Carbon-Starvation Induces Cross-Resistance to Thermal, Acid, and Oxidative Stress in Serratia marcescens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth Media, and Culture Conditions
| Strain Designations | Source of Isolation | Pigmentation | Other Relevant Characteristics | References |
|---|---|---|---|---|
| ATCC 13880 | Pond water | Mixed colony pigmentation at 30 °C; less pigmentation at 37 °C | Type strain of species; quality control strain; genome sequence complete | [3,29] |
| Db10 | Drosophila melanogaster | No pigmentation at either 30 °C or 37 °C | Invertebrate pathogen used in studies with host Caenorhabditis elegans; genome sequence complete | [30,31] |
| UWG6 | rhizosphere soil | Red pigmentation at 30 °C; pale pink at 37 °C | Positive for glucose fermentation and DNase activity; bile-resistant | This study |
2.2. Starvation-Induced Cross-Resistance Challenges
2.3. Long-Term Carbon-Starvation Survival
2.4. Statistical Analysis of Survival Data
3. Results
3.1. Starvation-Induced Cross-Resistance to Thermal Stress in S. marcescens ATCC 13880
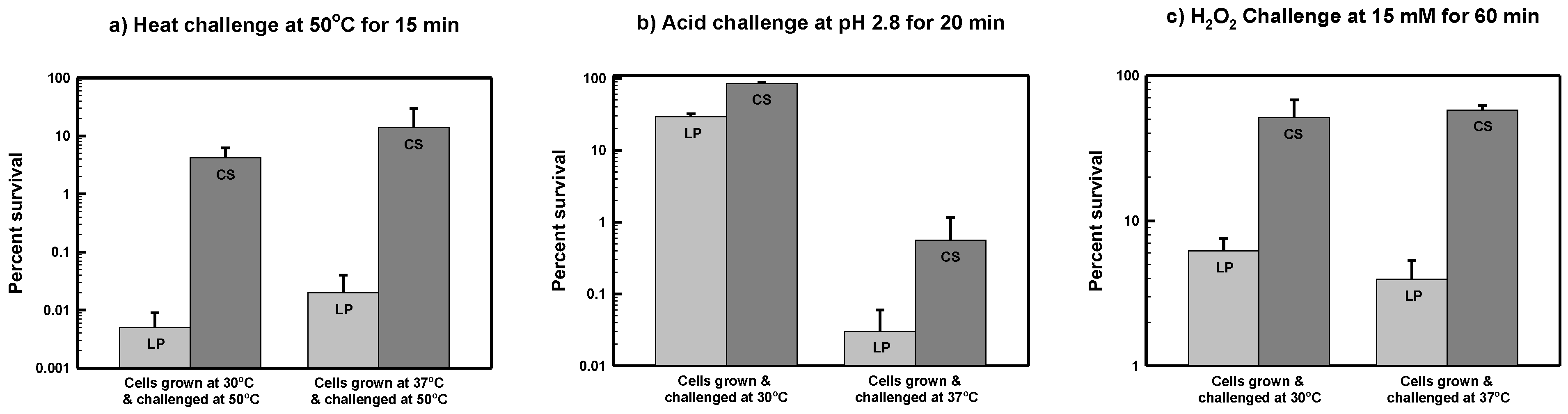
3.2. Starvation-Induced Cross-Resistance to Acid Stress in S. marcescens ATCC 13880
3.3. Starvation-Induced Cross-Resistance to Oxidative Stress in S. marcescens ATCC 13880
3.4. Starvation-Induced Cross-Resistance in Other Pigmented and Non-Pigmented Strains
| Thermal Challenge (15 min at 50 °C) | |||
| Strain UWG6 | Strain Db10 | ||
| 30 °C | 37 °C | 30 °C | 37 °C |
| LP = 0.040 ± 0.026 | LP = 7.5 ± 4.3 | LP = 0.020 ± 0.020 | LP = 0.21 ± 0.11 |
| CS = 38 ± 10 | CS = 35 ± 15 | CS = 3.1 ± 3.4 | CS = 44 ± 7.8 |
| Acid Challenge (20 min at pH 2.8) | |||
| Strain UWG6 | Strain Db10 | ||
| 30 °C | 37 °C | 30 °C | 37 °C |
| LP = 50 ± 30 | LP = 0.36 ± 0.33 | LP = 2.3 ± 2.2 | LP = 0.01 ± 0.01 |
| CS = 72 ± 16 | CS = 0.89 ± 0.087 | CS = 42 ± 7.6 | CS = 3.9 ± 2.2 |
| Hydrogen Peroxide Challenge (60 min at 15 mM) | |||
| Strain UWG6 | Strain Db10 | ||
| 30 °C | 37 °C | 30 °C | 37 °C |
| LP = 2.0 ± 1.5 | LP = 0.60 ± 0.28 | LP = 7.9 ± 2.5 | LP = 6.5 ± 5.0 |
| CS = 7.9 ± 1.7 | CS = 56 ± 22 | CS = 45 ± 20 | CS = 56 ± 13 |
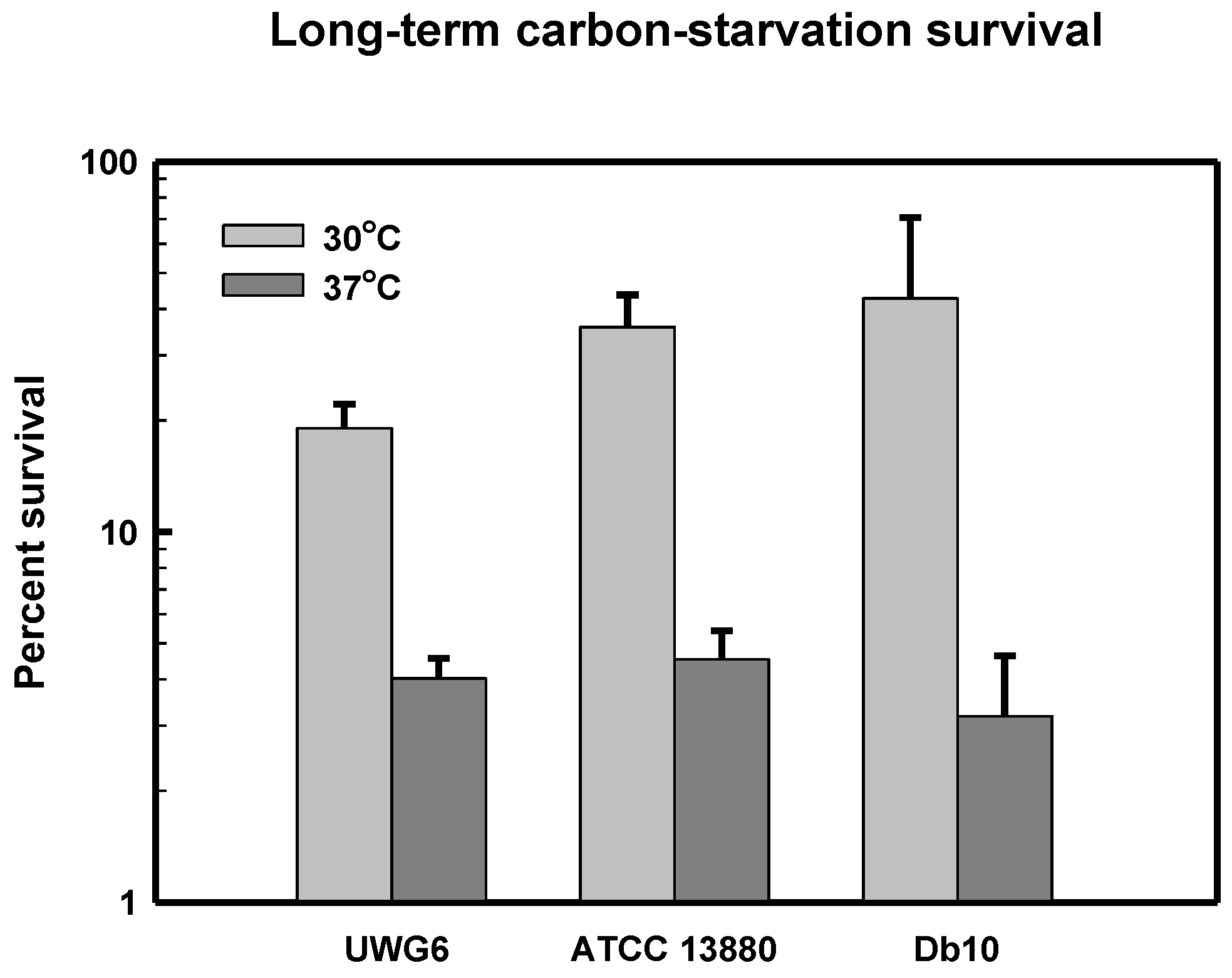
3.5. Long-Term Carbon-Starvation Survival in Pigmented and Non-Pigmented Strains
3.6. Stress-Induced Colony Phenotypes
| Strains | Type of Challenge | SIC Phenotypes |
|---|---|---|
| ATCC 13880 | Acid challenge | pigmentation only in the center of some colonies (“fish eye” phenotype) |
| Long-term carbon-starvation | non-pigmented, translucent colonies | |
| UWG6 | Thermal challenge | non-pigmented (white) colonies |
| Acid challenge | non-pigmented (white to translucent) colonies | |
