydrogen ions may be involved in biogenic weathering when organic acids are present in concentrations sufficient to produce low pH conditions. However, as noted later, the effectiveness of organic acids for rock weathering primarily comes from their ability to form organometal complexes where multivalent metals form ligands with carboxyl functional groups, not from hydronium ion activity [
46]. Hydronium ion attack may occur when carbonic acid is present as a result of the dissociation of biologically-produced CO
2. This gas is produced during the metabolic process of respiration, but in plants exposed to light, CO
2 is utilized as a substrate for photosynthesis, along with additional CO
2 obtained from the surrounding atmosphere. Biochemical processes vary between times of darkness and light, but on net balance, photosynthetic organisms consume CO
2 and produce O
2. For this reason, carbonic acid attack on minerals is not likely to be a major force on surfaces inhabited by algae or cyanophytes.
Hydronium ion attack may occur from the release of oxalic acid, derived from calcium oxalate present in the cells of many plants. In addition, oxalic acid may also serve as a chelating agent [
45]. Calcium oxalate occurs in the cells of many plants, from microscopic algae to giant gymnosperms, and in cyanophytes and fungi [
47,
48,
49]. Formed from glucose by an enzymatic process, calcium oxalate may accumulate to form considerable quantities of crystals within individual cells, a phenomenon that may provide physical protection against predation [
50]. Metabolically, calcium oxalate is a mechanism for regulating calcium levels in tissues and organs [
51,
52]. Oxalic acid release by lichens and fungi can be important for biogenic weathering, and this compound is known to occur in some microscopic algae [
53,
54,
55]. However, oxalic acid production is not well documented for microscopic algae and cyanophytes; for these microbes, the potential benefits of oxalate production are probably very different compared to larger life forms. For microbes that release organic acids as chelating agents to obtain nutrient elements, the most likely agents are carboxylic acids, as discussed below.
