Enrichment of Fusobacteria in Sea Surface Oil Slicks from the Deepwater Horizon Oil Spill
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sample Collection and Incubation
2.2. Barcoded Amplicon Pyrosequencing
2.3. Phylogenetic Tree
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kujawinski, E.B.; Kido Soule, M.C.; Valentine, D.L.; Boysen, A.K.; Longnecker, K.; Redmond, M.C. Fate of dispersants associated with the Deepwater Horizon oil spill. Environ. Sci. Technol. 2011, 45, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Lubchenco, J.; McNutt, M.K.; Dreyfus, G.; Murawski, S.A.; Kennedy, D.M.; Anastas, P.T.; Chu, S.; Hunter, T. Science in support of the Deepwater Horizon response. Proc. Natl. Acad. Sci. USA 2012, 109, 20212–20221. [Google Scholar] [CrossRef] [PubMed]
- McNutt, M.K.; Chen, S.; Lubchenco, J.; Hunter, T.; Dreyfus, G.; Murawski, S.A.; Kennedy, D.M. Applications of science and engineering to quantify and control the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20222–20228. [Google Scholar] [CrossRef] [PubMed]
- Bælum, J.; Borglin, S.; Chakraborty, R.; Fortney, J.L.; Lamendella, R.; Mason, O.U.; Auer, M.; Zemla, M.; Bill, M.; Conrad, M.E.; et al. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 2012, 14, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jasson, J.K.; Proobst, A.; Borglin, S.E.; Fortney, J.L. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 2010, 330, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, S.; Grim, S.; Sogin, M.; Bracco, A.; Crespo-Medina, M.; Joy, S.B. Diverse, rare microbial taxa responded to the Deepwater Horizon deep-sea hydrocarbon plume. ISME J. 2015, 121. [Google Scholar] [CrossRef] [PubMed]
- Redmond, M.C.; Valentine, D.L. Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20292–20297. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nigro, L.M.; Gutierrez, T.; D’Ambrosio, L.; Joye, S.B.; Highsmith, R.; Teske, A. Pulsed blooms and persistent oil-degrading bacterial populations in the water column during and after the Deepwater Horizon blowout. Deep-Sea Res. II 2014, 1, 14. [Google Scholar] [CrossRef]
- Gutierrez, T.; Singleton, D.R.; Berry, D.; Yang, T.; Aitken, M.D.; Teske, A. Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 2013, 7, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Hazen, T.C.; Prince, R.C.; Mahmoudi, N. Marine oil bioremediation. Feature Article. Environ. Sci. Technol. 2016, 50, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Kostka, J.E.; Hazen, T.C.; Sobecky, P.A. Microbial responses to the Deepwater Horizon oil spill: From coastal wetlands to the deep sea. Annu. Rev. Mar. Sci. 2015, 7, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Hazen, T.C. Oil biodegradation and bioremediation: A tale of the two worst spills in US history. Environ. Sci. Technol. 2011, 45, 6709–6715. [Google Scholar] [CrossRef] [PubMed]
- Operational Science Advisory Team (OSAT). Summary Report for Fate and Effects of Remnant Oil in the Beach Environment. Available online: http://www.restorethegulf.gov/sites/default/files/u316/OSAT-2%20Report%20no%20ltr.pdf (accessed on 10 February 2011).