| Long-term carbon-starvation | translucent colonies; some with pink centers |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, V.L. Serratia marcescens: Historical perspective and clinical review. N. Engl. J. Med. 1979, 300, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.A.; Grimont, F. The genus Serratia. Annu. Rev. Microbiol. 1978, 32, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, A.; Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Mahlen, S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.L.; Chauvet, S.; Andrès, E.; Aurouze, M.; Vallet, I.; Michel, G.P.; Uh, M.; Celli, J.; Filloux, A.; de Bentzmann, S.; et al. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 2003, 22, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, A.; Nagaya, Y.; Pradel, E.; Ooka, T.; Ogura, Y.; Katsura, K.; Kurokawa, K.; Oshima, K.; Hattori, M.; Parkhill, J.; et al. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol. Evol. 2014, 6, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Parment, P.A. The role of Serratia marcescens in soft contact lens associated ocular infections. Acta Ophthalmol. Scand. 1997, 75, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.B.; Zhu, H.; Cole, N.; Huynh, C.; Lam, S.; Willcox, M.D. Efficacy of contact lens multipurpose solutions against Serratia marcescens. Optom. Vis. Sci. 2007, 84, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Matin, A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol. Microbiol. 1991, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Spector, M.P. The starvation-stress response of Salmonella. Adv. Microb. Physiol. 1998, 40, 233–279. [Google Scholar] [PubMed]
- Spector, M.P.; Kenyon, W.J. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- Bennett, J.W.; Bentley, R. Seeing red: The story of prodigiosin. Adv. Appl. Microbiol. 2000, 47, 1–32. [Google Scholar] [PubMed]
- Carbonell, G.V.; Della Colleta, H.H.; Yano, T.; Darini, A.L.; Levy, C.E.; Fonseca, B.A. Clinical relevance and virulence factors of pigmented Serratia marcescens. FEMS Immunol. Med. Microbiol. 2000, 28, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.K.; Williamson, N.R.; Slater, H.; Cox, A.; Abbasi, S.; Foulds, I.; Simonsen, H.T.; Leeper, F.J.; Salmond, G.P. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology 2004, 150, 3547–3560. [Google Scholar] [CrossRef] [PubMed]
- Ruhen, R.W.; Wetherall, F.M. Colonial variation in Serratia marcescens together with antibiotic resistance. Pathology 1983, 15, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Paruchuri, D.K.; Harshey, R.M. Flagellar variation in Serratia marcescens is associated with color variation. J. Bacteriol. 1987, 169, 61–65. [Google Scholar] [PubMed]
