Transmission Dynamics of Trichomonas tenax: Host and Site Specificity, Zoonotic Potential, and Environmental Factors
Abstract
1. Introduction
2. Hosts
2.1. Host and Site Specificity
2.2. Zoonotic Potential
3. Factors Affecting Transmission and Prevalence
3.1. Factors Affecting Transmission
3.2. Transmission
4. Future Research Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dybicz, M.; Perkowski, K.; Baltaza, W.; Padzik, M.; Sędzikowska, A.; Chomicz, L. Molecular identification of Trichomonas tenax in the oral environment of domesticated animals in Poland–Potential effects of host diversity for human health. Ann. Agric. Environ. Med. 2018, 25, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte-Wolf, C.; Orrego, P.R.; Muñoz, C.; Herrera, D.; Bravo, J.; Gonzalez, J.; Varela, H.; Catalán, A.; Araya, J.E. Observational cross-sectional study of Trichomonas tenax in patients with periodontal disease attending a Chilean University Dental Clinic. BMC Oral Health 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, B.M.; Lee, J.J. Structure and division of Trichomonas tenax (O. F. Müller). Am. J. Epidemiol. 1959, 69, 177–201. [Google Scholar] [CrossRef]
- Nagao, E.; Yamamoto, A.; Igarashi, T.; Goto, N.; Sasa, R. Two distinct hemolysins in Trichomonas tenax ATCC 30207. Oral Microbiol. Immunol. 2000, 15, 355–359. [Google Scholar] [CrossRef]
- Matthew, M.A.; Yang, N.; Ketzis, J.; Mukaratirwa, S.; Yao, C. Trichomonas tenax: A neglected protozoan infection in the oral cavities of humans and dogs—A scoping review. Trop. Med. Infect. Dis. 2023, 8, 60. [Google Scholar] [CrossRef]
- Marty, M.; Lemaitre, M.; Kémoun, P.; Morrier, J.J.; Monsarrat, P. Trichomonas tenax and periodontal diseases: A concise review. Parasitology 2017, 144, 1417–1425. [Google Scholar] [CrossRef]
- Bisson, C.; Dridi, S.M.; Machouart, M. Assessment of the role of Trichomonas tenax in the etiopathogenesis of human periodontitis: A systematic review. PLoS ONE 2019, 14, e0226266. [Google Scholar] [CrossRef]
- Patel, N.; Colyer, A.; Harris, S.; Holcombe, L.; Andrew, P. The prevalence of canine oral protozoa and their association with periodontal disease. J. Eukaryot. Microbiol. 2017, 64, 286–292. [Google Scholar] [CrossRef]
- Mohamad, S.; Mohamed, Z. Chapter 4 Detection of Trichomonas tenax and Entamoeba gingivalis: A review. In Updates in Parasitology; Avid Science: Berlin, Germany, 2017; pp. 2–31. [Google Scholar]
- García-Huerta, O.E.; Chávez-Ruvalcaba, F.; Chávez-Ruvalcaba, M.I.; Chávez-Ruvalcaba, K.M.; Díaz-Alfaro, L. Periodontal Diseases-A Clinician’s Guide; IntechOpen: Rijeka, Croatia, 2012; pp. 1–14. [Google Scholar] [CrossRef]
- Ribeiro, L.C.; Santos, C.; Benchimol, M. Is Trichomonas tenax a parasite or a commensal? Protist 2015, 166, 196–210. [Google Scholar] [CrossRef]
- El Sibaei, M.M.; Abdel-Fattah, N.S.; Ahmed, S.A.; Abou-Seri, H.M. Growth kinetics, antigen profiling, and proteinase activity of Egyptian Trichomonas tenax isolates derived from patients having oral infections. Exp. Parasitol. 2012, 130, 416–422. [Google Scholar] [CrossRef]