- Niu, H.; Li, Z.; Lee, K.; Kepkay, P.; Mullin, J.V. Modelling the transport of oil-mineral-aggregates (OMAs) in the marine environment and assessment of their potential risks. Environ. Model. Assess. 2011, 16, 61–75. [Google Scholar] [CrossRef]
- Passow, U.; Ziervogel, K.; Asper, V.; Diercks, A. Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ. Res. Lett. 2012, 7, 035031. [Google Scholar] [CrossRef]
- Alldredge, A.L.; Cohen, Y. Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science 1987, 235, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Alldredge, A.L.; Silver, M.W. Characteristics, dynamics and significance of marine snow. Prog. Oceanogr. 1988, 20, 41–82. [Google Scholar] [CrossRef]
- Ploug, H. Cyanobacterial surface blooms formed by Aphanizomenon. sp. and Nodularia spumigena in the Baltic Sea: Small-scale fluxes, pH, and oxygen micro-environments. Limnol. Oceanogr. 2008, 53, 914–921. [Google Scholar] [CrossRef]
- Ploug, H.; Kühl, M.; Buchholz-Cleven, B.; Jørgensen, B.B. Anoxic aggregates—An ephemeral phenomenon in the pelagic environment? Aquat. Microb. Ecol. 1997, 13, 285–294. [Google Scholar] [CrossRef]
- Shanks, A.L.; Reeder, M.L. Reducing microzones and sulfide production in marine snow. Mar. Ecol. Prog. Ser. 1993, 96, 43–47. [Google Scholar] [CrossRef]
- Bianchi, M.; Marty, D.; Teyssié, J.L.; Fowler, S.W. Strictly aerobic and anaerobic bacteria associated with sinking particulate matter and zooplankton fecal pellets. Mar. Ecol. Prog. Ser. 1992, 88, 55–60. [Google Scholar] [CrossRef]
- Tillett, D.; Neilan, B.A. Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. J. Phycol. 2000, 36, 251–258. [Google Scholar] [CrossRef]
- Hamady, M.; Walker, J.J.; Harris, J.K.; Gold, N.J.; Knight, R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 2008, 5, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Kunin, V.; Engelbrektson, A.; Ochman, H.; Hugenholtz, P. Wrinkles in the rare biosphere: Pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 2010, 12, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar]
