Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Media
2.2. Bioinformatics Analyses of the gntR Gene and GntR Protein in P. plecoglossicida JUIM01
2.3. Construction of the Recombinant Strain to Express GntR
2.4. Expression and Purification of the Recombinant GntR Protein (PpGntR)
2.5. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS)
2.6. Circular Dichroism (CD) Far-Ultraviolet Scanning Analysis
2.7. Construction of the gntR-Deleted and gntR-Complemented Recombinants Derived from P. plecoglossicida JUIM01
2.8. Quantitative Real-Time PCR (RT-qPCR)
2.9. Electrophoretic Mobility Shift Assay (EMSA)
2.10. DNase I Footprinting Assay
2.11. Statistical Analysis
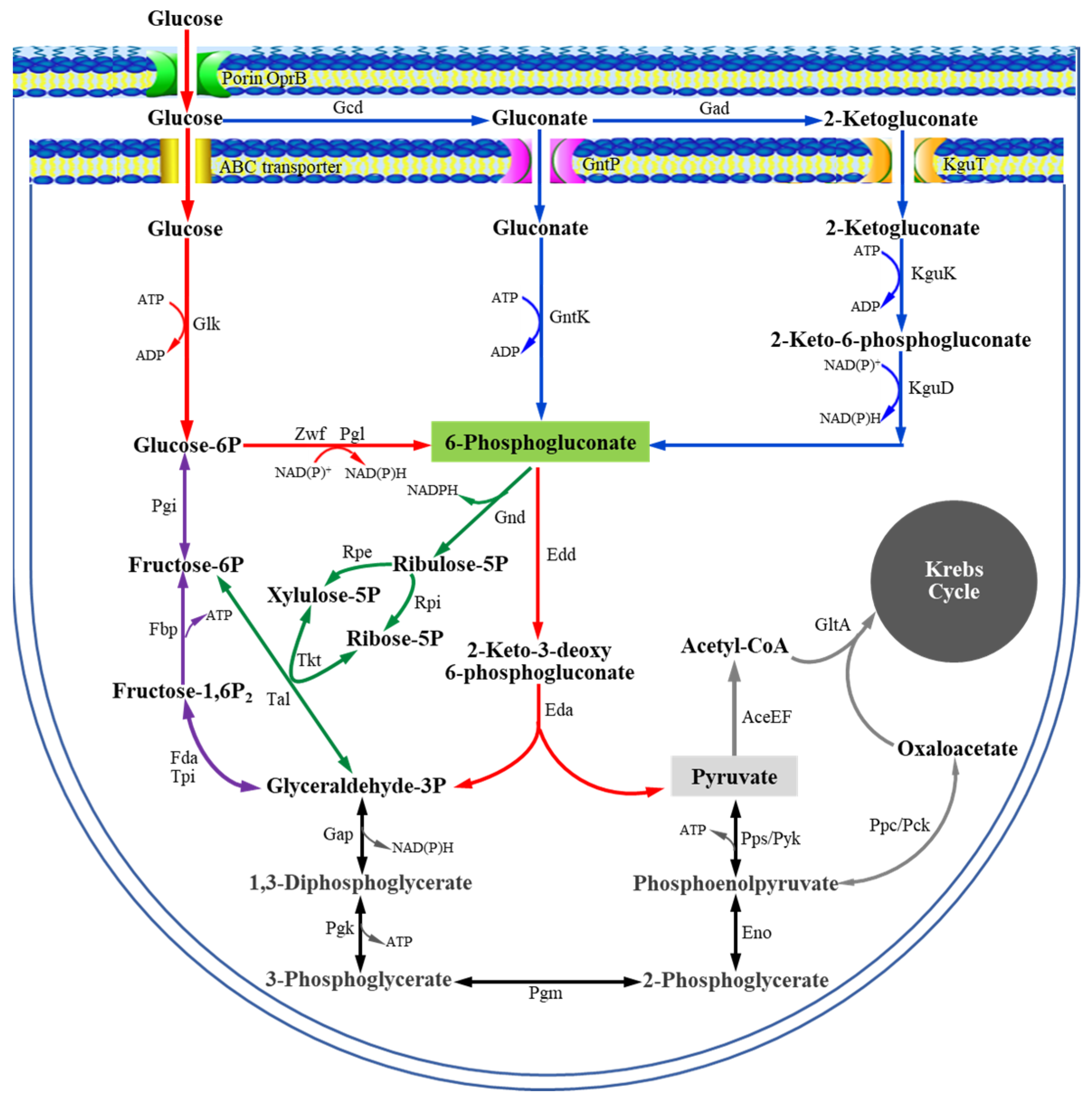
3. Results and Discussion
3.1. Cloning and Identification of the gntR Gene of P. plecoglossicida JUIM01
3.2. Heterogeneous Expression and Purification of the Recombinant GntR Protein (PpGntR)
3.3. Molecular Weight and Secondary Structure of PpGntR
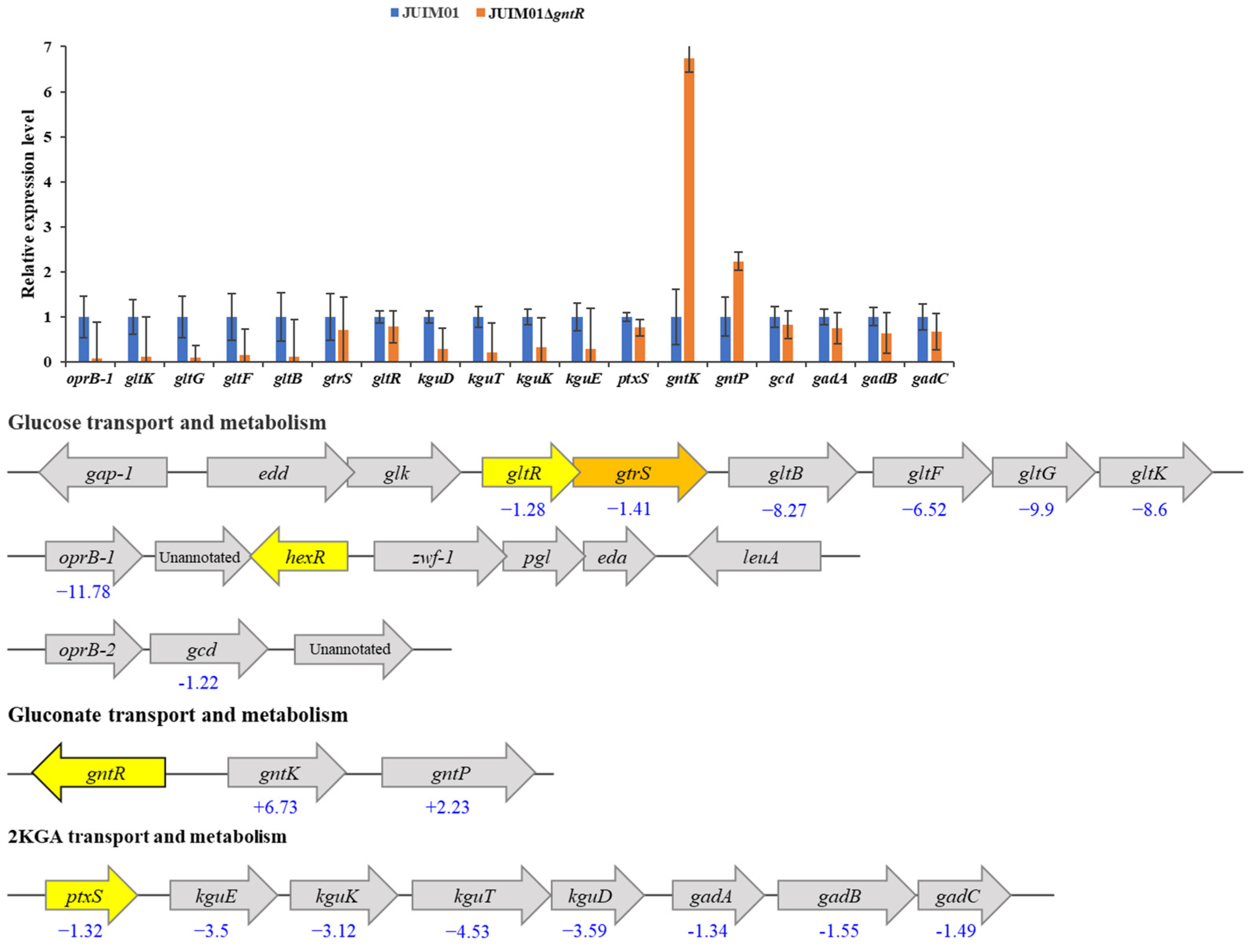
3.4. Effect of gntR Gene Deletion on Expression of Glucose/Gluoconate-Metabolism-Related Genes in P. plecoglossicida JUIM01
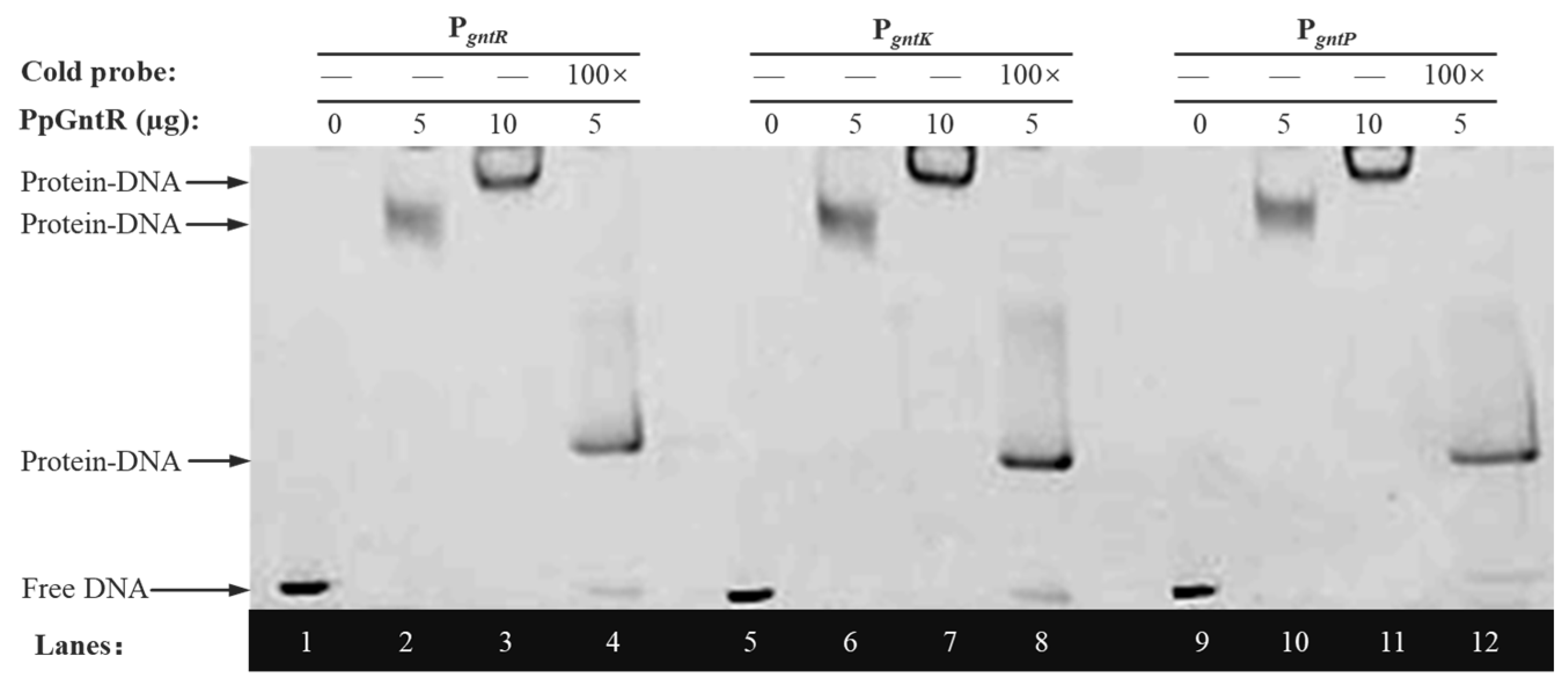
3.5. Specific Binding of PpGntR to the Promoter Region of the Potential Target Genes gntK and gntP
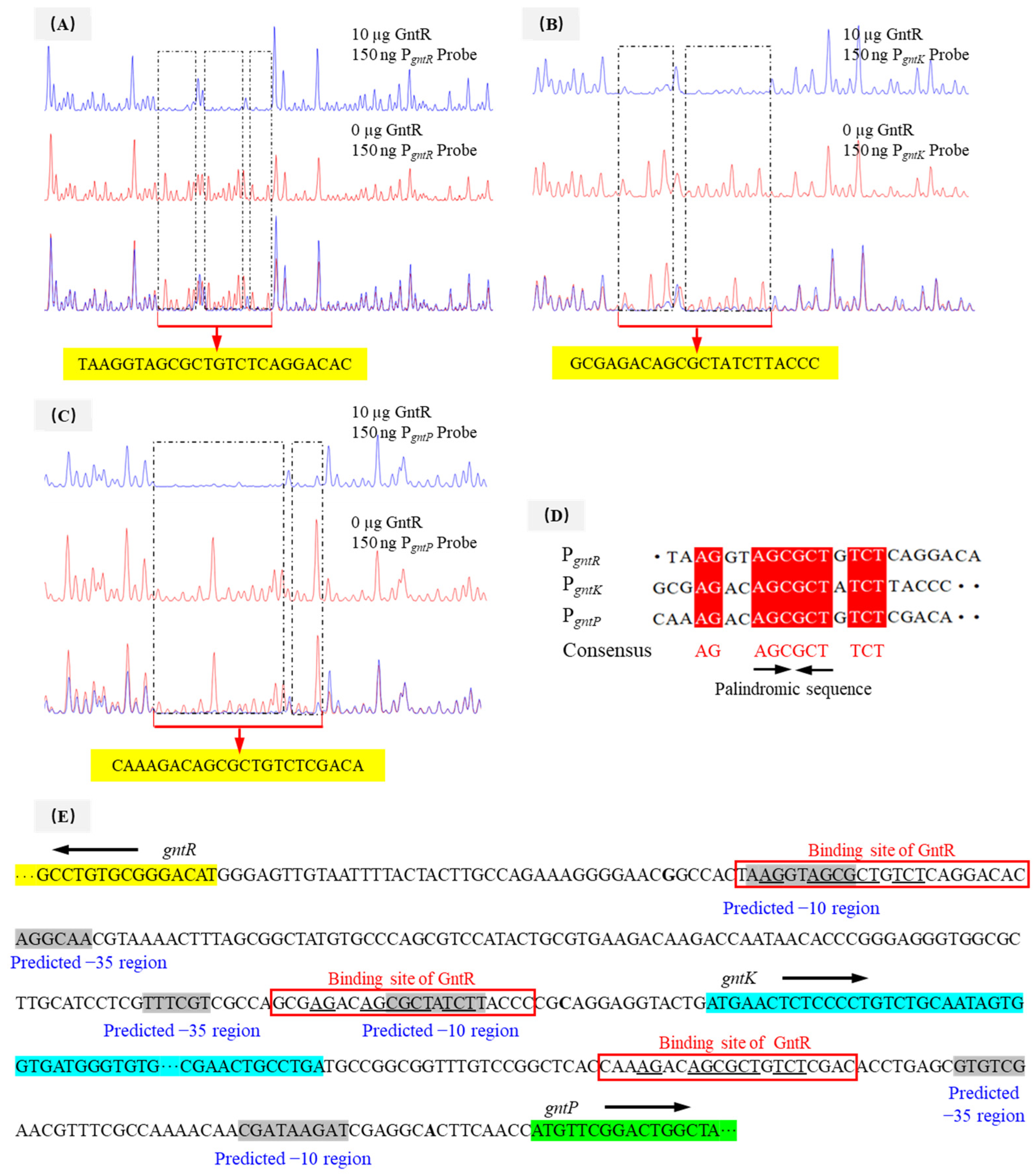
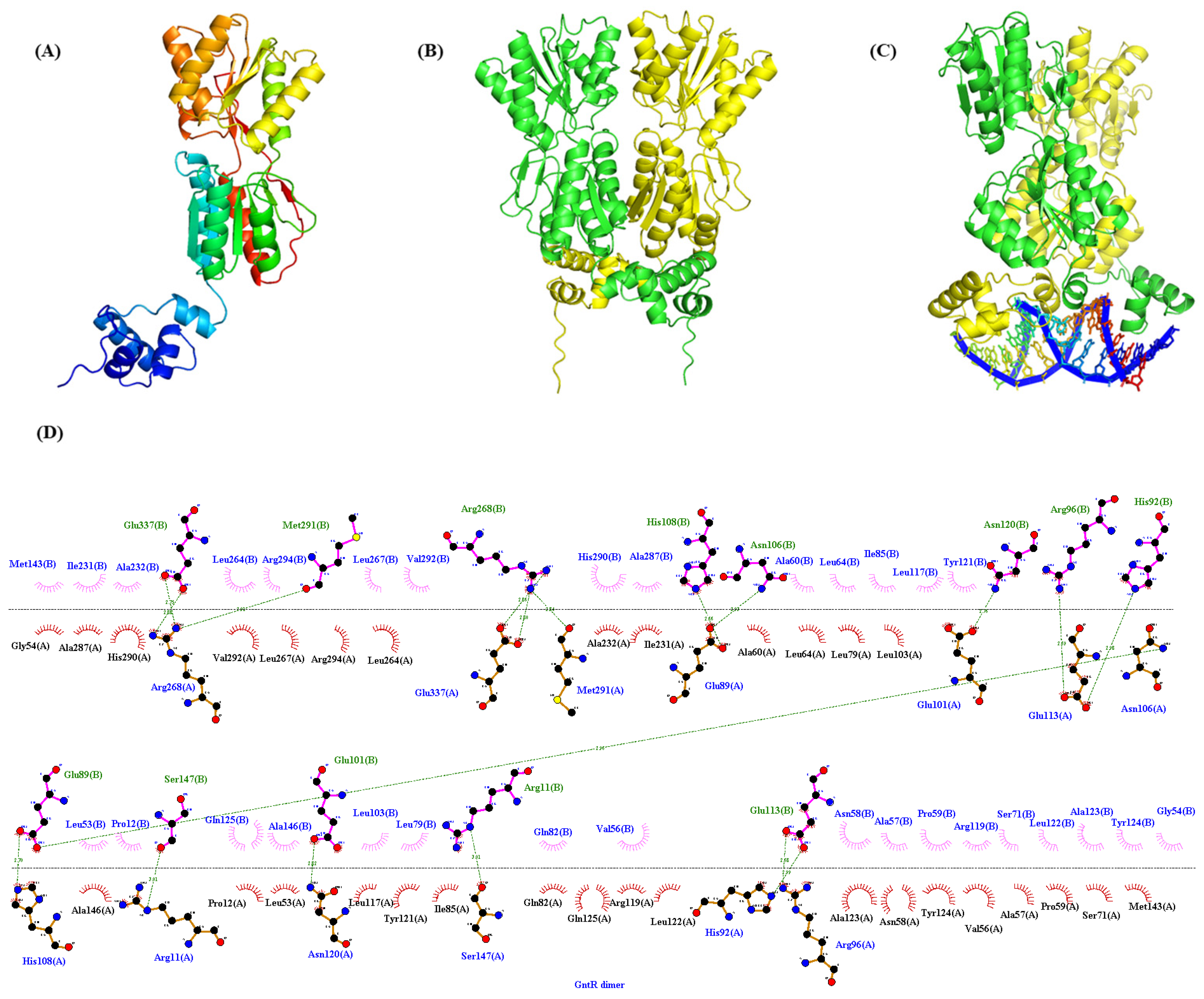
3.6. Identification of the Binding Sites of PpGntR to the Promoter Region of the Target Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopp, D.; Sunna, A. Alternative carbohydrate pathways–enzymes, functions and engineering. Crit. Rev. Biotechnol. 2020, 40, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Grüning, N.M.; Ralser, M. Glycolysis: How a 300 year long research journey that started with the desire to improve alcoholic beverages kept revolutionizing biochemistry. Curr. Opin. Syst. Biol. 2021, 28, 100380. [Google Scholar] [CrossRef]
