The Effects of Bacillus licheniformis on the Growth, Biofilm, Motility and Quorum Sensing of Salmonella typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Bacteria Strains, and Reagents
2.2. Determination of the MIC
2.3. Determination of the DIZ
2.4. Growth Curve Assay
2.5. Preparation of Biofilm
2.5.1. Preparation of Immature Biofilms
2.5.2. Preparation of Mature Biofilm
2.6. Crystal Violet Biofilm Assay
2.7. Biofilm Components
2.7.1. Exopolysaccharides Quantification Assay
2.7.2. Extracellular DNA (e-DNA) Quantification Assay
2.7.3. Protease Quantification Assay
2.8. Microscopic Observation
2.8.1. Optical Microscope Assay
2.8.2. Fluorescence Microscopy Assay
2.8.3. Scanning Electron Microscope (SEM) Assay
2.9. Motility Assay
2.10. Assay for AI-1 Activity of ST
2.11. Data Analysis Real-Time Polymerase Chain (PCR) Analysis
2.12. Data Analysis
3. Results
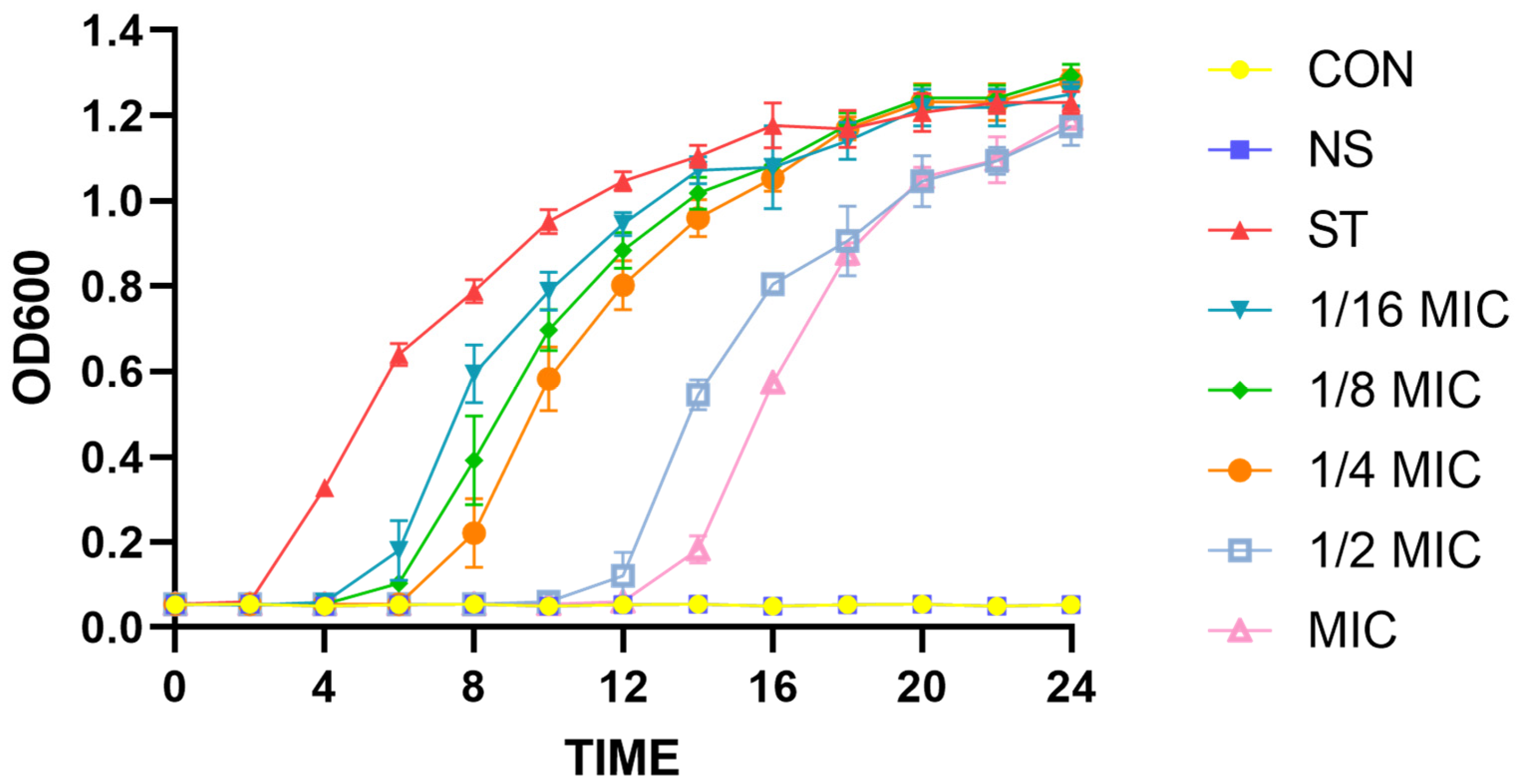
3.1. Effect of Lipopeptides on Growth of Salmonella typhimurium
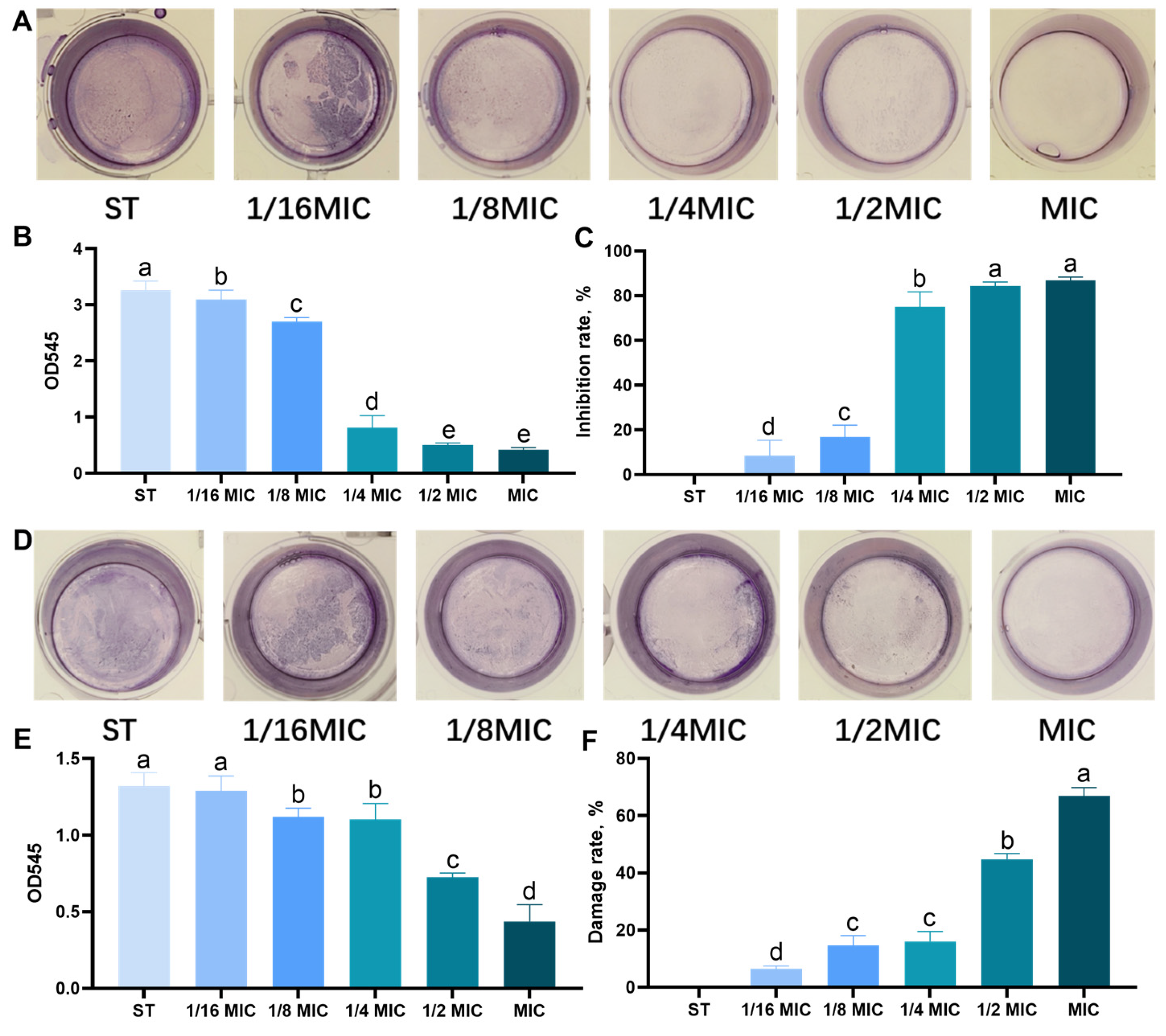
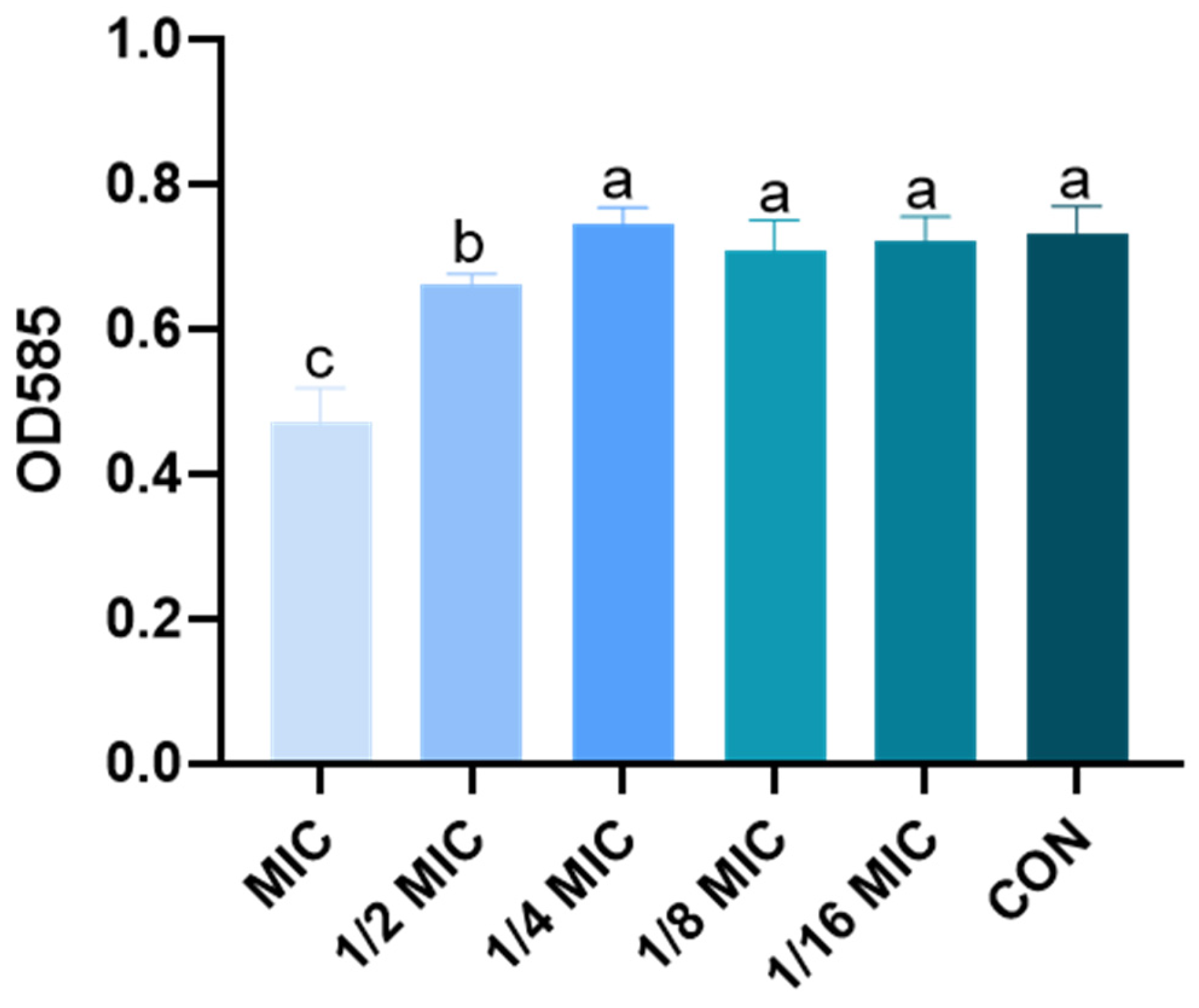
3.2. Effect of B. licheniformis on the Quantity of Biofilm of Salmonella typhimurium
3.3. Effect of B. licheniformis on Component of Biofilm of Salmonella typhimurium
3.3.1. Quantification of Exopolysaccharides
3.3.2. Quantification of e-DNA
3.3.3. Quantification of Protease
3.4. Effect of B. licheniformis on the Structure of Biofilm of Salmonella typhimurium
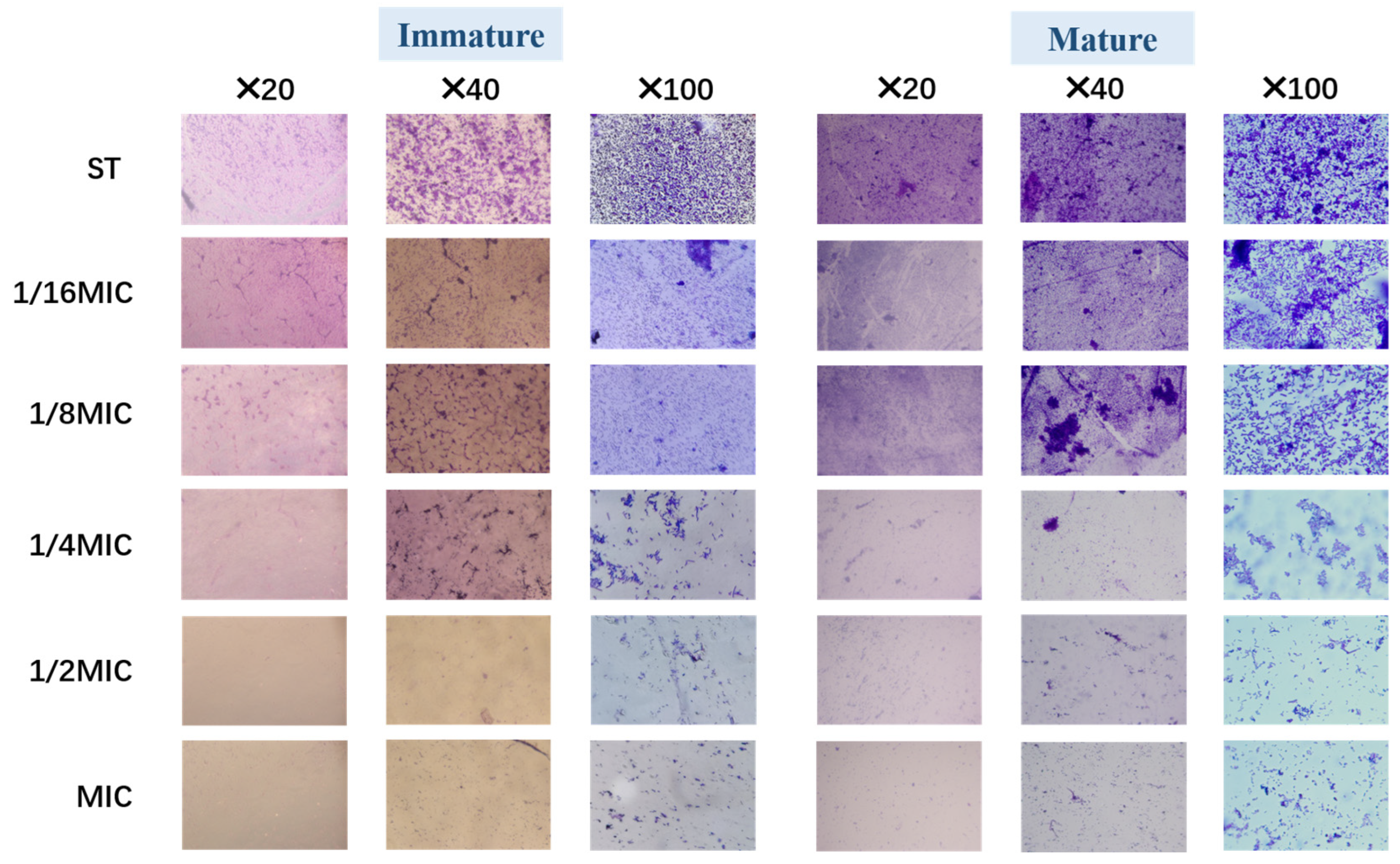
3.4.1. Optical Microscope
3.4.2. Confocal Fluorescence Microscope
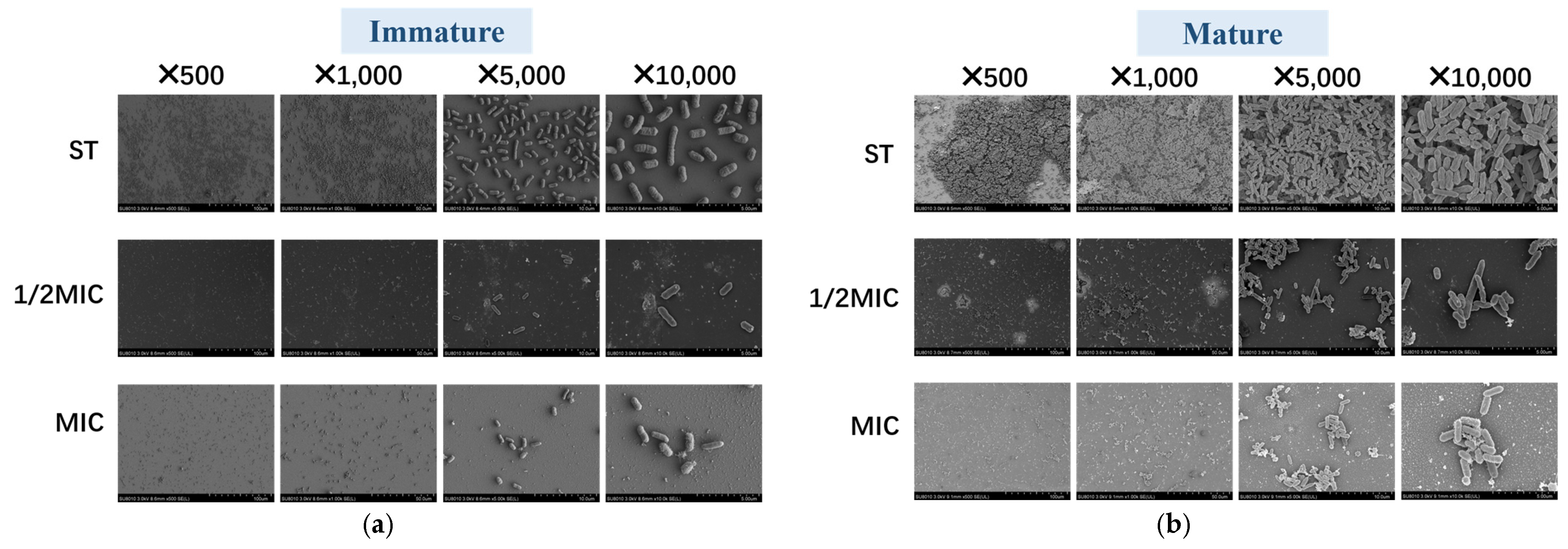
3.4.3. Scanning Electron Microscope
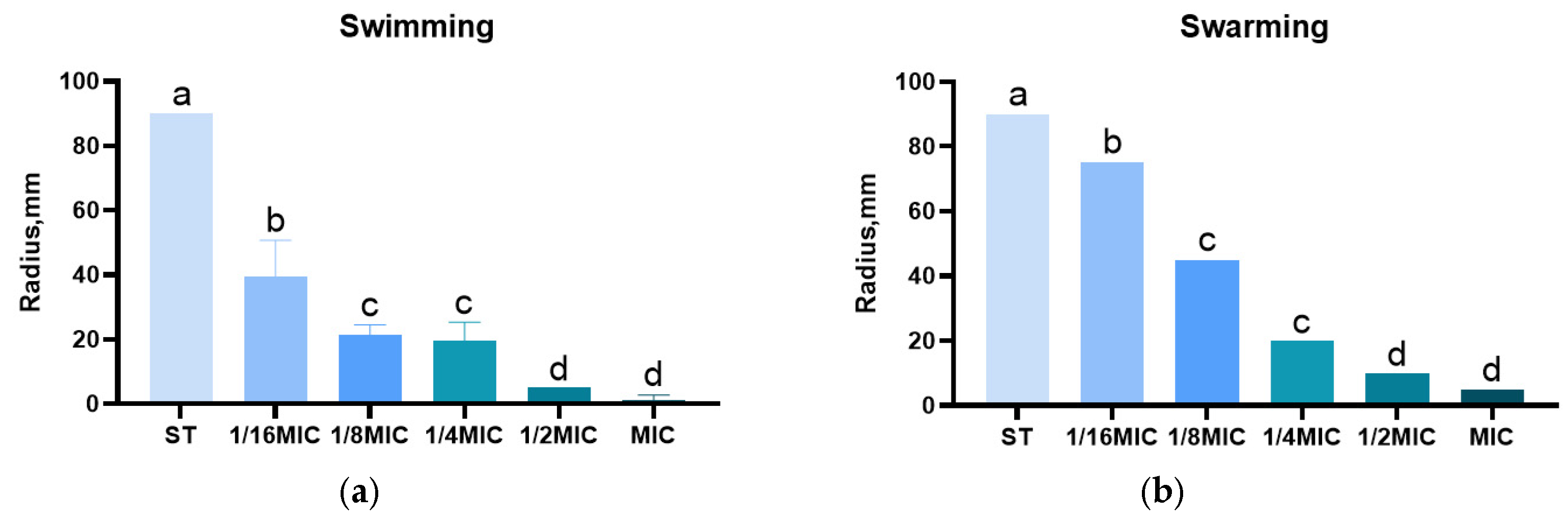
3.5. Effect of B. licheniformis on Salmonella typhimurium Migration
3.6. Effect of B. licheniformis on AI-1 Activity Produced
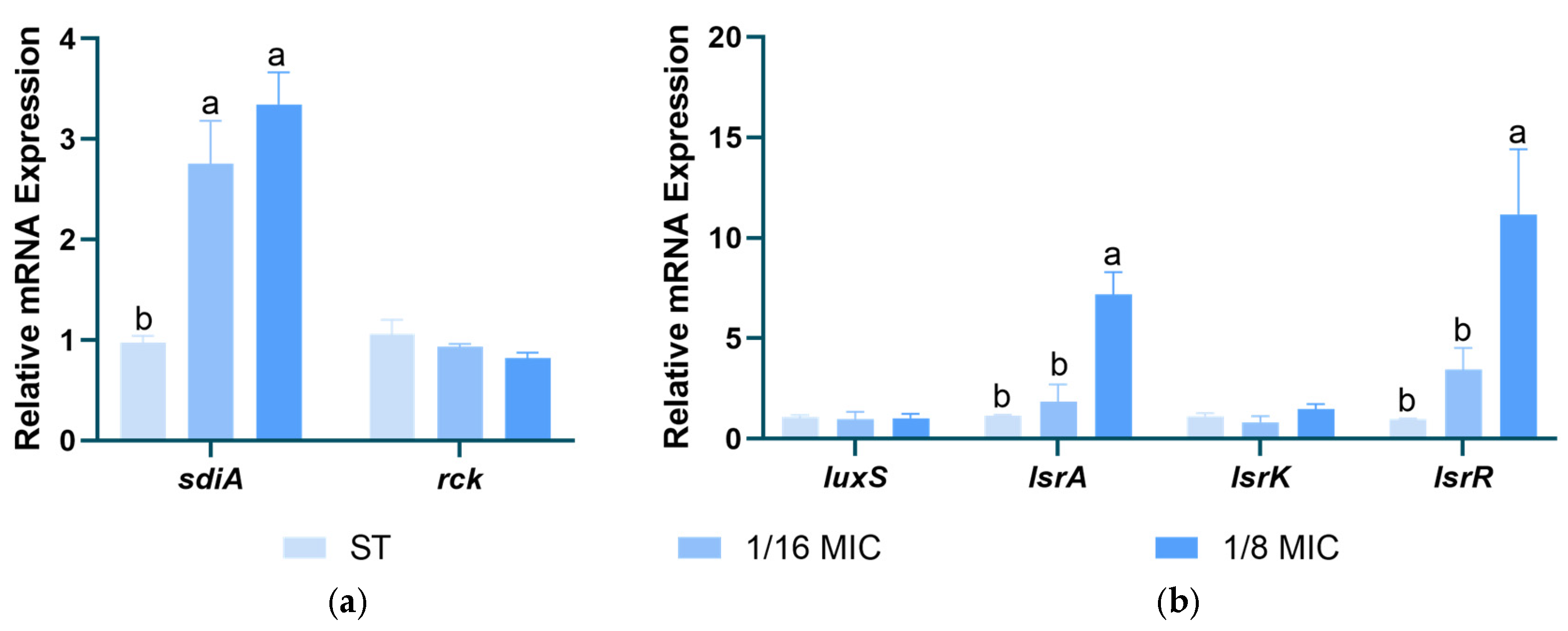