- Yamamoto, A.; Asaga, E.; Nagao, E.; Igarashi, T.; Goto, N. Characterization of the cathepsin B-like proteinases of Trichomonas tenax ATCC 30207. Oral Microbiol. Immunol. 2000, 15, 360–364. [Google Scholar] [CrossRef]
- Bózner, P.; Demeš, P. Cell-associated and extracellular proteolytic activity of an oral flagellate, Trichomonas tenax. Arch. Oral Biol. 1991, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Matthew, M.A.; Yao, C. Roles of cysteine proteases in biology and pathogenesis of parasites. Microorganisms 2023, 11, 1397. [Google Scholar] [CrossRef]
- Athari, A.; Soghandi, L.; Haghighi, A.; Kazemi, B. Prevalence of oral trichomoniasis in patients with periodontitis and gingivitis using PCR and direct smear. Iran. J. Public Health 2007, 36, 33–37. [Google Scholar]
- Mahdi, N.K.; al-Saeed, A.T. Trichomonas tenax in Basrah, Iraq. J. Pak. Med. Assoc. 1993, 43, 261–262. [Google Scholar]
- Mehr, A.K.; Zarandi, A.; Anush, K. Prevalence of oral Trichomonas tenax in periodontal lesions of down syndrome in Tabriz, Iran. J. Clin. Diagnostic Res. 2015, 9, 88–90. [Google Scholar] [CrossRef]
- Hong, Z.B.; Lai, Y.T.; Chen, C.H.; Chen, Y.J.; Chen, C.C.; Lin, W.C. Trichomonas tenax induces barrier defects and modulates the inflammatory cytotoxicity of gingival and pulmonary epithelial cells. Parasite 2023, 30, 193–202. [Google Scholar] [CrossRef]
- Diamond, L.S.; Bartgis, I.L. Axenic cultivation of Trichomonas tenax, the oral flagellate of man I. establishment of cultures. J. Protozool. 1962, 9, 442–444. [Google Scholar] [CrossRef]
- Kikuta, N.; Yamamoto, A.; Fukura, K.; Goto, N. Specific and sensitive detection of Trichomonas tenax by the polymerase chain reaction. Lett. Appl. Microbiol. 1997, 24, 193–197. [Google Scholar] [CrossRef]
- Matthew, M.A.; Christie, J.; Yang, N.; Yao, C. A loop-mediated isothermal amplification (LAMP) assay specific to Trichomonas tenax is suitable for use at point-of-care. Microorganisms 2022, 10, 594. [Google Scholar] [CrossRef]
- Eslahi, A.V.; Olfatifar, M.; Abdoli, A.; Houshmand, E.; Johkool, M.G.; Zarabadipour, M.; Abadi, P.A.; Ghorbani, A.; Mirzadeh, M.; Badri, M. The neglected role of Trichomonas tenax in oral diseases: A systematic review and meta-analysis. Acta Parasitol. 2021, 66, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Ketzis, J.K. Aberrant and accidental trichomonad flagellate infections: Rare or underdiagnosed? Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 64–72. [Google Scholar] [CrossRef]
- Dobell, C. The common flagellate of the human mouth, Trichomonas tenax (O.F.M.): Its discovery and its nomenclature. Parasitology 1939, 31, 138–146. [Google Scholar] [CrossRef]
- Goodey, T.; Wellings, A.W. Observations on Entamoeba gingivalis from the human mouth, with a note on the trichomonad flagellate Tetratrichomonas buccalis n. sp. Parasitology 1917, 9, 537–559. [Google Scholar] [CrossRef]
