Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.1.1. Main Chemical Reagents and Culture Media
2.1.2. Experimental Soil Samples
2.1.3. Biochemical Reagent
2.1.4. Experimental Instruments and Equipment
2.2. Soil Properties
2.3. Preparation of Soil Samples and Bacterial Suspensions
2.4. Experimental Design and Treatment
2.5. Extraction and Detection of Prometryn and Acetochlor
2.6. Pot Experiment Setup and Treatment
- (1)
- Control group (CK): Soil in the potting buckets was not inoculated with microorganisms and not sprayed with pesticides.
- (2)
- Treatment group 1 (PA): Mother liquor of prometryn and acetochlor was sprayed evenly into the potting buckets until the final concentration of both prometryn and acetochlor in the soil reached 50 mg·kg−1.
- (3)
- Treatment group 2 (NMJ): Potting buckets were evenly sprayed with prometryn and acetochlor mother liquor to a final concentration of 50 mg·kg−1 for each pesticide, followed by the addition of a bacterial suspension of strain NM-1 (concentration: 1 × 108 CFU·mL−1), which was mixed thoroughly.
2.7. Detection of Physiological Indexes of Soybean Seedlings
2.8. Determination of Soil Enzyme Activities
2.9. Soil DNA Extraction and High-Throughput Sequencing
2.10. Statistical Analysis
3. Results and Discussion
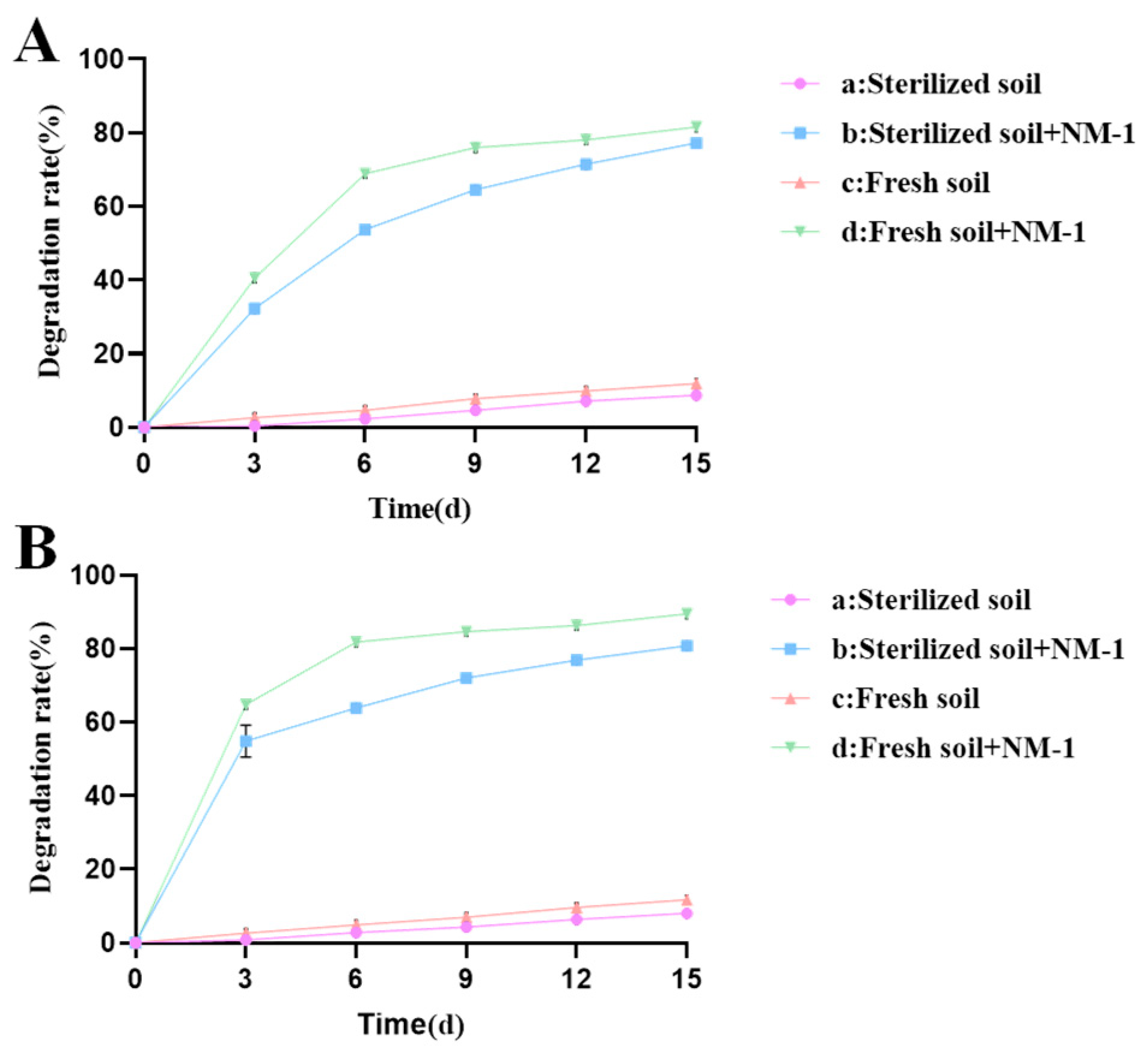
3.1. Bioremediation of Prometryn and Acetochlor Contaminated Soil by Strain NM-1
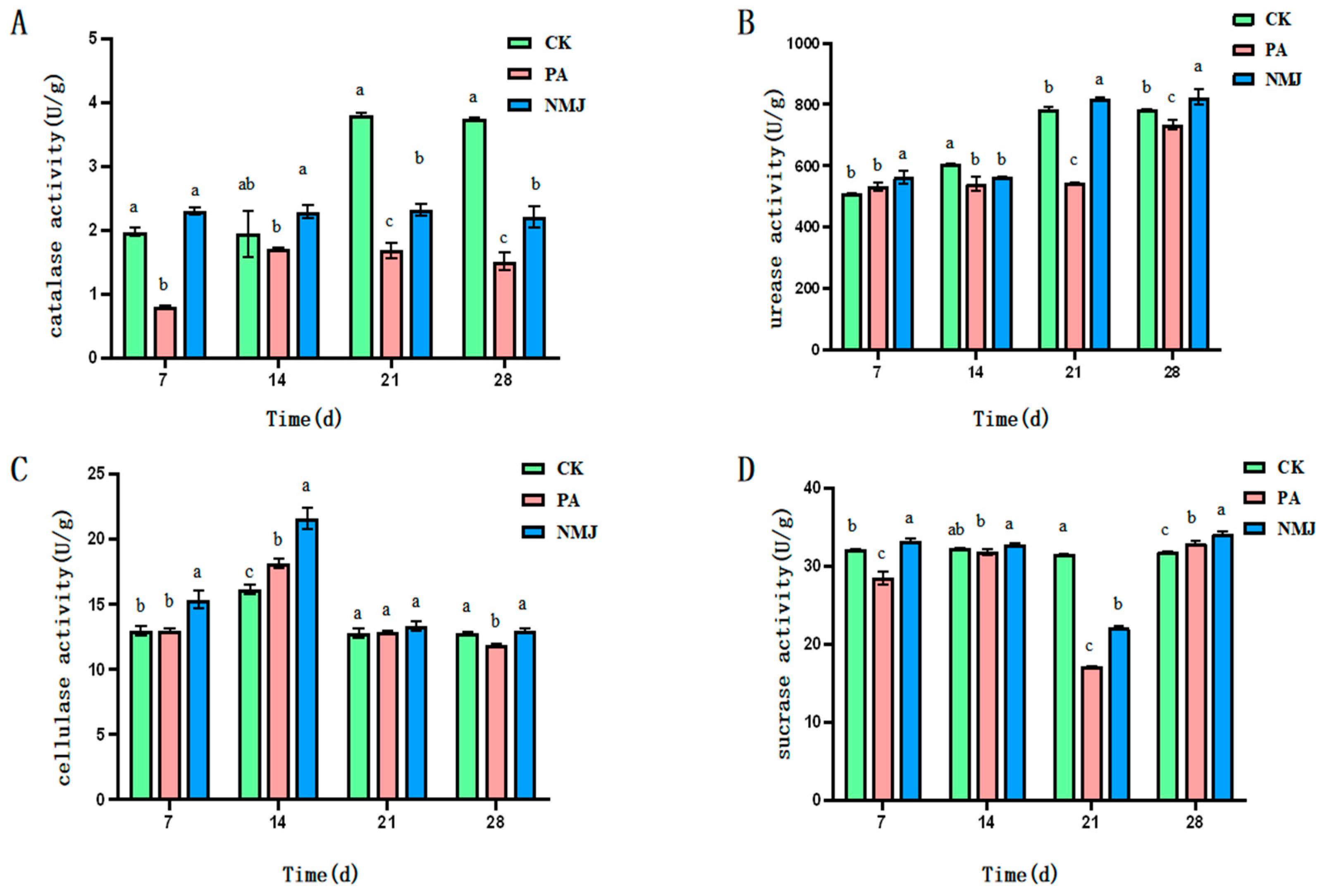
3.2. Effect of Strain NM-1 on Soil Enzyme Activity
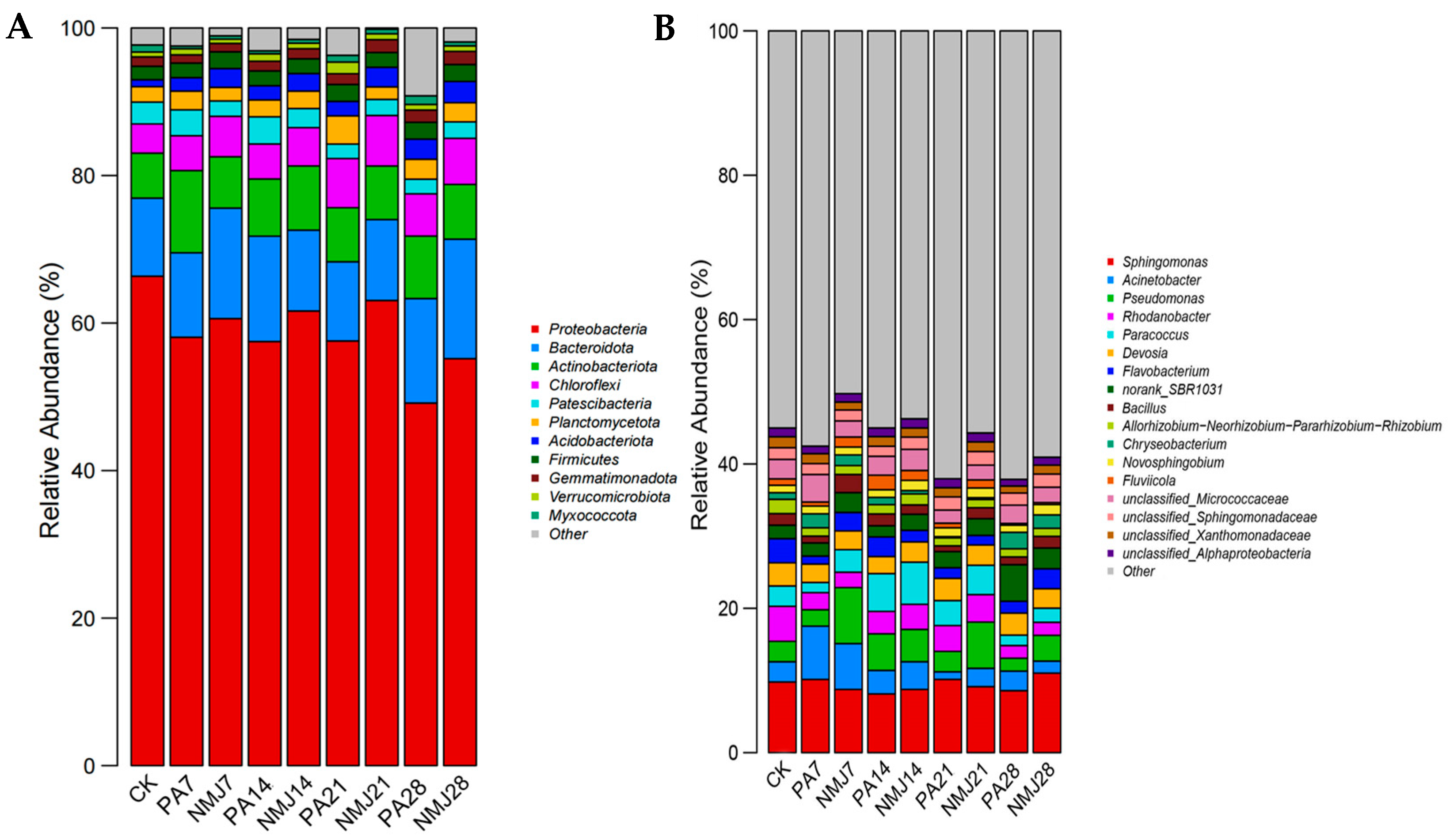
3.3. Effect of Strain NM-1 on Soil Bacterial Community Composition
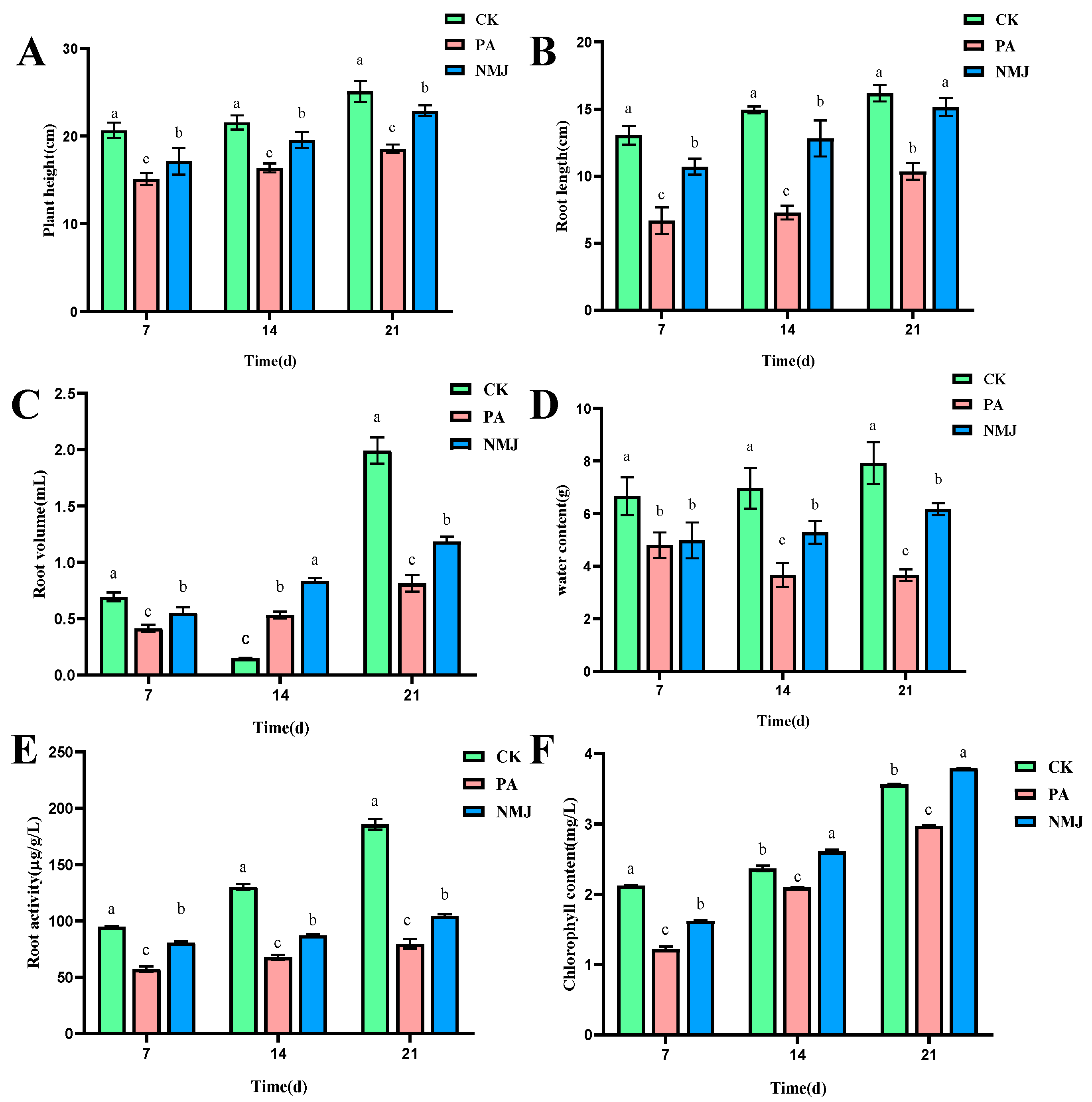
3.4. Effects of Strain NM-1 on Physiological and Ecological Indexes of Soybean Seedlings
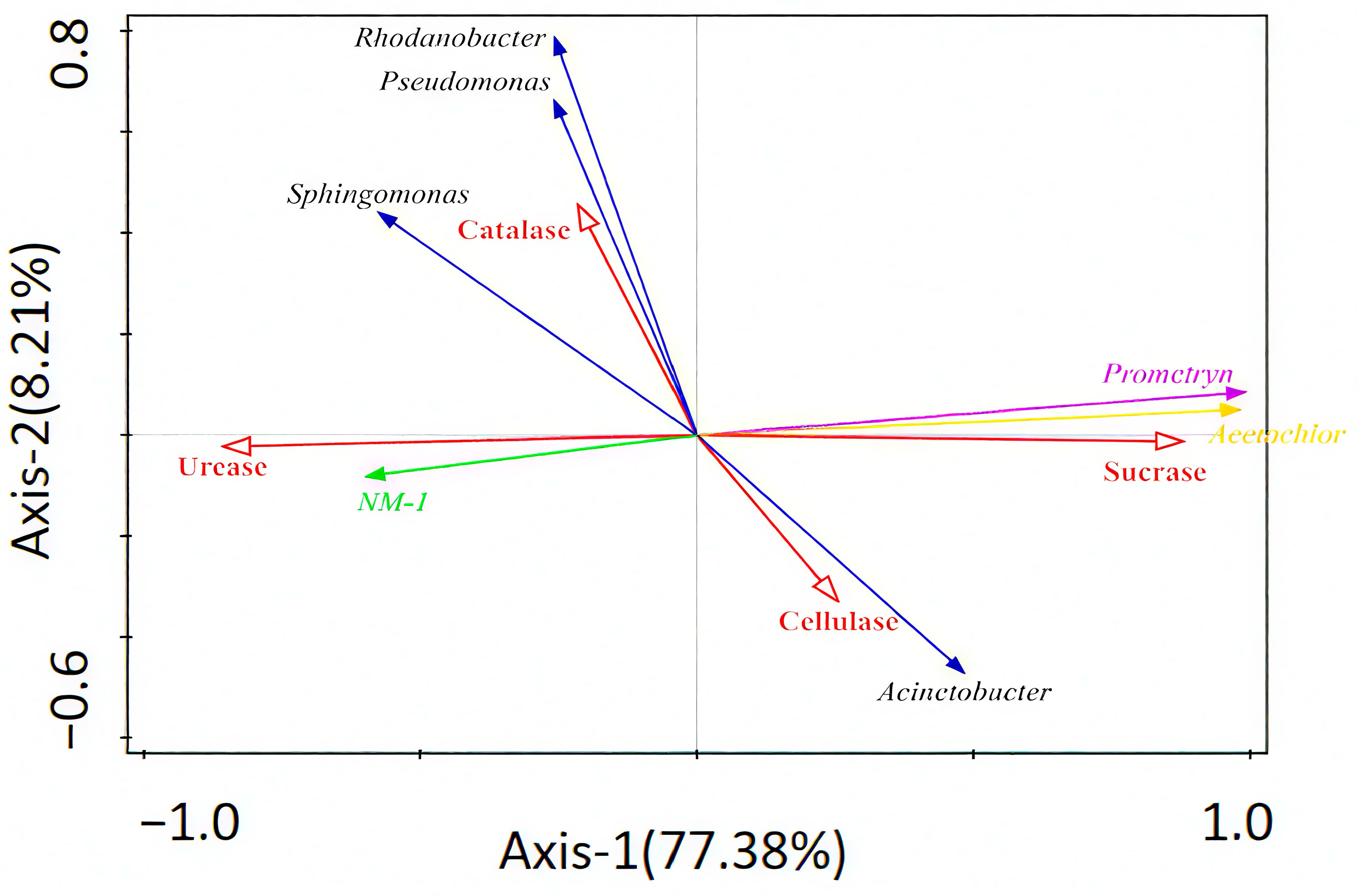
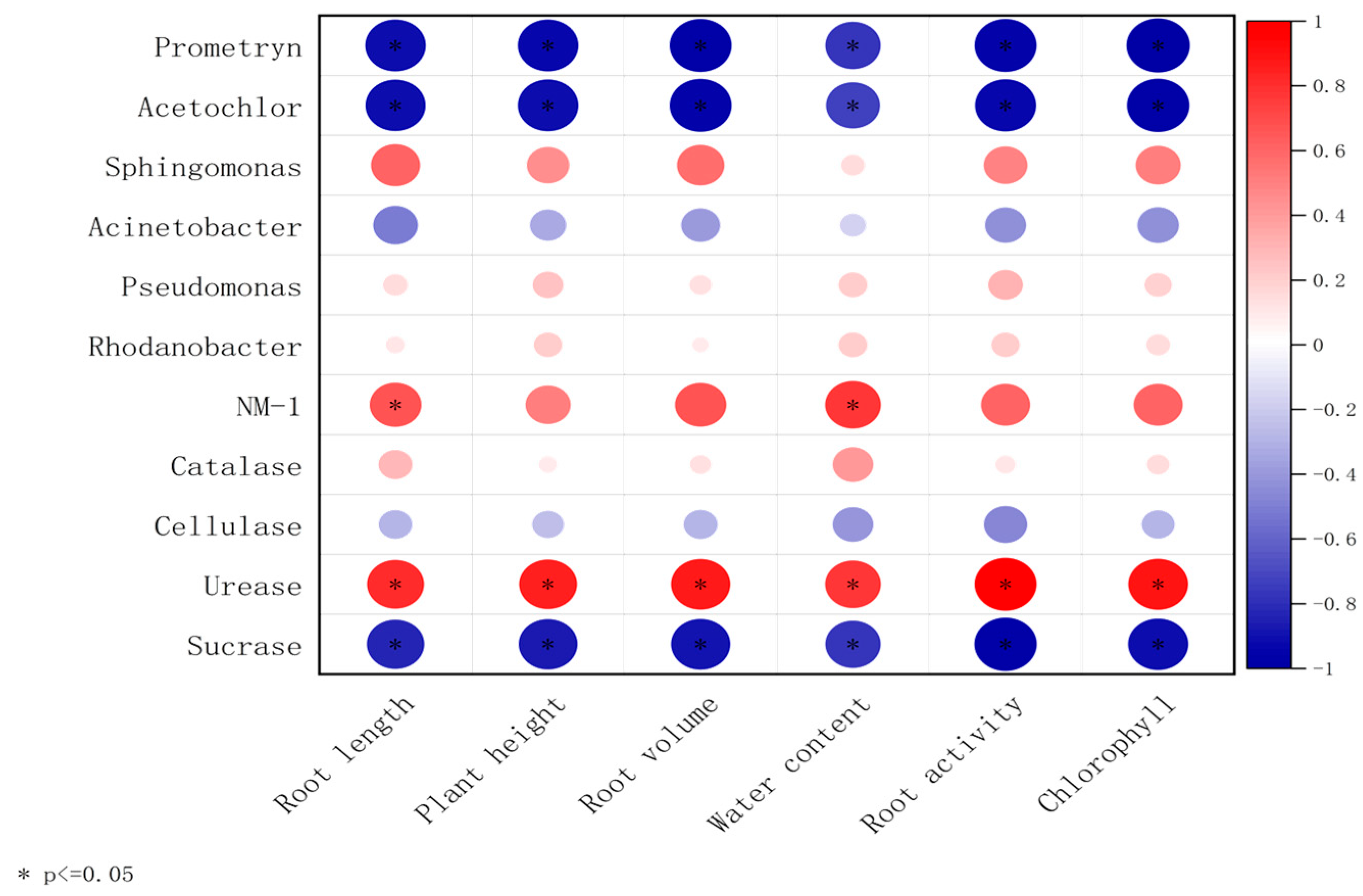
3.5. Correlation Analysis Between Strain NM-1, Soil Enzymes, Prometryn, Acetochlor, and Bacterial Communities
3.6. Microbe–Enzyme–Plant Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kniss, A. Long-term trends in the intensity and relative toxicity of herbicide use. Nat. Commun. 2017, 8, 14865. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, E.G.; Ren, Y.; Jin, S.; Zhang, T.; Liu, J.; Li, Z. Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects. Int. J. Environ. Res. Public Health 2017, 14, 1466. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Yang, Y.; Zong, Y.; Yang, Y.; Guo, M.; Zhang, Z.; Zhang, R.; Ru, S.; Zhang, X. Long-term exposure to prometryn damages the visual system and changes color preference of female zebrafish (Danio rerio). Chemosphere 2024, 363, 142835. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lv, J.; Deng, H.; Liu, Q.; Liang, S. Occurrence and Removal of Triazine Herbicides during Wastewater Treatment Processes and Their Environmental Impact on Aquatic Life. Int. J. Environ. Res. Public Health 2022, 19, 4557. [Google Scholar] [CrossRef] [PubMed]
- Boulahia, K.; Carol, P.; Planchais, S.; Abrous-Belbachir, O. Phaseolus ulgaris L. Seedlings Exposed to Prometryn Herbicide Contaminated Soil Trigger an Oxidative Stress Response. J. Agric. Food Chem. 2016, 64, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Min, N.; Park, H.; Hong, T.; An, G.; Song, G.; Lim, W. Developmental toxicity of prometryn induces mitochondrial dysfunction, oxidative stress, and failure of organogenesis in zebrafish (Danio rerio). J. Hazard. Mater. 2023, 443, 130202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, L.; Chen, H.; Huang, B.; Xu, J.; Li, Y.; Héroux, P.; Zhu, X.; Wu, Y.; Xia, D. Prometryn induces apoptotic cell death through cell cycle arrest and oxidative DNA damage. Toxicol. Res. 2019, 8, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.; Chen, S.; Zhao, W.; Zhao, Y.; Wang, X.; Zhang, Y. A urinary metabonomics analysis of long-term effect of acetochlor exposure on rats by ultra-performance liquid chromatography/mass spectrometry. Pestic. Biochem. Physiol. 2016, 128, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liang, T.; Zhai, H. Effects of acetochlor on the photosynthetic and fluorescence characteristics and chloroplast structure of grape leaves. Ying Yong Sheng Tai Xue Bao 2012, 23, 2185–2190. [Google Scholar] [PubMed]
- Zhang, Y.; Xue, W.; Long, R.; Yang, H.; Wei, W. Acetochlor affects zebrafish ovarian development by producing estrogen effects and inducing oxidative stress. Environ. Sci. Pollut. Res. Int. 2020, 27, 27688–27696. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, F.; Lu, J.; Wu, M.; Cheng, J.; Xu, W.; Li, Z.; Zhang, Y. Developmental and cardiovascular toxicities of acetochlor and its chiral isomers in zebrafish embryos through oxidative stress. Sci. Total Environ. 