- Ludwig, W.; Strunk, O.; Westram, R.; Richter, L.; Meier, H.; Yadhukumar, A.; Buchner, T.; Lai, S.; Steppi, G.; Jobb, W.; et al. ARB: A software environment for sequence data. Nucleic Acids Res. 2004, 32, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Berry, D.; Yang, T.; Mishamandani, S.; McKay, L.; Teske, A.; Aitken, M. Role of bacterial exopolysaccharides (EPS) in the fate of the oil released during the Deepwater Horizon oil spill. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; King, G.M. Isolation, characterization, and polyaromatic hydrocarbon degradation potential of aerobic bacteria from marine macrofaunal burrow sediments and description of Lutibacterium anuloederans gen. nov., sp. nov., and Cycloclasticus spirillensus sp. nov. Appl. Environ. Microbiol. 2001, 67, 5585–5592. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lai, Q.; Dong, C.; Shao, Z. Biodiversity of polycyclic aromatic hydrocarbon-degrading bacteria from deep sea sediments of the Middle Atlantic Ridge. Environ. Microbiol. 2008, 10, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Dyksterhouse, S.E.; Gray, J.P.; Herwig, R.P.; Cano Lara, J.; Staley, J.T. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 1995, 45, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Geiselbrecht, A.D.; Hedlund, B.P.; Tichi, M.A.; Staley, J.T. Isolation of marine polycyclic aromatic hydrocarbon (PAH)-degrading Cycloclasticus strains from the Gulf of Mexico and comparison of their PAH degradation ability with that of Puget Sound Cycloclasticus strains. Appl. Environ. Microbiol. 1998, 64, 4703–4710. [Google Scholar] [PubMed]
- Gutierrez, T.; Biddle, J.F.; Teske, A.; Aitken, M.D. Cultivation-dependent and cultivation-independent characterization of hydrocarbon-degrading bacteria in Guaymas Basin sediments. Front. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lai, Q.; Cui, Z.; Tan, T.; Shao, Z. A pyrene-degrading consortium from deep-sea sediment of the West Pacific and its key member Cycloclasticus sp. P1. Environ. Microbiol. 2008, 10, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Holm-Hansen, O.; Sutcliffe, W.H. Jr.; Sharp, J. Measurement of deoxyribonucleic acid in the ocean and its ecological significance. Limnol. Oceanogr. 1968, 13, 507–514. [Google Scholar]