- Hersh, S.M. Pulmonary trichomoniasis and Trichomonas tenax. J. Med. Microbiol. 1985, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, Y.; Yang, Y.; Yang, W.; Lin, J.; Cao, K. Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: A case report. BMC Infect. Dis. 2021, 21, 842. [Google Scholar] [CrossRef]
- Cai, D.H.; Fang, X.L. Pyopneumothorax caused by Trichomonas tenax and Porphyromonas endodontalis coinfection in a patient with previous cerebral infarction: A case report and literature review. Infect. Drug Resist. 2022, 15, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Mallat, H.; Podglajen, I.; Lavarde, V.; Mainardi, J.L.; Frappler, J.; Cornet, M. Molecular characterization of Trichomonas tenax causing pulmonary infection. J. Clin. Microbiol. 2004, 42, 3886–3887. [Google Scholar] [CrossRef]
- Duboucher, C.; Caby, S.; Chabé, M.; Gantois, N.; Delgado-Viscogliosi, P.; Pierce, R.; Capron, M.; Dei-Cas, E.; Viscogliosi, É. Human pulmonary trichomonoses. Press. Medicale 2007, 36, 835–839. [Google Scholar] [CrossRef]
- Duboucher, C.; Mogenet, M.; Perie, G. Salivary trichomoniasis: A case report of infestation of a submaxillary gland by Trichomonas tenax. Arch. Pathol. Lab. Med. 1995, 119, 277–279. [Google Scholar]
- Duboucher, C.; Farto-Bensasson, F.; Chéron, M.; Peltier, J.Y.; Beaufils, F.; Périé, G. Lymph node infection by Trichomonas tenax: Report of a case with co-infection by Mycobacterium tuberculosis. Hum. Pathol. 2000, 31, 1317–1321. [Google Scholar] [CrossRef]
- Fedorych, P.V.; Mavrov, G.I.; Osinska, T.V.; Shcherbakova, Y.V. Protozoan genital invasions caused by the representatives of Trichomonas and Giardia. Wiad. Lek. 2020, 73, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Brosh-Nissimov, T.; Hindiyeh, M.; Azar, R.; Smollan, G.; Belausov, N.; Mandelboim, M.; Rahav, G.; Keller, N.; Gefen-Halevi, S. A false-positive Trichomonas vaginalis result due to Trichomonas tenax presence in clinical specimens may reveal a possible T. tenax urogenital infection. Clin. Microbiol. Infect. 2019, 25, 123–124. [Google Scholar] [CrossRef]
- Hegner, R.; Ratcliffe, H. Trichomonads from the mouth of the dog. J. Parasitol. 1927, 14, 51. [Google Scholar] [CrossRef]
- Kellerová, P.; Tachezy, J. Zoonotic Trichomonas tenax and a new trichomonad species, Trichomonas brixi n. sp., from the oral cavities of dogs and cats. Int. J. Parasitol. 2017, 47, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Łojszczyk-Szczepaniak, A.; Tomczuk, K.; Skrzypek, T.; Lisiak, B.; Abd-Al-Hammza Abbass, Z. Canine Trichomonas tenax mandibular gland infestation. Acta Vet. Scand. 2016, 58, 15. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, J.; Wang, F.; Li, H.; Zhao, X. Prevalence of Trichomonas spp. in domestic pigeons in Shandong Province, China, and genotyping by restriction fragment length polymorphism. Vet. J. 2016, 211, 88–93. [Google Scholar] [CrossRef]
- Landman, W.J.M.; Gantois, N.; Sawant, M.; Majoor, F.A.; van Eck, J.H.H.; Viscogliosi, E. Prevalence of trichomonads in the cloaca of wild wetland birds in the Netherlands. Avian Pathol. 2021, 50, 465–476. [Google Scholar] [CrossRef]