2023, 896, 165296. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Huang, Y.; Huang, Y.; Yang, Y.; Zhao, Y.; Martyniuk, C.J. Toxicity assessment of the herbicide acetochlor in the human liver carcinoma (HepG2) cell line. Chemosphere 2020, 243, 125345. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, H.W.; Bhatt, P. Microbial adaptation and impact into the pesticide’s degradation. Arch. Microbiol. 2022, 204, 288. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wu, Z.; Wei, M.; Liu, X.; He, Y.; Ye, B.C. Bacillus subtilis SL-13 biochar formulation promotes pepper plant growth and soil improvement. Can. J. Microbiol. 2019, 65, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Ding, M.Y.; Xiao, C.Y.; Shen, Y.W.; Wang, Y.Y.; Li, H.T.; Liu, R.M.; Gao, J.G. Isolation and Identification of Pseudomonas sp. Strain DY-1 from Agricultural Soil and Its Degradation Effect on Prometryne. Curr. Microbiol. 2021, 78, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dong, W.; Wang, F.; Li, J.; Shen, W.; Li, Y.; Cui, Z. Degradation of acetochlor by a bacterial consortium of Rhodococcus sp.T3-1, Delftia sp.T3-6 and Sphingobium sp.MEA3-1. Lett. Appl. Microbiol. 2014, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Qiu, J.; Ma, Y.; Zhou, S. Isolation, identification, and acetochlor-degrading potential of a novel Rhodococcus sp. MZ-3. J. Environ. Sci. Health B 2016, 51, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, L.; Liu, X.; Shao, K.; Li, X.Z.; Li, R.X. Soil Enzyme Activity in Picea schrenkiana and Its Relationship with Environmental Factors in the Tianshan Mountains, Xinjiang. Huan Jing Ke Xue 2021, 42, 403–410. (In Chinese) [Google Scholar] [PubMed]
- Tan, X.; Nie, Y.; Ma, X.; Guo, Z.; Liu, Y.; Tian, H.; Megharaj, M.; Shen, W.; He, W. Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci. Total Environ. 2021, 770, 144500. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total Environ. 2020, 741, 140446. [Google Scholar] [CrossRef] [PubMed]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef] [PubMed]