- Traub, W.H.; Eiden, A.; Leonhard, B.; Bauer, D. Phenotypic variation of clinical Serratia marcescens isolates repeatedly recovered from individual patients. Zentralbl. Bakteiol. 1996, 284, 124–135. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Holanda, A.R.; Lustosa, G.P.; Nóbrega, S.M.; Santana, W.J.; Souza, L.B.; Coutinho, H.D. Virulence factors and resistance mechanisms of Serratia marcescens: A short review. Acta Microbiol. Immunol. Hung. 2006, 53, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Friman, V.P.; Hiltunen, T.; Jalasvuori, M.; Lindstedt, C.; Laanto, E.; Örmälä, A.M.; Laakso, J.; Mappes, J.; Bamford, J.K. High temperature and bacteriophages can indirectly select for bacterial pathogenicity in environmental reservoirs. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Ketola, T.; Mikonranta, L.; Zhang, J.; Saarinen, K.; Örmälä, A.M.; Friman, V.P.; Mappes, J.; Laakso, J. Fluctuating temperature leads to evolution of thermal generalism and preadaptation to novel environments. Evolution 2013, 67, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Costerton, J.W. Prolonged survival of Serratia marcescens in chlorhexidine. Appl. Environ. Microbiol. 1981, 42, 1093–1102. [Google Scholar] [PubMed]
- Nakashima, A.K.; Highsmith, A.K.; Martone, W.J. Survival of Serratia marcescens in benzalkonium chloride and in multiple-dose medication vials: Relationship to epidemic septic arthritis. J. Clin. Microbiol. 1987, 25, 1019–1021. [Google Scholar] [PubMed]
- Szewzyk, U.; Szewzyk, R.; Stenström, T.A. Growth and survival of Serratia marcescens under aerobic and anaerobic conditions in the presence of materials from blood bags. J. Clin. Microbiol. 1993, 31, 1826–1830. [Google Scholar] [PubMed]
- Parment, P.A.; Colucci, B.; Nyström, B. The efficacy of soft contact lens disinfection solutions against Serratia marcescens and Pseudomonas aeruginosa. Acta Ophthalmol. Scand. 1996, 74, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Langsrud, S.; Møretrø, T.; Sundheim, G. Characterization of Serratia marcescens surviving in disinfecting footbaths. J. Appl. Microbiol. 2003, 95, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, H.; Kobayashi, J.M.; Ohyama, T.; Okabe, N. A nosocomial outbreak of febrile bloodstream infection caused by heparinized-saline contaminated with Serratia marcescens, Tokyo, 2002. Jpn. J. Infect. Dis. 2004, 57, 189–192. [Google Scholar] [PubMed]