- Martínez-Herrero, M.C.; Garijo-Toledo, M.M.; Liebhart, D.; Ganas, P.; Martínez-Díaz, R.A.; Ponce-Gordo, F.; Carrero-Ruiz, A.; Hess, M.; Gómez-Muñoz, M.T. Novel avian oropharyngeal trichomonads isolated from European turtle doves (Streptopelia turtur) and racing pigeons (Columba livia): Genetic and morphometric characterisation of clonal cultures. Infect. Genet. Evol. 2017, 55, 93–103. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Halverson, B.A.; Sudjadi, S.T.; Purnomo; Masbar, S. Parasites found in the mouths of inhabitants of three villages of South Kalimantan (Borneo), Indonesia. Trop. Geogr. Med. 1984, 36, 57–59. [Google Scholar]
- Ali Mohammed, S.A.; Mohsen Alwaaly, A.B. Prevalence Trichomonas tenax in Karbala Governorate. J. Phys. Conf. Ser. 2019, 1294, 062030. [Google Scholar] [CrossRef]
- Fadhil Ali Malaa, S.; Abd Ali Abd Aun Jwad, B.; Khalis Al-Masoudi, H. Assessment of Entamoeba gingivalis and Trichomonas tenax in patients with chronic diseases and its correlation with some risk factors. Arch. Razi Inst. 2022, 77, 77–82. [Google Scholar] [CrossRef]
- Norberg, C.M.B.M. Entamoeba gingivalis (Gros, 1849) and Trichomonas tenax (Muller, 1773) oral infections in patients from Baixada Fluminense, Province of Rio de Janeiro, Brazil. Sci. J. Public Health 2014, 2, 288–292. [Google Scholar] [CrossRef]
- Dybicz, M.; Perkowski, K.; Sędzikowska, A.; Baltaza, W.; Chomicz, L. Studies on prevalence of infection with Trichomonas tenax identified by molecular techniques–In respect to oral health of patients with various systemic disease requiring immunosuppressive therapy. Ann. Parasitol. 2018, 64, 193–197. [Google Scholar]
- Wantland, W.W.; Wantland, E.M.; Winquist, D.L. Collection, identification, and cultivation of oral protozoa. J. Dent. Res. 1963, 42, 1234–1241. [Google Scholar] [CrossRef]
- Asai, S.; Hayashi, A.; Nakamura, Y.; Kato, M.; Sato, M.; Nitta, H.; Namikawa, I. Growth of Trichomonas tenax in tissue culture medium containing complement and antiserum to the accompanying bacteria. Jpn. J. Oral Biol. 1986, 28, 731–736. [Google Scholar] [CrossRef]
- Hinshaw, H.C. Experimental infection of dogs with Endamoeba gingivalis and Trichomonas buccalis of human mouth. Proc. Soc. Exp. Biol. Med. 1928, 25, 430. [Google Scholar] [CrossRef]
- Maritz, J.M.; Land, K.M.; Carlton, J.M.; Hirt, R.P. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014, 30, 333–341. [Google Scholar] [CrossRef]
- Ito, N.; Itoh, N.; Nakanishi, R.; Kameshima, S.; Kimura, Y. Molecular prevalence and zoonotic potential of trichomonads from oral cavities in household dogs. J. Eukaryot. Microbiol. 2023, 70, e12941. [Google Scholar] [CrossRef]
- Stoyanov, S.; Tasinov, O.; Dimitrova, T.; Yaneva, G. Prevalence of Trichomonas tenax in the population affected by periodontal disease—A review. Appl. Sci. 2024, 14, 2666. [Google Scholar] [CrossRef]