In general, the dominant chemical process for biogenic weathering is chelation, a process where organic molecules react with multi-valent ions on mineral surfaces, solubilizing these elements to produce organometal complexes. The principal chelating agents are organic acids belonging to two groups: high molecular weight (HMWOA) and low molecular weight (LMWOA).
High molecular weight organic acids (HMWOA) are primarily degradation products of dead plant matter, producing humic and fulvic acids that are present in soils, peat, and water. Because they are degradation products, these organic acids are exceptions to a definition of biochemicals as compounds that are produced by living organisms. They are mentioned here because they originate from true biochemical precursors, and because they potentially provide a source of soluble nutrients for living cells.
Humic acids have an aromatic hydrocarbon framework with associated phenolic and carboxylic functional groups that can react with divalent and trivalent metals to produce chelate complexes. Fulvic acids have structural similarities to humic acids, but they are lower in molecular weight, and are more biologically reactive [
56]. These compounds are important for the breakdown of minerals in soils [
57,
58].
Low molecular weight organic acids (LMWOA) include members of two groups: carboxylic acids produced during the citric acid cycle (Krebs cycle), and phenolic acids (lichenic acids). Lichenic acids are phenolic compounds formed as secondary metabolic products.
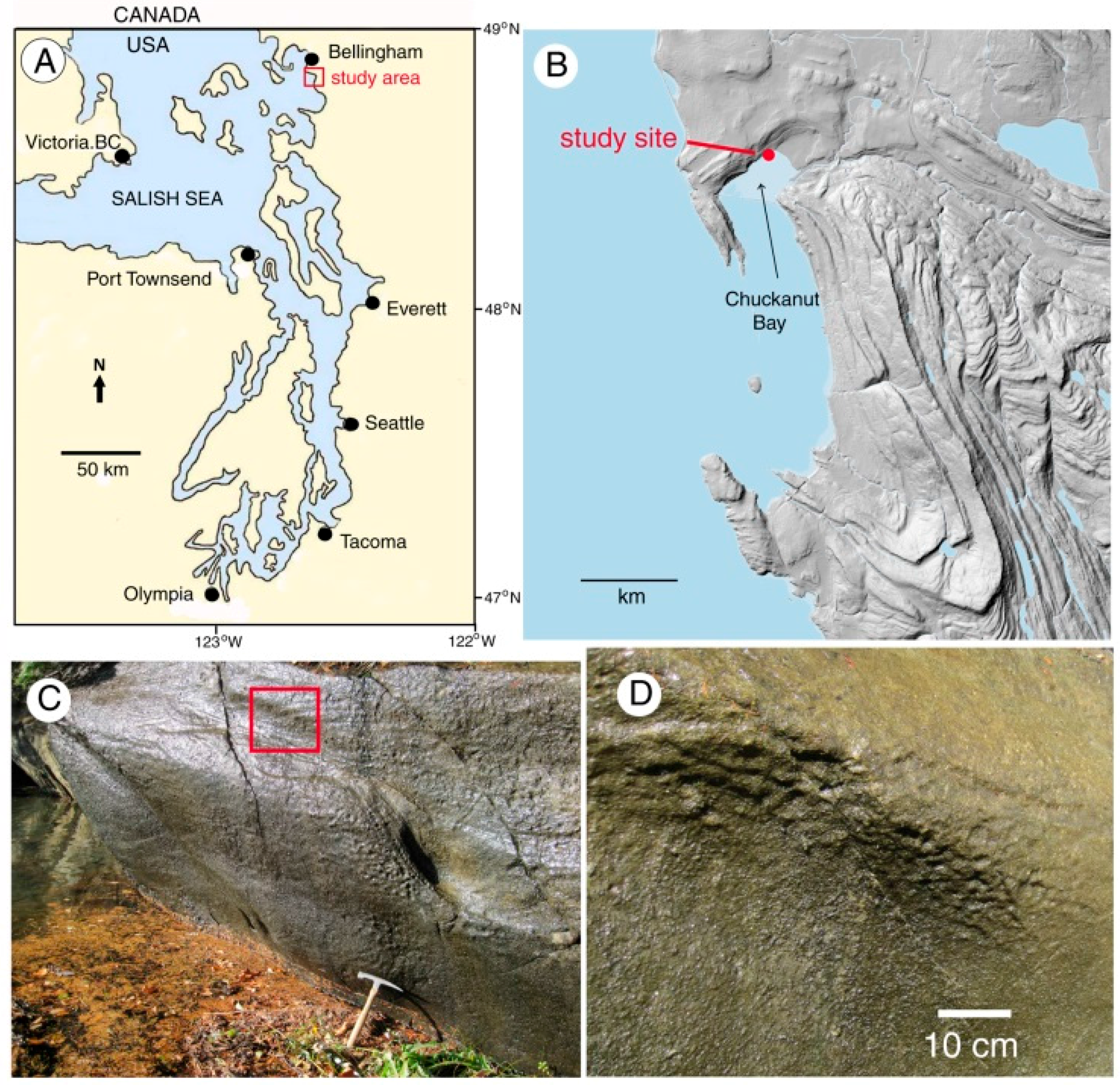
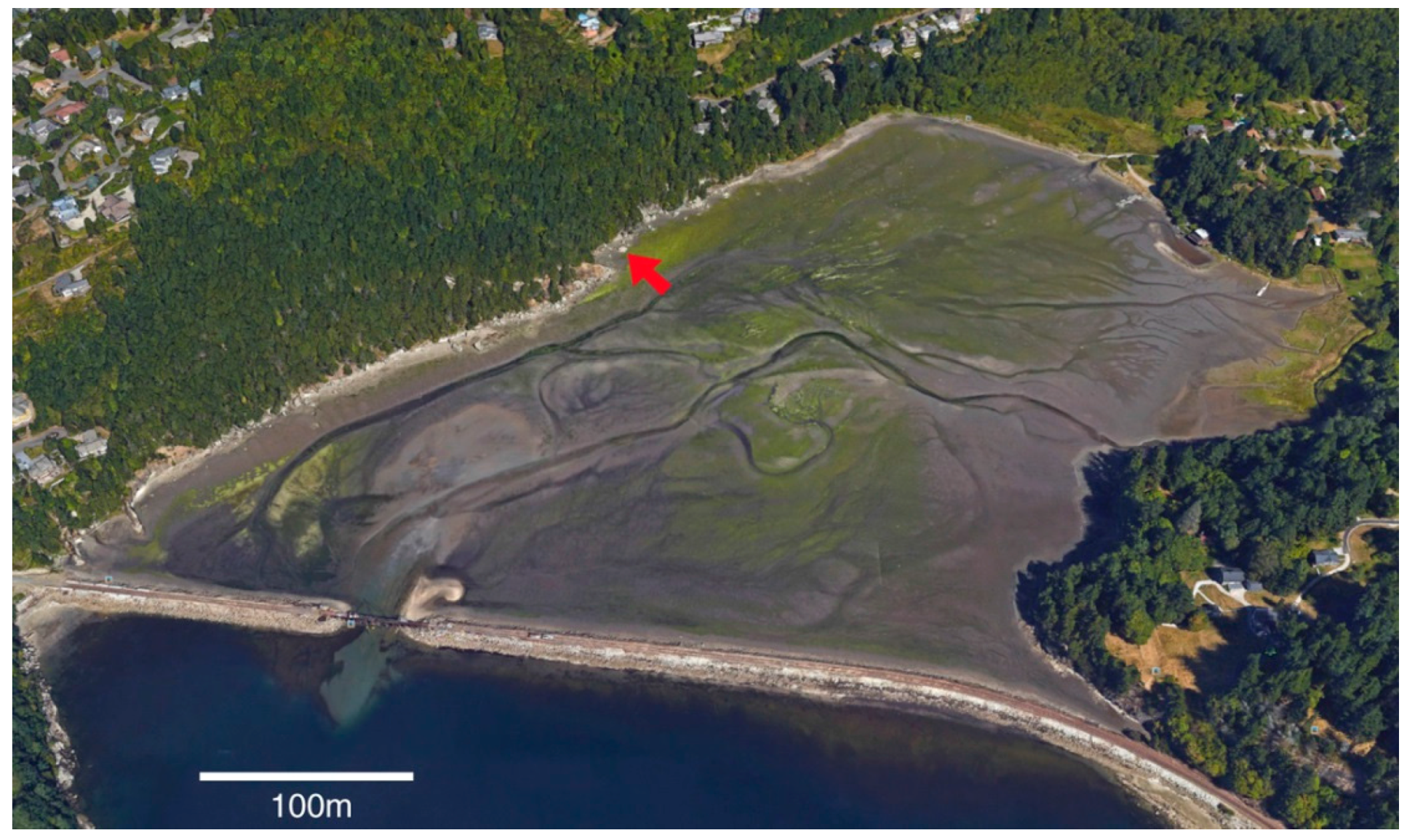
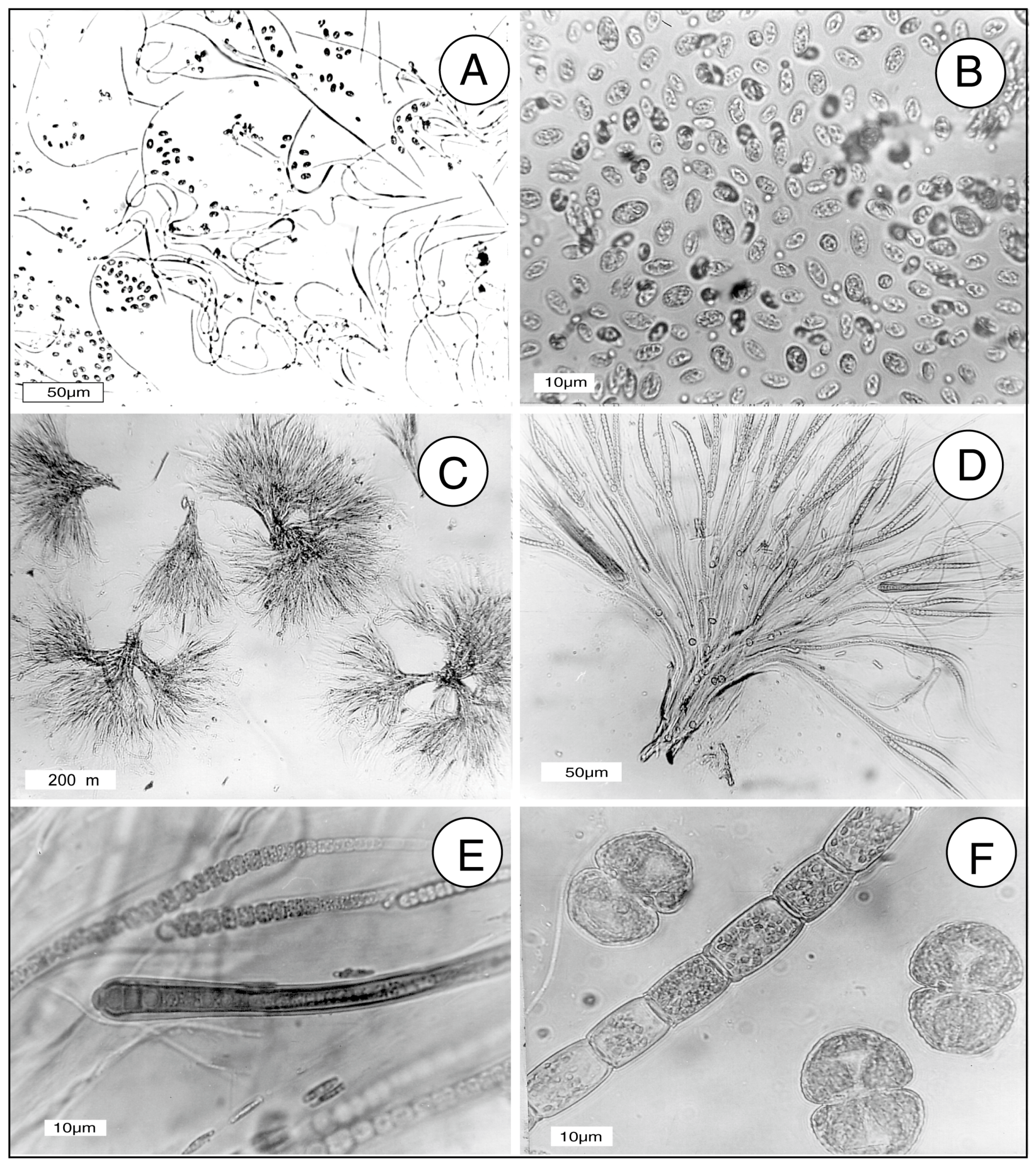