- Rouhi-Kelarlou, T.; Golchin, A.; Toularoud, A.A.S. Ecotoxicological impact of butisanstar and clopyralid herbicides on soil microbial respiration and the enzymatic activities. Chemosphere 2024, 357, 142029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, Q.; Xiao, C.; Ding, M.; Liang, D.; Li, H.; Liu, R. Impact of Paenarthrobacter ureafaciens ZF1 on the soil enzyme activity and microbial community during the bioremediation of atrazine-contaminated soils. BMC Microbiol. 2022, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, W.; Yao, Y.; Li, H.; Wang, Q.; Niu, B. Microbial interactions within beneficial consortia promote soil health. Sci. Total Environ. 2023, 900, 165801. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Zhang, H.W.; Wang, J.; Li, X.Y.; Li, X.; Su, Z.C. High bioremediation potential of strain Chenggangzhangella methanolivorans CHL1 for soil polluted with metsulfuron-methyl or tribenuron-methyl in a pot experiment. Environ. Sci. Pollut. Res. Int. 2021, 28, 4731–4738. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liu, D.; Li, B.; Gao, Y.T.; Fan, Y.; Wang, H.B. Residue Determination of Prometryne and Acetochlor in Soil and Water by High Performance Liquid Chromatography. Adv. Mater. Res. 2013, 2606, 2340–2343. [Google Scholar] [CrossRef]
- Parveen, S.; Bhatti, I.A.; Ashar, A.; Javed, T.; Mohsin, M.; Hussain, M.T.; Khan, M.I.; Naz, S.; Iqbal, M. Synthesis, characterization and photocatalytic performance of iron molybdate (Fe2(MoO4)3) for the degradation of endosulfan pesticide. Mater. Res. Express 2020, 7, 035016. [Google Scholar] [CrossRef]
- GB/T 36197-2018; Standardized Methods for Soil Bioremediation Pot Experiments. China Standards Press: Beijing, China, 2018.
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Ma, J.; Liu, J. Response of Alkali Stress to Physiological Indexes of Oat Seedlings. J. Southwest Univ. Nat. Sci. Ed. 2021, 43, 58–65. [Google Scholar]
- Du, Y.; Zhang, Q.; Yu, M.; Yin, M.; Chen, F. Effect of sodium alginate-gelatin-polyvinyl pyrrolidone microspheres on cucumber plants, soil, and microbial communities under lead stress. Int. J. Biol. Macromol. 2023, 247, 125688. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, M.; Zhang, M.; Fang, X.; Wang, H.; Lv, J.; Shi, F. Topography- and depth-dependent rhizosphere microbial community characteristics drive ecosystem multifunctionality in Juglans mandshurica forest. Sci. Total Environ. 2024, 949, 175070. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Zheng, J.; Qin, J.; Ou, C.; Liao, Q.; Si, M.; Yang, Z.; Yang, W. Phase transformation of Cr(VI) host-mineral driven by citric acid-aided mechanochemical approach for advanced remediation of chromium ore processing residue-contaminated soil. J. Hazard. Mater. 2024, 461, 132530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Sun, X.W.; Hu, F.; Li, H.X. Isolation, screening and identification of prometryne-degrading bacteria and their degrading characteristics. Huan Jing Ke Xue 2013, 34, 2894–2898. [Google Scholar] [PubMed]
- Liu, J.; Hua, R.; Lv, P.; Tang, J.; Wang, Y.; Cao, H.; Wu, X.; Li, Q.X. Novel hydrolytic de-methylthiolation of the s-triazine herbicide prometryn by Leucobacter sp. JW-1. Sci. Total Environ. 2017, 579, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, Q.; Wang, G.X.; Ni, H.Y.; He, J.; Yan, X.; Gu, J.G.; Li, S.P. Sphingomonas chloroacetimidivorans sp. nov., a chloroacetamide herbicide-degrading bacterium isolated from activated sludge. Antonie Van Leeuwenhoek 2015, 108, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, R.; Zhu, J.; Zhang, J.; He, J.; Li, S.; Jiang, J. Degradation of the chloroacetamide herbicide butachlor by Catellibacterium caeni sp. nov NMA-1(T). Int. Biodeterior. Biodegrad. 2012, 73, 16–22. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, M.; Lu, P. Effects of pyrene on the structure and metabolic function of soil microbial communities. Environ. Pollut. 2022, 305, 119301. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, E.; Shi, H.; Wang, T.; Ma, Y.; Fulmer, A.; Adams, C. Comprehensive screening study of pesticide degradation via oxidation and hydrolysis. J. Agric. Food Chem. 2012, 60, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; An, N.; Jiang, D.; Cao, B.; Jiang, Z.; Yan, Y.; Ming, C.; Meng, Q.; Han, W. Short-term response of soil enzyme activities and bacterial communities in black soil to a herbicide mixture: Atrazine and Acetochlor. Appl. Soil Ecol. 2023, 181, 104652. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Laohakunjit, N.; KerNMhoechuen, O.; Kyu, K.L.; Ratanakhanokchai, K. Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol. 2013, 58, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, X.; Zhang, T.; He, W.; Song, F. Effects of the long-term application of atrazine on soil enzyme activity and bacterial community structure in farmlands in China. Environ. Pollut. 2020, 262, 114264. [Google Scholar] [CrossRef] [PubMed]