3.7. Effect of B. licheniformis on Salmonella typhimurium QS Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.J.; Li, J.; Zhang, X.; Gao, F.; Benton, C.S.; Andam, C.P. Diverse lineages of multidrug resistant clinical Salmonella enterica and a cryptic outbreak in New Hampshire, USA revealed from a year-long genomic surveillance. Infect. Genet. Evol. 2021, 87, 104645. [Google Scholar] [CrossRef] [PubMed]
- Laptev, G.Y.; Filippova, V.A.; Kochish, I.I.; Yildirim, E.A.; Ilina, L.A.; Dubrovin, A.V.; Brazhnik, E.A.; Novikova, N.I.; Novikova, O.B.; Dmitrieva, M.E.; et al. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella enteritidis and fed a phytobiotic. Animals 2019, 9, 615. [Google Scholar] [CrossRef]
- Marin, C.; Hernandiz, A.; Lainez, M. Biofilm development capacity of Salmonella strains isolated in poultry risk factors and their resistance against disinfectants. Poult. Sci. 2009, 88, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, E.; Nesse, L.L.; Hauck, R.; Windhorst, D.; Hafez, H.M.; Vestby, L.K. Biofilm building capacity of Salmonella enterica strains from the poultry farm environment. FEMS Immunol. Med. Microbiol. 2012, 65, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Otta, S.K.; Karunasagar, I.; Karunasagar, I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 2001, 64, 367–372. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.O.; Hoiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Gradel, K.O.; Randall, L.; Sayers, A.R.; Davies, R.H. Possible associations between salmonella persistence in poultry houses and resistance to commonly used disinfectants and a putative role of mar. Vet. Microbiol. 2005, 107, 127–138. [Google Scholar] [CrossRef]