In this report, the study site is a sandstone outcrop saturated with groundwater. Fresh-water algae inhabit the exposed surface. In this environment, biogenic weathering is most likely caused by the chelating effects of LMWOA generated during the citric acid cycle (Krebs cycle). This reaction sequence is a fundamental process for carbohydrate oxidation for all aerobic organisms. Pyruvate produced from glucose during anaerobic glycolysis is utilized during the aerobic Krebs cycle, where intermediate metabolites are citrate, isocitrate, oxalyosuccinate, ketoglutarate, succinate, fumarate, malate, and oxalyacetate. These tricarboxylic acids all have the potential to be effective chelating agents for multivalent metals because of their multiple carboxyl functional groups.
The release of metabolic intermediates would seem to be detrimental to efficient metabolism; the benefit may be that that the release of LMWOA gives an organism the ability to obtain nutritional elements from nearby mineral surfaces. However, the energetic efficiency of metabolism may be very different for organisms that live directly on bare rock or unweathered soil, compared to organisms that are epiphytic or parasitic, or plants rooted in fertile soils. In the latter instances, mineral nutrients are obtained either from a host, or from organic chelates formed by reactions of minerals with dead organic matter (HMWOA). In contrast, organisms dwelling in barren environments need to obtain essential elements from a primary source, e.g., rock-forming minerals.
The most abundant tricarboxylic cycle acid to be released outside cells is usually citric acid (C
6H
8O
7). Indeed, the commercial production of citric acid typically relies on the fermentation of various sugars by the fungus
Aspergillus niger [
59,
60,
61]. Experiments involving the effectiveness of fungal culture in mineral dissolution indicate that solubilization can be caused by the production of citric acid by the organisms [
24].
For LMWOA, the nature of the chemical reaction varies with the chemical composition of the minerals and the organic acids. In sulfides and oxides, solubilization occurs when metal cations are removed from the lattice. For silicates, the decomposition sequence encountered under inorganic conditions may be quite different