- Hofstad, T. The genus Fusobacterium. In The Prokaryotes, 2nd ed.; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.-H., Eds.; Springer-Verlag: New York, NY, USA, 1991; pp. 4114–4126. [Google Scholar]
- Beazley, M.J.; Martinez, R.J.; Rajan, S.; Powell, J.; Piceno, Y.M.; Tom, Y.M.; Tom, L.M.; Andersen, G.L.; Hazen, T.C.; van Nostrand, J.D. Microbial Community Analysis of a Coastal Salt Marsh Affected by the Deepwater Horizon Oil Spill. PLoS ONE 2012, 7, e41305. [Google Scholar] [CrossRef]
- Liu, C. Geomicrobiology of Louisiana Coastal Marshes before and after the Deepwater Horizon Oil Spill. Masters’s Thesis, The Department of Geology and Geophysics, Louisiana State University, Baton Rouge, LA, USA, 2011. [Google Scholar]
- Orcutt, B.N.; Joye, S.B.; Kleindienst, S.; Knittel, K.; Ramette, A.; Reitz, A.; Samarkin, V.; Treude, T.; Boetius, A. Impact of natural oil and higher hydrocarbons on microbial diversity, distribution, and activity in Gulf of Mexico cold-seep sediments. Deep-Sea Res. II 2010, 57, 2008–2010. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Ke, W.J.; Sun, X.-B.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Comparison of bacterial community in aqueous and oil phases of water-flooded petroleum reservoirs using pyrosequencing and clone library approaches. Appl. Microbiol. Biotechnol. 2014, 98, 4209–4221. [Google Scholar] [CrossRef] [PubMed]
- Wrzodek, C.; Dräger, A.; Zell, A. KEGGtranslator: Visualizing and converting the KEGG PATHWAY database to various formats. Bioinformatics 2011, 27, 2314–2315. [Google Scholar] [CrossRef] [PubMed]
- Arnosti, C.; Ziervogel, K.; Yang, T.; Teske, A. Oil-derived marine aggregates—Hot spots of polysaccharide degradation by specialized bacterial communities. Deep Sea Res. II 2015. [Google Scholar] [CrossRef]