- Hinshaw, H.C. Correlation of protozoan infections of human mouth with extent of certain lesions in pyorrhea alveolaris. Proc. Soc. Exp. Biol. Med. 1926, 24, 71–73. [Google Scholar] [CrossRef]
- Ghabanchi, J.; Zibaei, M.; Afkar, M.D.; Sarbazie, A.H. Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, Southern Iran. Indian J. Dent. Res. 2010, 21, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaimi, F.H.A.; Alajeely, A.A.A.; Ail, Y.M. Incidence of Entamoeba gingivalis and Trichomonas tenax in periodontitis and gingivitis patients who Attended to private clincs in Babylon Province. Med.-Leg. Update 2020, 20, 906–910. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Dolińska, E.; Milewski, R.; Pietruska, M.J.; Gumińska, K.; Prysak, N.; Tarasewicz, T.; Janica, M.; Pietruska, M. Periodontitis-related knowledge and its relationship with oral health behavior among adult patients seeking professional periodontal care. J. Clin. Med. 2022, 11, 1517. [Google Scholar] [CrossRef]
- Meabed, E.; Henin, R. Prevalence of Entamoeba gingivalis and Trichomonas tenax among patients suffering from chronic systemic diseases in Egypt. Afro-Egypt. J. Infect. Endem. Dis. 2022, 12, 390–401. [Google Scholar] [CrossRef]
- Kostara, I.; Carageorgiou, H.; Varonos, D.; Tzannetis, S. Growth and survival of Trichomonas vaginalis. J. Med. Microbiol. 1998, 47, 555–560. [Google Scholar] [CrossRef]
- Amin, A.; Neubauer, C.; Liebhart, D.; Grabensteiner, E.; Hess, M. Axenization and optimization of in vitro growth of clonal cultures of Tetratrichomonas gallinarum and Trichomonas gallinae. Exp. Parasitol. 2010, 124, 202–208. [Google Scholar] [CrossRef]
- Tompkins, E.L.; Beltran, T.A.; Gelner, E.J.; Farmer, A.R. Prevalence and risk factors for Trichomonas Vaginalis infection among adults in the U.S., 2013–2014. PLoS ONE 2020, 15, e0234704. [Google Scholar] [CrossRef]
- Barbosa, M.D.S.; Andrade de Souza, I.B.; Schnaufer, E.C.D.S.; Silva, L.F.d.; Maymone Gonçalves, C.C.; Simionatto, S.; Marchioro, S.B. Prevalence and factors associated with Trichomonas vaginalis infection in indigenous Brazilian women. PLoS ONE 2020, 15, e0240323. [Google Scholar] [CrossRef]
- Oladokun, A.O.; Opeodu, O.I.; Lawal, A.O.; Falade, M.O. Entamoeba gingivalis and Trichomonas tenax in periodontal disease. Microbiol. Res. J. Int. 2021, 31, 61–72. [Google Scholar] [CrossRef]
- Ibrahim, S.; Sc, B.; Sc, M. Evaluation of Entamoeba gingivalis and Trichomonas tenax in patients with periodontitis and gingivitis and its correlation with some risk factors. J. Baghdad Coll. Dent. 2012, 24, 158–162. [Google Scholar]
- Al-Hasnawy, M.H.; Rabee, A.H. A review on Trichomonas species infection in humans and animals in Iraq. Iraqi J. Vet. Sci. 2023, 37, 305–313. [Google Scholar] [CrossRef]