from the sequence that occurs in the presence of organic acids. The alteration of biotite provides an example. A common inorganic pathway for the weathering of biotite involves the transformation to vermiculate when hydrated cations cause interlayer expansion. In the presence of organic acids, metal ions in the octahedral layer are removed, leaving behind a fragile matrix of amorphous silica. This relict silicate lattice will disintegrate upon mechanical disturbance of the mineral grain [
62].
Studies of dissolution of iron from ferruginous minerals and from granodiorite show that organic acids of biologic origin can dissolve iron from the lattices of certain minerals within minutes [
63]. The amount of solubilization bears no direct correlation to the pH of the aqueous solutions, evidence that the dissolution of metal cations is the result of chelation, not the action of hydronium ions produced by the dissociation of these organic acids [
46].
Solubilization of minerals in the presence of organic chelating agents may be enhanced relative to solubility under inorganic conditions. Most minerals show small but measurable solubility in pure water, but equilibrium is normally reached before significant lattice damage occurs. However, organic compounds present in solution may react with ions as they are released from the mineral, producing soluble organic complexes. Such reactions would push the equilibrium toward the release of additional ions, so that the mineral could continue to dissolve indefinitely without reaching a saturation point.
The bioavailability of Fe commonly involves chelation effects, but this is not the only mechanism. In aquatic and marine environments, phytoplankton may obtain this micronutrient from unchelated Fe [
64,
65]. Iron bioavailability may also be facilitated by the reduction effects of organic ligands [
66,
67]. This is a reminder that although the present study provides data for understanding iron bioavailability for microbes growing on rock surfaces, it is not a model for global Fe uptake.