- Yang, T.; Speare, K.; McKay, L.; MacGregor, B.J.; Joye, S.B.; Teske, A. Following the oil fallout: Bacterial community succession in Gulf of Mexico seafloor sediment after the 2010 Deepwater Horizon blowout. Front. Microbiol. 2016. submitted. [Google Scholar]
- Fuchs, B.M.; Spring, S.; Teeling, H.; Quast, C.; Wulf, J.; Schattenhofer, M.; Yan, S.; Ferriera, S.; Johnson, J.; Glöckner, F.O.; Amann, R. Characterization of a marine gamma proteo-bacterium capable of aerobic anoxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Joye, S.B.; MacDonald, I.R.; Leifer, I.; Asper, V. Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nat. Geosci. 2011, 4, 160–164. [Google Scholar] [CrossRef]
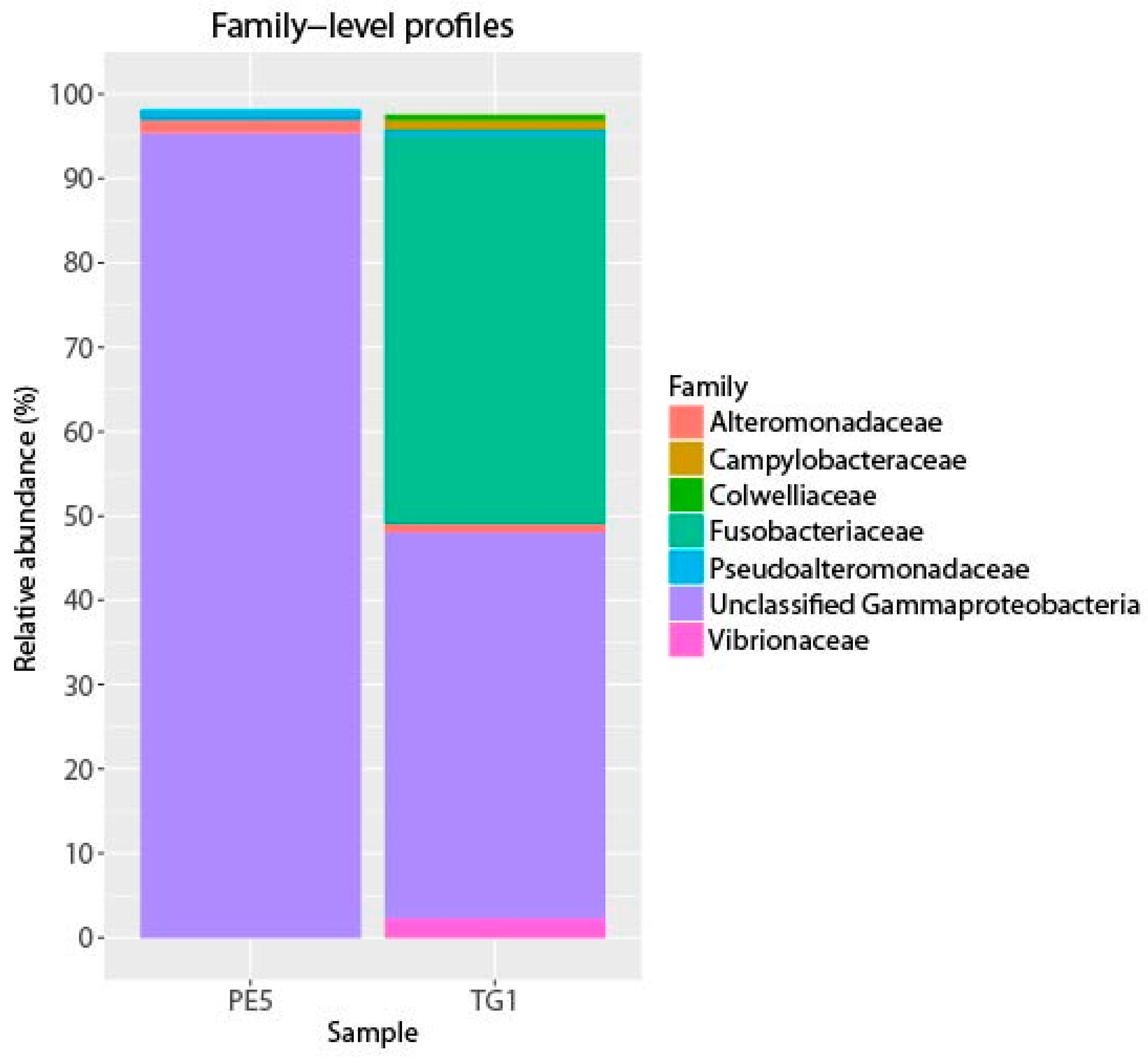
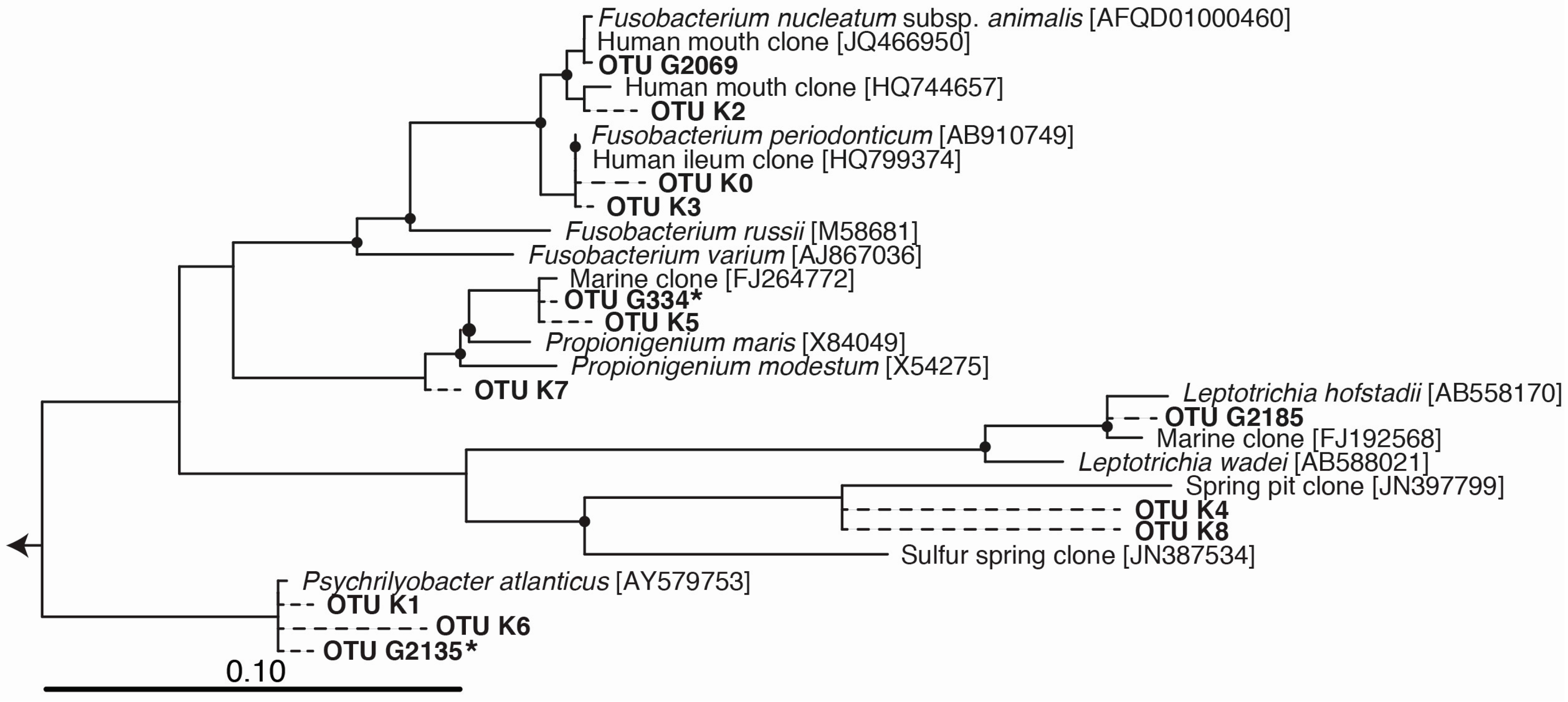
| Phylogenetic Affiliation | PE5 Incubation | TG1 Incubation | ||
|---|---|---|---|---|
| % Relative Abundance | No. of Sequences | % Relative Abundance | No. of Sequences | |
| Alphaproteobacteria | ||||
| Oceanibaculum | 0.00 | 0 | 0.00 | 1 |
| Rhodospirillales | 0.00 | 2 | 0.00 | 1 |
| Roseobacter | 0.06 | 28 | 0.01 | 3 |
| SAR11 (candidatus Pelagibacter) | 0.02 | 8 | 0.00 | 1 |
| SAR11 (SAR407) | 0.03 | 14 | 0.00 | 0 |
| SAR11 (SAR464) | 0.00 | 0 | 0.00 | 1 |
| SAR11 (unnamed) | 0.00 | 0 | 0.00 | 1 |
| Unclassified | 0.00 | 2 | 0.00 | 0 |
| Gammaproteobacteria | ||||
| Acinetobacter | 0.00 | 0 | 0.00 | 0 |
| Agarivorans | 0.00 | 2 | 0.03 | 15 |
| AGG47 related | 0.00 | 1 | 0.00 | 0 |
| Alcanivorax | 0.00 | 1 | 0.00 | 0 |
| Alteromonadales | 0.09 | 41 | 0.00 | 1 |
| Alteromonas | 0.73 | 349 | 0.01 | 3 |
| Arctic 96BD19 | 0.00 | 0 | 0.00 | 1 |
| Colwellia | 0.09 | 41 | 0.18 | 84 |
| Colwellia related | 0.02 | 8 | 0.01 | 7 |
| Congregibacter and relatives | 0.03 | 13 | 0.46 | 219 |
| Cycloclasticus | 47.03 | 22,333 | 21.78 | 10,285 |
| Cycloclasticus related | 2.24 | 564 | 1.19 | 439 |
| DWH plume group | 0.04 | 9 | 0.02 | 6 |
| Halomonas | 0.15 | 36 | 0.00 | 0 |
| Idiomarina | 0.00 | 0 | 0.00 | 1 |
| Marinimicrobium | 0.00 | 0 | 0.00 | 1 |
| Marinobacter | 0.02 | 5 | 0.01 | 2 |
| Marinomonas | 0.00 | 0 | 0.05 | 19 |