- Davies, R.; Breslin, M. Observations on salmonella contamination of commercial laying farms before and after cleaning and disinfection. Vet. Rec. 2003, 152, 283–287. [Google Scholar] [CrossRef]
- Moretro, T.; Heir, E.; Nesse, L.L.; Vestby, L.K.; Langsrud, S. Control of salmonella in food related environments by chemical disinfection. Food Res. Int. 2012, 45, 532–544. [Google Scholar] [CrossRef]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan, K.S.; Sharifi, S.; Memar, M.Y.; Zununi, V.S. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; Cruz, A.G.D. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.Y.; Kuk, J.H.; Moon, J.H.; Cho, J.I.; Kim, Y.C.; Park, K.H. Identification and antimicrobial activity of phenylacetic acid produced by bacillus licheniformis isolated from fermented soybean, chungkook-jang. Curr. Microbiol. 2004, 48, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Padmaja, R.J.; Halami, P.M. Diversity in the antibacterial potential of probiotic cultures bacillus licheniformis mcc2514 and bacillus licheniformis mcc2512. Res. Microbiol. 2015, 166, 546–554. [Google Scholar] [CrossRef]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Damare, S.R.; Nancharaiah, Y.V.; Ramaiah, N.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Disruption of microbial biofilms by an extracellular protein isolated from epibiotic tropical marine strain of bacillus licheniformis. PLoS ONE 2013, 8, e64501. [Google Scholar] [CrossRef]
- Vinoj, G.; Vaseeharan, B.; Thomas, S.; Spiers, A.J.; Shanthi, S. Quorum-quenching activity of the ahl-lactonase from bacillus licheniformis dahb1 inhibits vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar. Biotechnol. 2014, 16, 707–715. [Google Scholar] [CrossRef]