- Kohlstedt, M.; Wittmann, C. GC-MS-based 13C metabolic flux analysis resolves the parallel and cyclic glucose metabolism of Pseudomonas putida KT2440 and Pseudomonas aeruginosa PAO1. Metab. Eng. 2019, 54, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Nikel, P.I.; Chavarría, M.; Fuhrer, T.; Sauer, U.; de Lorenzo, V. Pseudomonas putida KT2440 strain metabolizes glucose through a cycle formed by enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and pentose phosphate pathways. J. Biol. Chem. 2015, 290, 25920–25932. [Google Scholar] [CrossRef]
- Sasnow, S.S.; Wei, H.; Aristilde, L. Bypasses in intracellular glucose metabolism in iron-limited Pseudomonas putida. MicrobiologyOpen 2016, 5, 3–20. [Google Scholar] [CrossRef]
- del Castillo, T.; Ramos, J.L.; Rodríguez-Herva, J.J.; Fuhrer, T.; Sauer, U.; Duque, E. Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: Genomic and flux analysis. J. Bacteriol. 2007, 189, 5142–5152. [Google Scholar] [CrossRef]
- del Castillo, T.; Duque, E.; Ramos, J.L. A set of activators and repressors control peripheral glucose pathways in Pseudomonas putida to yield a common central intermediate. J. Bacteriol. 2008, 190, 2331–2339. [Google Scholar] [CrossRef]
- Tlemçani, L.L.; Corroler, D.; Barillier, D.; Mosrati, R. Physiological states and energetic adaptation during growth of Pseudomonas putida mt-2 on glucose. Arch. Microbiol. 2008, 190, 141–150. [Google Scholar] [CrossRef]
- Wang, D.M.; Sun, L.; Sun, W.J.; Cui, F.J.; Gong, J.S.; Zhang, X.M.; Shi, J.S.; Xu, Z.H. Purification, characterization and gene identification of a membrane-bound glucose dehydrogenase from 2-keto-D-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01. Int. J. Biol. Macromol. 2018, 118, 534–541. [Google Scholar] [CrossRef]