- Sunenshine, R.H.; Tan, E.T.; Terashita, D.M.; Jensen, B.J.; Kacica, M.A.; Sickbert-Bennett, E.E.; Noble-Wang, J.A.; Palmieri, M.J.; Bopp, D.J.; Jernigan, D.B.; et al. A multistate outbreak of Serratia marcescens bloodstream infection associated with contaminated intravenous magnesium sulfate from a compounding pharmacy. Clin. Infect Dis. 2007, 45, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Spector, M.P. How Salmonella survive against the odds. Annu. Rev. Microbiol. 1995, 49, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Daligault, H.E.; Davenport, K.W.; Minoque, T.D.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Coyne, S.R.; Gibbons, H.S.; Jaissle, J.; Rosenzweig, C.N.; et al. Genome assembly of Serratia marcescens type strain ATCC 13880. Genome Announc. 2014. [Google Scholar] [CrossRef] [PubMed]
- Flyg, C.; Kenne, K.; Boman, H.G. Insect pathogenic properties of Serratia marcescens: Phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J. Gen. Microbiol. 1980, 120, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, H.; Ewbank, J.J. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 2004, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, W.J.; Sayers, D.G.; Humphreys, S.; Roberts, M.; Spector, M.P. The starvation-stress response of Salmonella enterica serovar Typhimurium requires sigma(E)-, but not CpxR-regulated extracytoplasmic functions. Microbiology 2002, 148, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rius, N.; Solé, M.; Francia, A.; Lorén, J.G. Buffering capacity of pigmented and nonpigmented strains of Serratia marcescens. Appl. Environ. Microbiol. 1994, 60, 2152–2154. [Google Scholar] [PubMed]
- Tanikawa, T.; Nakagawa, Y.; Matsuyama, T. Transcriptional downregulator hexS controlling prodigiosin and serrawettin W1 biosynthesis in Serratia marcescens. Microbiol. Immunol. 2006, 50, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Dimmick, R.L. Effect of 3 percent hydrogen peroxide on the viability of Serratia marcescens. J. Bacteriol. 1996, 91, 925–929. [Google Scholar]
- Bayliss, C.E.; Waites, W.M. Resistance of Serratia marcescens to hydrogen peroxide. J. Appl. Bacteriol. 1981, 50, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Robbe-Saule, V.; Coynault, C.; Ibanez-Ruiz, M.; Hermant, D.; Norel, F. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS). Mol. Microbiol. 2001, 39, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.W.; Cai, Y.J.; Liao, X.R.; Zhang, F.; Zhang, D.B. Production, characterization, cloning and sequence analysis of a monofunctional catalase from Serratia marcescens SYBC08. J. Basic Microbiol. 2011, 51, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.P.; Goldschmidt, M.E.; Gott, C.L. Inhibition by temperature of the terminal step in biosynthesis of prodigiosin. Biochem. Biophys. Res. Commun. 1965, 19, 177–181. [Google Scholar] [CrossRef]