- Li, Y.; Wangjiang, T.; Sun, Z.; Shi, L.; Chen, S.; Chen, L.; Guo, X.; Wu, W.; Xiong, G.; Wang, L. Inhibition mechanism of crude lipopeptide from Bacillus subtilis against Aeromonas veronii growth, biofilm formation, and spoilage of channel catfish flesh. Food Microbiol. 2024, 120, 104489. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Barbarossa, A.; Rosato, A.; Tardugno, R.; Carrieri, A.; Corbo, F.; Limongelli, F.; Fumarola, L.; Fracchiolla, G.; Carocci, A. Antibiofilm effects of plant extracts against Staphylococcus aureus. Microorganisms 2025, 13, 454. [Google Scholar] [CrossRef]
- Tao, W.; Li, W.; Aweya, J.J.; Lin, R.; Jin, R.; Liang, D.; Ren, Z.; Yang, S. Bacillus subtilis fermented shrimp waste isolated peptide, pvq9, and its antimicrobial mechanism on four gram-positive foodborne bacteria. Food Microbiol. 2025, 125, 104654. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhang, X.; Ding, H.; Chen, Y.; He, W.; Wei, Y.; Chen, W.; Chan, Y.K.; Shi, Y.; Huang, D.; et al. Engineered probiotic bio-heterojunction with robust antibiofilm modality via “eating” extracellular polymeric substances for wound regeneration. Adv. Mater. 2024, 36, e2402530. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.Y.; Yuan, M.; Cui, Z.Q.; Wu, Z.M.; Yu, Z.J.; Song, K.; Tang, B.; Fu, B.D. Rutin inhibits quorum sensing, biofilm formation and virulence genes in avian pathogenic Escherichia coli. Microb. Pathog. 2018, 119, 54–59. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Koop, L.; Wong, Y.K.; Ahmed, S.; Siddiqui, K.S.; Manefield, M. Influence of calcium in extracellular dna mediated bacterial aggregation and biofilm formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In vitro antimicrobial activity of essential oils against Salmonella enterica serotypes enteritidis and typhimurium strains isolated from poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Tenke, P.; Riedl, C.R.; Jones, G.L.; Williams, G.J.; Stickler, D.; Nagy, E. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int. J. Antimicrob. Agents 2004, 23 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; Lopez, M.; Prieto, M.; Alvarez-Ordonez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Malaczewska, J.; Kaczorek-Lukowska, E. Nisin-a lantibiotic with immunomodulatory properties: A review. Peptides 2021, 137, 170479. [Google Scholar] [CrossRef]
- Surin, H.N.; Kacirova, J.; Sondorova, M.; Selianova, S.; Mucha, R.; Madar, M. Inhibitory effect of Bacillus licheniformis strains isolated from canine oral cavity. Life 2022, 12, 1238. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic bacilli inhibit Salmonella biofilm formation without killing planktonic cells. Front. Microbiol. 2021, 12, 615328. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, S.; Han, J.; Wu, J.; You, L.; Shi, X.; Wang, S. Synergistic antibacterial activity and mechanism of action of nisin/carvacrol combination against Staphylococcus aureus and their application in the infecting pasteurized milk. Food Chem. 2022, 380, 132009. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Rizzo, M.G.; De Pasquale, C.; Ferlazzo, G.; Caccamo, M.T.; Magazu, S.; Guglielmino, S.; Gugliandolo, C. Lichenysin-like polypeptide production by Bacillus licheniformis b3-15 and its antiadhesive and antibiofilm properties. Microorganisms 2023, 11, 1842. [Google Scholar] [CrossRef]
- Seviour, T.; Derlon, N.; Dueholm, M.S.; Flemming, H.C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.; Lotti, T.; Malpei, M.F.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A review of the role of extracellular polymeric substances (eps) in wastewater treatment systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Feng, L.; Wu, Y.; Zhu, J. Extracellular matrix affects mature biofilm and stress resistance of psychrotrophic spoilage Pseudomonas at cold temperature. Food Microbiol. 2023, 112, 104214. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef] [PubMed]