- Wang, D.M.; Sun, L.; Sun, W.J.; Cui, F.J.; Gong, J.S.; Zhang, X.M.; Shi, J.S.; Xu, Z.H. A membrane-bound gluconate Dehydrogenase from 2-keto-D-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01: Purification, characterization, and gene identification. Appl. Biochem. Biotechnol. 2019, 188, 897–913. [Google Scholar] [CrossRef]
- Yu, S.Q.; Lai, B.; Plan, M.R.; Hodson, M.P.; Lestari, E.A.; Song, H.; Krömer, J.O. Improved performance of Pseudomonas putida in a bioelectrochemical system through overexpression of periplasmic glucose dehydrogenase. Biotechnol. Bioeng. 2018, 115, 145–155. [Google Scholar] [CrossRef]
- Wang, D.M.; Chen, X.; Guo, H.; Wang, Q.H.; Sun, L.; Sun, W.J. Exploring the response mechanism of Pseudomonas plecoglossicida to high-temperature stress by transcriptomic analyses for 2-keto gluconic acid production. Food Biosci. 2024, 62, 105063. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.J.; Zhou, Y.Z.; Peng, Z.; Cui, F.J.; Zhou, Q.; Man, Z.W.; Guo, J.; Sun, W.J. Can cadmium-contaminated rice be used to produce food additive sodium erythorbate? Food Chem. 2025, 462, 140923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Shen, Y.; Xu, Y.; Balan, V. Directing cell catalysis of glucose to 2-keto-D-gluconic acid using Gluconobacter oxydans NL71. Process Biochem. 2020, 94, 365–369. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Cai, W.; Liu, L.; Du, G.C.; Chen, J.; Zhou, J.W. Efficient biosynthesis of 2-keto-D-gluconic acid by fed-batch culture of metabolically engineered Gluconobacter japonicus. Synth. Syst. Biotechnol. 2019, 4, 134–141. [Google Scholar] [CrossRef]
- Udaondo, Z.; Ramos, J.L.; Segura, A.; Krell, T.; Daddaoua, A. Regulation of carbohydrate degradation pathways in Pseudomonas involves a versatile set of transcriptional regulators. Microb. Biotechnol. 2018, 11, 442–454. [Google Scholar] [CrossRef]