| Moritella | 0.00 | 1 | 0.10 | 37 |
| Neptunomonas | 0.00 | 0 | 0.01 | 2 |
| Oceanobacter | 0.00 | 0 | 0.00 | 1 |
| Oceanospirillales | 0.03 | 7 | 0.03 | 10 |
| Oceanospirillum | 0.00 | 0 | 0.02 | 8 |
| Oleispira | 0.03 | 7 | 0.03 | 10 |
| Pseudoalteromonas | 1.17 | 287 | 0.71 | 260 |
| SAR86 | 0.02 | 4 | 0.00 | 0 |
| Shewanella | 0.00 | 0 | 0.02 | 8 |
| SUP05 Arctic related | 0.00 | 1 | 0.00 | 1 |
| Unidentified | 0.05 | 13 | 0.39 | 142 |
| Vibrionales | 0.02 | 5 | 1.82 | 654 |
| ZD0417 | 0.00 | 1 | 0.00 | 0 |
| Bacteroidetes | 0.43 | 104 | 0.64 | 227 |
| Cyanobacteria | 0.30 | 73 | 0.01 | 3 |
| Deltaproteobacteria | 0.05 | 12 | 0.03 | 9 |
| Epsilonproteobacteria | 0.04 | 10 | 0.75 | 264 |
| Firmicutes | 0.00 | 0 | 0.28 | 98 |
| Fusobacteria | 0.00 | 0 | 31.46 | 10,926 |
| Actinobacteria | 0.01 | 4 | 0.00 | 0 |
| Lentisphaerae | 0.00 | 0 | 0.08 | 20 |
| Planctomycetes | 0.02 | 4 | 0.00 | 0 |
| Fibrobacteres/Acidobacteria | 0.00 | 0 | 0.00 | 1 |
| Firmicutes | 0.02 | 5 | 0.00 | 0 |
| Unclassified bacteria | 0.02 | 4 | 0.01 | 3 |
| Total sequences | 23,999 | 23,775 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, T.; Berry, D.; Teske, A.; Aitken, M.D. Enrichment of Fusobacteria in Sea Surface Oil Slicks from the Deepwater Horizon Oil Spill. Microorganisms 2016, 4, 24. https://doi.org/10.3390/microorganisms4030024
Gutierrez T, Berry D, Teske A, Aitken MD. Enrichment of Fusobacteria in Sea Surface Oil Slicks from the Deepwater Horizon Oil Spill. Microorganisms. 2016; 4(3):24. https://doi.org/10.3390/microorganisms4030024
Chicago/Turabian StyleGutierrez, Tony, David Berry, Andreas Teske, and Michael D. Aitken. 2016. "Enrichment of Fusobacteria in Sea Surface Oil Slicks from the Deepwater Horizon Oil Spill" Microorganisms 4, no. 3: 24. https://doi.org/10.3390/microorganisms4030024
APA StyleGutierrez, T., Berry, D., Teske, A., & Aitken, M. D. (2016). Enrichment of Fusobacteria in Sea Surface Oil Slicks from the Deepwater Horizon Oil Spill. Microorganisms, 4(3), 24. https://doi.org/10.3390/microorganisms4030024