- Wenrich, D.H. The species of Trichomonas in man. J. Parasitol. 1947, 33, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Willcox, R.R. Epidemiological aspects of human trichomoniasis. Br. J. Vener. Dis. 1960, 36, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Crucitti, T.; Jespers, V.; Mulenga, C.; Khondowe, S.; Vandepitte, J.; Buvé, A. Trichomonas vaginalis is highly prevalent in adolescent girls, pregnant women, and commercial sex workers in Ndola, Zambia. Sex. Transm. Dis. 2010, 37, 223–227. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Anderson, N.L.; Johnson, C.K.; Fender, S.; Heckly, S.; Metzler, M.; Nave, P.; Yim, J. Clinical signs and histopathologic findings associated with a newly recognized protozoal disease (Trichomonas gallinae) in free-ranging house finches (Carpodacus mexicanus). J. Zoo Wildl. Med. 2010, 41, 249–254. [Google Scholar] [CrossRef]
- McBurney, S.; Kelly-Clark, W.K.; Forzán, M.J.; Vanderstichel, R.; Teather, K.; Greenwood, S.J. Persistence of Trichomonas gallinae in birdseed. Avian Dis. 2017, 61, 311–315. [Google Scholar] [CrossRef]
- Crucitti, T.; Jespers, V.; Mulenga, C.; Khondowe, S.; Vandepitte, J.; Buvé, A. Non-sexual transmission of Trichomonas vaginalis in adolescent girls attending school in Ndola, Zambia. PLoS ONE 2011, 6, e16310. [Google Scholar] [CrossRef] [PubMed]
- Torres-Machorro, A.L.; Hernández, R.; Alderete, J.F.; López-Villaseñor, I. Comparative analyses among the Trichomonas vaginalis, Trichomonas tenax, and Tritrichomonas foetus 5S ribosomal RNA genes. Curr. Genet. 2009, 55, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Hall, C.A. Physiology, Osmosis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
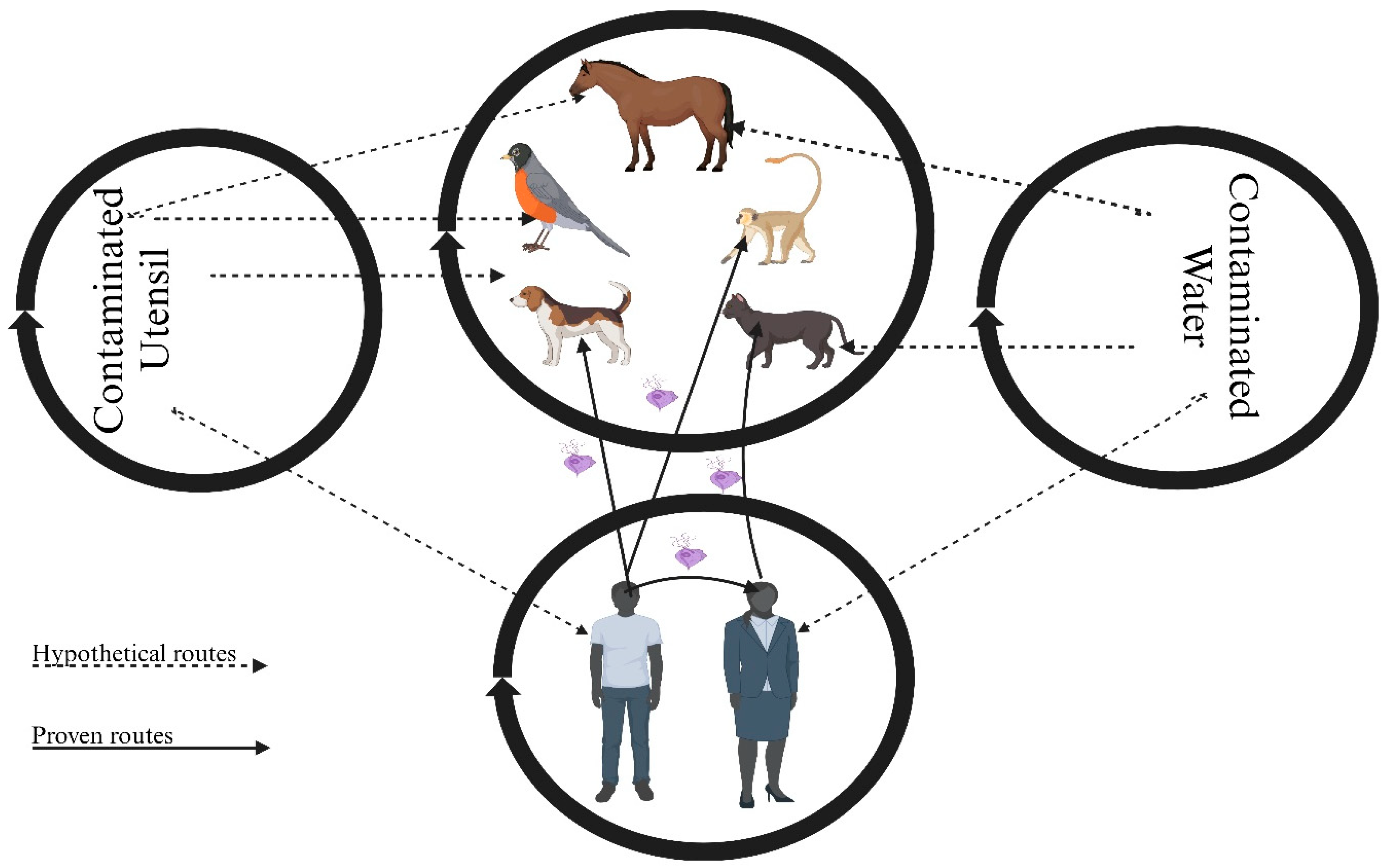
| Host | Site | Type(s) of Study/Studies | Region(s) | Route of Transmission | Reference(s) * |
|---|---|---|---|---|---|
| Humans | Oral cavity | Cross-sectional, experimental, case–control | South America, Europe, Asia, Africa | Direct contact | [2,17,18,19,20,21,27,42,43,44,45,46] |
| Humans | Lung | Retrospective, case report | Europe, Asia | Unknown | [27,28,29,30] |
| Humans | Lymph node | Case report | Europe | Unknown | [33] |
| Humans | Submaxillary gland | Case report | Europe | Unknown | [32] |
| Humans | Urogenital tract | Systemic review, case report | Europe | Unknown | [34,35] |
| Dogs | Oral cavity | Cross-sectional | Europe, North America | Direct contact | [1,8,36,37] |
| Dogs | Mandibular gland | Case report | Europe | Unknown | [38] |
| Cats | Oral cavity | Cross-sectional | Europe | Direct contact | [1,37] |
| Horses | Oral cavity | Cross-sectional | Europe | Unknown | [1] |
| Birds | Cloaca | Cross-sectional | Asia, Europe | Unknown | [39,40,41] |
| Socioeconomic Factors | ||||
|---|---|---|---|---|
| Age | Older individuals | Older individuals tend to have weaker immune systems, making them more susceptible to T. tenax infection. Additionally, older individuals tend to have poor oral hygiene due to their inability to take care of themselves. | Iraq | [44,55] |
| Sex | Males | Males tend to have a higher prevalence than females, possibly due to poorer oral hygiene (females tend to care more about their oral hygiene than males) or hormonal influences (difference in hormones, e.g., testosterone vs. estrogen). | Iraq | [55] |