- Daddaoua, A.; Krell, T.; Alfonso, C.; Morel, B.; Ramos, J.L. Compartmentalized glucose metabolism in Pseudomonas putida is controlled by the PtxS repressor. J. Bacteriol. 2010, 192, 4357–4366. [Google Scholar] [CrossRef]
- Daddaoua, A.; Fillet, S.; Fernández, M.; Udaondo, Z.; Krell, T.; Ramos, J.L. Genes for carbon metabolism and the ToxA virulence factor in Pseudomonas aeruginosa are regulated through molecular interactions of PtxR and PtxS. PLoS ONE 2012, 7, e39390. [Google Scholar] [CrossRef]
- Daddaoua, A.; Krell, T.; Ramos, J.L. Transcriptional control by two interacting regulatory proteins: Identification of the PtxS binding site at PtxR. Nucleic Acids Res. 2013, 41, 10150–10156. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.M.; Sun, W.J.; Zhang, X.F.; Cui, F.J.; Su, C.; Zhang, X.M.; Xu, G.Q.; Shi, J.S.; Xu, Z.H. Characterization of a transcriptional regulator PtxS from Pseudomonas plecoglossicida for regulating 2-ketogluconic acid metabolism. Int. J. Biol. Macromol. 2021, 174, 330–338. [Google Scholar] [CrossRef]
- Daddaoua, A.; Krell, T.; Ramos, J.L. Regulation of glucose metabolism in Pseudomonas: The phosphorylative branch and Entner-Doudoroff enzymes are regulated by a repressor containing a sugar isomerase domain. J. Biol. Chem. 2009, 284, 21360–21368. [Google Scholar] [CrossRef] [PubMed]
- Daddaoua, A.; Corral-Lugo, A.; Ramos, J.L.; Krell, T. Identification of GntR as regulator of the glucose metabolism in Pseudomonas aeruginosa. Environ. Microbiol. 2017, 19, 3721–3733. [Google Scholar] [CrossRef]
- Chen, W.B.; Ma, R.; Feng, Y.; Xiao, Y.Z.; Sekowska, A.; Danchin, A.; You, C.H. GnuR represses the expression of glucose and gluconate catabolism in Pseudomonas putida KT2440. Microb. Biotechnol. 2024, 17, e70059. [Google Scholar] [CrossRef]