- Ramphal, R.; Lhermitte, M.; Filliat, M.; Roussel, P. The binding of anti-pseudomonal antibiotics to macromolecules from cystic fibrosis sputum. J. Antimicrob. Chemother. 1988, 22, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Nilsson, M.; Jensen, P.O.; Hoiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular dna shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef]
- Gloag, E.S.; Turnbull, L.; Huang, A.; Vallotton, P.; Wang, H.; Nolan, L.M.; Mililli, L.; Hunt, C.; Lu, J.; Osvath, S.R.; et al. Self-organization of bacterial biofilms is facilitated by extracellular dna. Proc. Natl. Acad. Sci. USA 2013, 110, 11541–11546. [Google Scholar] [CrossRef] [PubMed]
- Finkel, S.E.; Kolter, R. Dna as a nutrient: Novel role for bacterial competence gene homologs. J. Bacteriol. 2001, 183, 6288–6293. [Google Scholar] [CrossRef]
- Pleva, P.; Bartosova, L.; Macalova, D.; Zalesakova, L.; Sedlarikova, J.; Janalikova, M. Biofilm formation reduction by eugenol and thymol on biodegradable food packaging material. Foods 2021, 11, 2. [Google Scholar] [CrossRef]
- Grobas, I.; Polin, M.; Asally, M. Swarming bacteria undergo localized dynamic phase transition to form stress-induced biofilms. eLife 2021, 10, e62632. [Google Scholar] [CrossRef]
- Fraser, G.M.; Hughes, C. Swarming motility. Curr. Opin. Microbiol. 1999, 2, 630–635. [Google Scholar] [CrossRef]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and bacterial pathogenicity. J. Basic Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Deditius, J.A.; Felgner, S.; Sporing, I.; Kuhne, C.; Frahm, M.; Rohde, M.; Weiss, S.; Erhardt, M. Characterization of novel factors involved in swimming and swarming motility in Salmonella enterica serovar typhimurium. PLoS ONE 2015, 10, e135351. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, M.; Yi, G.; Liao, L.; Cheng, Q.; Zhu, J.; Zhang, B.; Wang, Y.; Chen, Y.; Zeng, M. Screening strategies for quorum sensing inhibitors in combating bacterial infections. J. Pharm. Anal. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sabag-Daigle, A.; Soares, J.A.; Smith, J.N.; Elmasry, M.E.; Ahmer, B.M.M. The acyl homoserine lactone receptor, SdiA, of Escherichia coli and Salmonella enterica serovar typhimurium does not respond to indole. Appl. Environ. Microbiol. 2012, 78, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, D.; Kim, M.; Park, J.; Lim, S.; Ryu, S. Lsrr-mediated quorum sensing controls invasiveness of salmonella typhimurium by regulating spi-1 and flagella genes. PLoS ONE 2012, 7, e37059. [Google Scholar] [CrossRef]
- Gong, C.; Jiang, X. Application of bacteriophages to reduce salmonella attachment and biofilms on hard surfaces. Poult. Sci. 2017, 96, 1838–1848. [Google Scholar] [CrossRef]
- Speranza, B.; Corbo, M.R.; Sinigaglia, M. Effects of nutritional and environmental conditions on Salmonella sp. biofilm formation. J. Food Sci. 2011, 76, M12–M16. [Google Scholar] [CrossRef]
- Xiao, X.; Qin, S.; Cui, T.; Liu, J.; Wu, Y.; Zhong, Y.; Yang, C. Bacillus licheniformis suppresses Clostridium perfringens infection via modulating inflammatory response, antioxidant status, inflammasome activation and microbial homeostasis in broilers. Poult. Sci. 2024, 103, 104222. [Google Scholar] [CrossRef]


| Gene Product | Primer Sequence (5′–3′) | Accession Number |
|---|---|---|
| sdiA | F: CGTACCACTTATCCTCCGGC R: TCTGCGTAATCCGAAACGCT | KF381283.1 |
| rck | F: AACGTACTGGTCGCTGATGG R: ATGCTCCTTCACTTCAGCCC | KX807610.1 |