- Sole, M.; Rius, N.; Francia, A.; Loren, J.G. The effect of pH on prodigiosin production by non-proliferating cells of Serratia marcescens. Lett. Appl. Microbiol. 1994, 19, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Deacon, W.E. An observation on the longevity of Serratia marcescens (B. prodigiosus). Science 1932, 76, 274. [Google Scholar] [CrossRef] [PubMed]
- Rettger, L.F.; Sherrick, J.L. Studies on bacterial variation. J. Med. Res. 1911, 24, 265–284. [Google Scholar] [PubMed]
- Reed, G.B. Independent variation of several characteristics in Serratia marcescens. J. Bacteriol. 1937, 34, 255–266. [Google Scholar] [PubMed]
- Bunting, M.I. A description of some color variants produced by Serratia marcescens, strain 274. J. Bacteriol. 1940, 40, 57–68. [Google Scholar] [PubMed]
- Klauck, E.; Typas, A.; Hengge, R. The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli. Sci. Prog. 2007, 90, 103–127. [Google Scholar] [PubMed]
- Sharma, U.K.; Chatterji, D. Transcriptional switching in Escherichia coli during stress and starvation by modulation of sigma activity. FEMS Microbiol. Rev. 2010, 34, 646–657. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, C.R.; Gabriel, W.M.; Turk, A.K.; Libby, S.J.; Fang, F.C.; Spector, M.P. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J. Bacteriol. 1994, 176, 4610–4616. [Google Scholar] [PubMed]
- Hengge-Aronis, R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002, 66, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Tormo, A.; Navarro-Lloréns, J.M. Polymorphism in the yclC-rpoS region in enterobacteria. Curr. Microbiol. 2003, 46, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, T.; Spira, B. Variation in stress response within a bacterial species and the indirect costs of stress resistance. Ann. N. Y. Acad. Sci. 2007, 1113, 105–113. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittman, J.R.; Kline, L.C.; Kenyon, W.J. Carbon-Starvation Induces Cross-Resistance to Thermal, Acid, and Oxidative Stress in Serratia marcescens. Microorganisms 2015, 3, 746-758. https://doi.org/10.3390/microorganisms3040746
Pittman JR, Kline LC, Kenyon WJ. Carbon-Starvation Induces Cross-Resistance to Thermal, Acid, and Oxidative Stress in Serratia marcescens. Microorganisms. 2015; 3(4):746-758. https://doi.org/10.3390/microorganisms3040746
Chicago/Turabian StylePittman, Joseph R., La’Kesha C. Kline, and William J. Kenyon. 2015. "Carbon-Starvation Induces Cross-Resistance to Thermal, Acid, and Oxidative Stress in Serratia marcescens" Microorganisms 3, no. 4: 746-758. https://doi.org/10.3390/microorganisms3040746
APA StylePittman, J. R., Kline, L. C., & Kenyon, W. J. (2015). Carbon-Starvation Induces Cross-Resistance to Thermal, Acid, and Oxidative Stress in Serratia marcescens. Microorganisms, 3(4), 746-758. https://doi.org/10.3390/microorganisms3040746