- Daddaoua, A.; Molina-Santiago, C.; de la Torre, J.; Krell, T.; Ramos, J.L. GtrS and GltR form a two-component system: The central role of 2-ketogluconate in the expression of exotoxin A and glucose catabolic enzymes in Pseudomonas aeruginosa. Nucleic Acids Res. 2014, 42, 7654–7665. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, Q.; Lan, L. Glucose-binding of periplasmic protein GltB activates GtrS-GltR two-component system in Pseudomonas aeruginosa. Microorganisms 2021, 9, 447. [Google Scholar] [CrossRef]
- Haydon, D.J.; Guest, J.R. A new family of bacterial regulatory proteins. FEMS Microbiol. Lett. 1991, 79, 291–295. [Google Scholar] [CrossRef]
- Jain, D. Allosteric control of transcription in GntR family of transcription regulators: A structural overview. IUBMB Life 2015, 67, 556–563. [Google Scholar] [CrossRef]
- Suvorova, I.A.; Korostelev, Y.D.; Gelfand, M.S. GntR family of bacterial transcription factors and their DNA binding motifs: Structure, positioning and co-evolution. PLoS ONE 2015, 10, e0132618. [Google Scholar] [CrossRef] [PubMed]
- Rigali, S.; Derouaux, A.; Giannotta, F.; Dusart, J. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J. Biol. Chem. 2002, 277, 12507–12515. [Google Scholar] [CrossRef]
- Tsypik, O.; Makitrynskyy, R.; Bera, A.; Song, L.J.; Wohlleben, W.; Fedorenko, V.; Ostash, B. Role of GntR family regulatory gene SCO1678 in gluconate metabolism in Streptomyces coelicolor M145. BioMed Res. Int. 2017, 2017, 9529501. [Google Scholar] [CrossRef]
- Li, Z.B.; Xiang, Z.T.; Zeng, J.M.; Li, Y.Q.; Li, J.Y. A GntR family transcription factor in Streptococcus mutans regulates biofilm formation and expression of multiple sugar transporter genes. Front. Microbiol. 2019, 9, 3224. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Nair, D.T. Spacing between core recognition motifs determines relative orientation of AraR monomers on bipartite operators. Nucleic Acids Res. 2013, 41, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Frunzke, J.; Engels, V.; Hasenbein, S.; Gätgens, C.; Bott, M. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol. Microbiol. 2008, 67, 305–322. [Google Scholar] [CrossRef]