- Aponte, H.; Herrera, W.; Cameron, C.; Black, H.; Meier, S.; Paolini, J.; Tapia, Y.; Cornejo, P. Alteration of enzyme activities and functional diversity of a soil contaminated with copper and arsenic. Ecotoxicol. Environ. Saf. 2020, 192, 110264. [Google Scholar] [CrossRef] [PubMed]
- Maphuhla, N.G.; Lewu, F.; Oyedeji, O. The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site. Int. J. Environ. Res. Public Health 2021, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.X.; Li, Y.; Peng, S.Y.; Wei, L.; Zhang, B.; Deng, X.Y.; Zhong, M.; Cheng, X. Cadmium biosorption and mechanism investigation using two cadmium-tolerant microorganisms isolated from rhizosphere soil of rice. J. Hazard. Mater. 2024, 470, 134134. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, C.; Li, L.; Han, R.; Shen, X.; Han, G.; Wang, J.; Nie, M.; Zhou, X.; Du, H.; et al. Effect of Compound Fertilizer on Foxtail Millet Productivity and Soil Environment. Plants 2024, 13, 3167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, H.; Liu, X.; Mao, L.; Zhang, L.; Zhu, L.; Wu, C.; Wu, W. Enhanced remediation of acetochlor-contaminated soils using phosphate-modified biochar: Impacts on environmental fate, microbial communities, and plant health. Sci. Total Environ. 2024, 956, 177359. [Google Scholar] [CrossRef] [PubMed]
- Polti, M.A.; Aparicio, J.D.; Benimeli, C.S.; Amoroso, M.J. Simultaneous bioremediation of Cr(VI) and lindane in soil by actinobacteria. Int. Biodeterior. Biodegrad. 2014, 88, 48–55. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Yao, L.; Li, N.; Cao, Q.; Dai, C.; Zhang, J.; He, Q.; He, J. Biodegradation of pendimethalin by Bacillus subtilis Y3. J. Environ. Sci. 2016, 41, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules 2019, 24, 3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Li, X.; Wang, H.; Su, Z.; Wang, X.; Zhang, H. Dynamic changes in microbial communities during the bioremediation of herbicide (chlorimuron-ethyl and atrazine) contaminated soils by combined degrading bacteria. PLoS ONE 2018, 13, e0194753. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.X.; Sun, G.D.; Liu, Z.P. Degradation of polycyclic aromatic hydrocarbons in soil mesocosms by microbial/plant bioaugmentation: Performance and mechanism. Chemosphere 2018, 198, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ma, L.; Wei, R.; Xu, L.; Ma, Y.; Chen, Z.; Dang, J.; Ma, S.; Li, S. Physiology, Biochemistry, and Transcriptomics Jointly Reveal the Phytotoxicity Mechanism of Acetochlor on Pisum sativum L. Environ. Toxicol. Chem. 2024, 43, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, E.I. The Role of Chlorophyll in Photosynthesis. Sci. Am. 1965, 213, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Fu, J.; Hu, S.; Qu, J. Degradation of acetochlor and beneficial effect of phosphate-solubilizing Bacillus sp. ACD-9 on maize seedlings. 3 Biotech 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed]
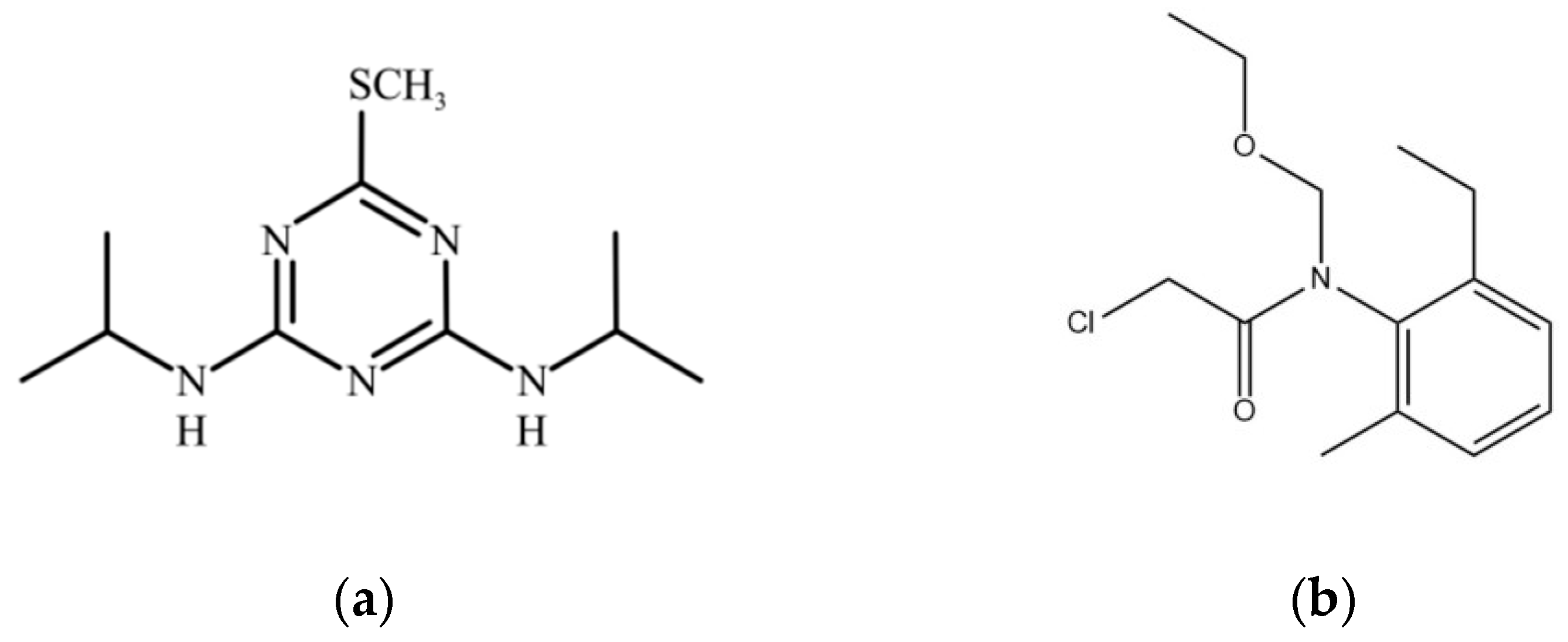
| Properties | Corresponding Characteristics of Prometryn | Corresponding Characteristics of Acetochlor |
|---|---|---|
| molecular formula | C10H19N5S | C14H20ClNO2 |
| molecular weight | 241.36 | 269.77 |
| flash point | 2 °C | 110 °C |
| boiling point | 309.64 °C | 134 °C |
| melting point | 118–120 °C | <0 °C |
| Density | 1.157 g/cm3 | 1.1 g/cm3 |
| solubility | 48.27 mg/L (20 °C) | 223 mg/L (25 °C) |
| Oral LD50 of rats | 3150–3750 mg/kg | 2148 mg/kg |
| LD50 of rabbits | >10,200 mg/kg | 794 mg/kg |
| Biochemical Reagent | Company | City | Country |
|---|---|---|---|
| 2 × Easy Taq Master Mix | Nanjing Novalza Biotechnology Co., Ltd. | Nanjing | China |
| ClonExpress II One-Step Cloning Kit | Nanjing Novalza Biotechnology Co., Ltd. | Nanjing | China |
| DL5000 DNA Marker | Nanjing Novalza Biotechnology Co., Ltd. | Nanjing | China |
| FlyCut® Hind III | NEB (Beijing) Co., Ltd. | Beijing | China |
| FlyCut® BamH I | NEB (Beijing) Co., Ltd. | Beijing | China |
| FlyCut® Not I | NEB (Beijing) Co., Ltd. | Beijing | China |
| Physiological indicator test tube | Huan Kai Technology Company | Guangzhou | China |
| Soil Enzyme Kit | Huan Kai Technology Company | Guangzhou | China |
| Instrument Name | Manufacturer | City | Country |
|---|---|---|---|
| Electronic balance | Electronic balance Pu Chun Measurement Instrument Co., Ltd. | Shanghai | China |
| Autoclave | TOMY, Japan | Tokyo | Japan |