| Income | Limited access to dental care due to poverty Overpopulation and fast-paced lifestyles | Lower-income individuals do not have the financial resources to pay for oral hygiene care and, as a result, tend to have weaker immune systems and poor oral hygiene, increasing the risk of T. tenax infection. Higher-income individuals live in more populated areas and live busier lives, and as a result, they come into contact with more people and may neglect their oral hygiene due to being too occupied. This increases the risk of T. tenax infection. | Saudi Arabia | [56] |
| Smoking | Smokers | Smoking is deemed to weaken the host immune system and damage the oral cavity, thus increasing the risk of T. tenax infection and periodontal disease. | Iraq | [44] |
| Public awareness | Lack of oral hygiene awareness | A lack of education and awareness on the importance of good oral hygiene practices leads to poor practices and risky behavior, increasing the chances of T. tenax infection. | Poland | [57] |
| Micro-Environmental Factors | ||||
| Temperature | Warm environments | Trichomonas tenax reproduces best at 35 °C compared to other temperatures, which means that its growth is affected by temperature, which indirectly impacts its transmission. | Iran, Poland, Egypt | [18,46,58] |
| pH | Slightly neutral or neutral pH | Trichomonas tenax reproduces best at a pH of 6.5–7 compared to other pHs, which means its growth is affected by the pH of the environment, which indirectly impacts its transmission. | Iran, Poland, Egypt | [18,46,58] |
| Oral hygiene practices | Poor oral hygiene | Poor oral hygiene significantly impacts transmission since T. tenax thrives in conditions where the oral cavity is poorly maintained. | Poland, Chile | [1,2] |
| Changes in oral micro-environments | Acute or chronic | Changes in the oral micro-environment can either promote or inhibit the growth of T. tenax. | St. Kitts, France, Iran | [5,7,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthew, M.; Ketzis, J.; Mukaratirwa, S.; Yao, C. Transmission Dynamics of Trichomonas tenax: Host and Site Specificity, Zoonotic Potential, and Environmental Factors. Microorganisms 2025, 13, 1475. https://doi.org/10.3390/microorganisms13071475
Matthew M, Ketzis J, Mukaratirwa S, Yao C. Transmission Dynamics of Trichomonas tenax: Host and Site Specificity, Zoonotic Potential, and Environmental Factors. Microorganisms. 2025; 13(7):1475. https://doi.org/10.3390/microorganisms13071475
Chicago/Turabian StyleMatthew, Maurice, Jennifer Ketzis, Samson Mukaratirwa, and Chaoqun Yao. 2025. "Transmission Dynamics of Trichomonas tenax: Host and Site Specificity, Zoonotic Potential, and Environmental Factors" Microorganisms 13, no. 7: 1475. https://doi.org/10.3390/microorganisms13071475
APA StyleMatthew, M., Ketzis, J., Mukaratirwa, S., & Yao, C. (2025). Transmission Dynamics of Trichomonas tenax: Host and Site Specificity, Zoonotic Potential, and Environmental Factors. Microorganisms, 13(7), 1475. https://doi.org/10.3390/microorganisms13071475