- Roy, S.; Patra, T.; Golder, T.; Chatterjee, S.; Koley, H.; Nandy, R.K. Characterization of the gluconate utilization system of Vibrio cholerae with special reference to virulence modulation. Pathog. Dis. 2016, 74, ftw085. [Google Scholar] [CrossRef]
- Tong, S.; Porco, A.; Isturiz, T.; Conway, T. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J. Bacteriol. 1996, 178, 3260–3269. [Google Scholar] [CrossRef]
- Izu, H.; Adachi, O.; Yamada, M. Gene organization and transcriptional regulation of the gntRKU operon involved in gluconate uptake and catabolism of Escherichia coli. J. Mol. Biol. 1997, 267, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Zhang, Q.N.; Li, L.L.; Qu, M.X.; Zan, X.Y.; Cui, F.J.; Zhou, Q.; Wang, D.M.; Sun, L. The functional characterization of the 6-phosphogluconate dehydratase operon in 2-ketogluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01. Foods 2024, 13, 3444. [Google Scholar] [CrossRef]
- Sun, W.J.; Wang, Q.H.; Luan, F.; Man, Z.W.; Cui, F.J.; Qi, X.H. The role of kguT gene in 2-ketogluconate-producing Pseudomonas plecoglossicida JUIM01. Appl. Biochem. Biotechnol. 2019, 187, 965–974. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Cain, A.K.; Nolan, L.M.; Sullivan, G.J.; Whitchurch, C.B.; Filloux, A.; Parkhill, J. Complete genome sequence of Pseudomonas aeruginosa reference strain PAK. Microbiol. Resour. Announc. 2019, 8, e00865-19. [Google Scholar] [CrossRef]