| Constant temperature shaking incubator | Zhicheng Analytical Instrument Manufacturing Co., Ltd. | Shanghai | China |
| UV-Vis spectrophotometer | Hitachi, Ltd. | Tokyo | Japan |
| Ultra-micro spectrophotometer | Implen | Munich | Germany |
| Low-temperature high-speed desktop centrifuge | Beckman Coulter, Inc. | Brea, CA | USA |
| Vortex mixer | QIAGEN | Hilden | Germany |
| Bacterial constant temperature incubator | Yiheng Scientific Instruments Co., Ltd. | Shanghai | China |
| Nucleic acid electrophoresis apparatus | Liuyi Instrument Factory | Beijing | China |
| PCR machine | Bio-Rad Laboratories, Inc. | Hercules, CA | USA |
| UV clean bench | Sujing Group Antai Company | Shanghai | China |
| Rotary evaporator | Shanghai Qiuzuo Scientific Instrument Co., Ltd. | Shanghai | China |
| pH meter | Bante Instruments Co., Ltd. | Shanghai | China |
| High-performance liquid chromatography (HPLC) | Agilent Technologies, Inc. | Santa Clara, CA | USA |
| Automatic snowflake ice machine | Xueke Electric Appliance Co., Ltd. | Ningbo | China |
| Ultrasonic cell disruptor | Xinzhi Biotechnology Co., Ltd. | Ningbo | China |
| Digital display constant temperature water bath | Shuoguang Electronic Technology Co., Ltd. | Tianjin | China |
| Artificial climate chamber | Yiheng Scientific Instruments Co., Ltd. | Shanghai | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Fu, Q.; Xu, J.; Niu, X.; Liu, L.; Wang, J.; Quan, J.; Yu, Q.; Chi, B.; Li, H.; et al. Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor. Microorganisms 2025, 13, 1698. https://doi.org/10.3390/microorganisms13071698
Cheng Y, Fu Q, Xu J, Niu X, Liu L, Wang J, Quan J, Yu Q, Chi B, Li H, et al. Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor. Microorganisms. 2025; 13(7):1698. https://doi.org/10.3390/microorganisms13071698
Chicago/Turabian StyleCheng, Yue, Qian Fu, Junjia Xu, Xinhua Niu, Lin Liu, Jiaqi Wang, Jingwen Quan, Qingyue Yu, Baoyan Chi, Haitao Li, and et al. 2025. "Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor" Microorganisms 13, no. 7: 1698. https://doi.org/10.3390/microorganisms13071698
APA StyleCheng, Y., Fu, Q., Xu, J., Niu, X., Liu, L., Wang, J., Quan, J., Yu, Q., Chi, B., Li, H., & Liu, R. (2025). Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor. Microorganisms, 13(7), 1698. https://doi.org/10.3390/microorganisms13071698