| luxS | F: ACGCTTGAGCATCTGTTTGC R: CTGCACTTTCAGCACATCCG | KF381282.1 |
| lsrA | F: GTCCCTGCAAGCCAAACAAG R: GGTGTGCGTTGCAGTCATTT | CP099973.1 |
| lsrK | F: AAAAGCTAGTGCGCTGGGAT R: GGGATGTCGTTAAGCCACCA | CP099973.1 |
| LsrR | F: AAATAGCGTGCGGGATGTGA R: GAATATCGCCTACTGCGCCT | CP099973.1 |
| 16sRNA | F: CGATGTCTACTTGGAGGTTGTG R: CTCTGGAAAGTTCTGTGGATGTC | NR074910.1 |
| DIZ (mm) | B. licheniformis | Neomycin Sulfate | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 6.25 mg/mL | 12.5 mg/mL | 25 mg/mL | 50 mg/mL | 100 mg/mL | 100 μg/mL | ||
| Salmonella typhimurium | 11.56 ± 1.74 e | 13.79 ± 1.37 d | 16.26 ± 1.49 c | 18.31 ± 1.56 b | 19.98 ± 1.38 a | 13.43 ± 1.75 d | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Xu, H.; Zhang, M.; Xu, B.; Dai, B.; Yang, C. The Effects of Bacillus licheniformis on the Growth, Biofilm, Motility and Quorum Sensing of Salmonella typhimurium. Microorganisms 2025, 13, 1540. https://doi.org/10.3390/microorganisms13071540
Peng W, Xu H, Zhang M, Xu B, Dai B, Yang C. The Effects of Bacillus licheniformis on the Growth, Biofilm, Motility and Quorum Sensing of Salmonella typhimurium. Microorganisms. 2025; 13(7):1540. https://doi.org/10.3390/microorganisms13071540
Chicago/Turabian StylePeng, Wenwen, Haocheng Xu, Meiting Zhang, Baoyang Xu, Bing Dai, and Caimei Yang. 2025. "The Effects of Bacillus licheniformis on the Growth, Biofilm, Motility and Quorum Sensing of Salmonella typhimurium" Microorganisms 13, no. 7: 1540. https://doi.org/10.3390/microorganisms13071540
APA StylePeng, W., Xu, H., Zhang, M., Xu, B., Dai, B., & Yang, C. (2025). The Effects of Bacillus licheniformis on the Growth, Biofilm, Motility and Quorum Sensing of Salmonella typhimurium. Microorganisms, 13(7), 1540. https://doi.org/10.3390/microorganisms13071540