- Kelly, S.M.; Price, N.C. The application of circular dichroism to studies of protein folding and unfolding. Biochim. Biophys. Acta 1997, 1338, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Su, R.; Zhang, W.; Chen, J. Purification and the secondary structure of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the alcalase hydrolysate of seahorse protein. J. Food Sci. Technol. 2020, 57, 3927–3934. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.L.; Hager, P.; Phibbs, P., Jr.; Ochsner, U.; Vasil, M.L.; Hamood, A.N. Characterization of the 2-ketogluconate utilization operon in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 2000, 37, 561–573. [Google Scholar] [CrossRef]
- Sun, L.; Yang, W.Q.; Li, L.L.; Wang, D.M.; Zan, X.Y.; Cui, F.J.; Qi, X.H.; Sun, L.; Sun, W.J. Characterization and transcriptional regulation of the 2-ketogluconate utilization operon in Pseudomonas plecoglossicida. Microorganisms 2024, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, R.; Nogales, J.; Rojo, F. The Crc/CrcZ-CrcY global regulatory system helps the integration of gluconeogenic and glycolytic metabolism in Pseudomonas putida. Environ. Microbiol. 2015, 17, 3362–3378. [Google Scholar] [CrossRef]
- Tan, K.; McCue, L.A.; Stormo, G.D. Making connections between novel transcription factors and their DNA motifs. Genome Res. 2005, 15, 312–320. [Google Scholar] [CrossRef][Green Version]
- Martínez-Antonio, A.; Collado-Vides, J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 2003, 6, 482–489. [Google Scholar] [CrossRef]
- Istiqomah, D.; Joko, T.; Ogawa, N. A brief overview of LacI-family transcriptional regulators in bacteria. Rev. Agric. Sci. 2023, 11, 310–325. [Google Scholar] [CrossRef]
| Strains and Plasmids | Description | Source |
|---|---|---|
| Strains | ||
| P. plecoglossicida JUIM01 | A 2-ketogluconate industrial producing strain | Our Lab |
| E. coli JM109 | General cloning strain | TaKaRa |
| E. coli BL21(DE3) | General expressing strain | TaKaRa |
| E. coli JM109/pK18mobsacB-ΔgntR | JM109 containing vector pK18mobsacB-ΔgntR | This work |
| P. plecoglossicida JUIM01ΔgntR | gntR-knockout mutant of JUIM01 | This work |
| P. plecoglossicida JUIM01ΔgntR-gntR | gntR-complemented strain of JUIM01ΔgntR | This work |
| E. coli JM109/pMD20-T-gntR | JM109 containing vector pMD20-T-gntR | This work |
| E. coli BL21(DE3)/pET-28a(+) | BL21(DE3) containing vector pET-28a(+) | Our Lab |
| E. coli BL21(DE3)/pET-28a(+)-gntR | BL21(DE3) containing recombinant vector pET-28a(+)-gntR | This work |
| Plasmids | ||
| pK18mobsacB | Mobilizable E. coli vector, Kanr, Sucs | Our Lab |
| pET-28a(+) | Expression vector, carrying an N-terminal His-Tag/thrombin/T7-Tag and C-terminal His-Tag, Kanr | Our Lab |
| pMD20-T | T-vector, 2736 bp, Ampr, lacZ | TaKaRa |
| pBBR1MCS-2 | E. coli-Pseudomonas shuttle vector, Kanr | Our Lab |
| pK18mobsacB-ΔgntR | pK18mobsacB containing incomplete gntR sequence of JUIM01 | This work |
| pBB-gntR | pBBR1MCS-2 containing the gntR of JUIM01 | This work |
| pMD20-T-gntR | pMD20-T containing the gntR of JUIM01 | This work |
| pET-28a(+)-gntR | pET-28a(+) containing the gntR of JUIM01 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, M.; Li, L.; Zan, X.; Cui, F.; Sun, L.; Sun, W. Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01. Microorganisms 2025, 13, 1395. https://doi.org/10.3390/microorganisms13061395
Qu M, Li L, Zan X, Cui F, Sun L, Sun W. Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01. Microorganisms. 2025; 13(6):1395. https://doi.org/10.3390/microorganisms13061395
Chicago/Turabian StyleQu, Mengxin, Lulu Li, Xinyi Zan, Fengjie Cui, Lei Sun, and Wenjing Sun. 2025. "Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01" Microorganisms 13, no. 6: 1395. https://doi.org/10.3390/microorganisms13061395
APA StyleQu, M., Li, L., Zan, X., Cui, F., Sun, L., & Sun, W. (2025). Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01. Microorganisms, 13(6), 1395. https://doi.org/10.3390/microorganisms13061395







