Interactive Effects of Precipitation and Nitrogen on Soil Microbial Communities in a Desert Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Sample Collection
2.4. PLFA Determination and Soil Parameters Measurements
2.5. Statistics and Analysis of Data
3. Results
3.1. Effects of Increased Precipitation and N Deposition on Soil Physicochemical Properties
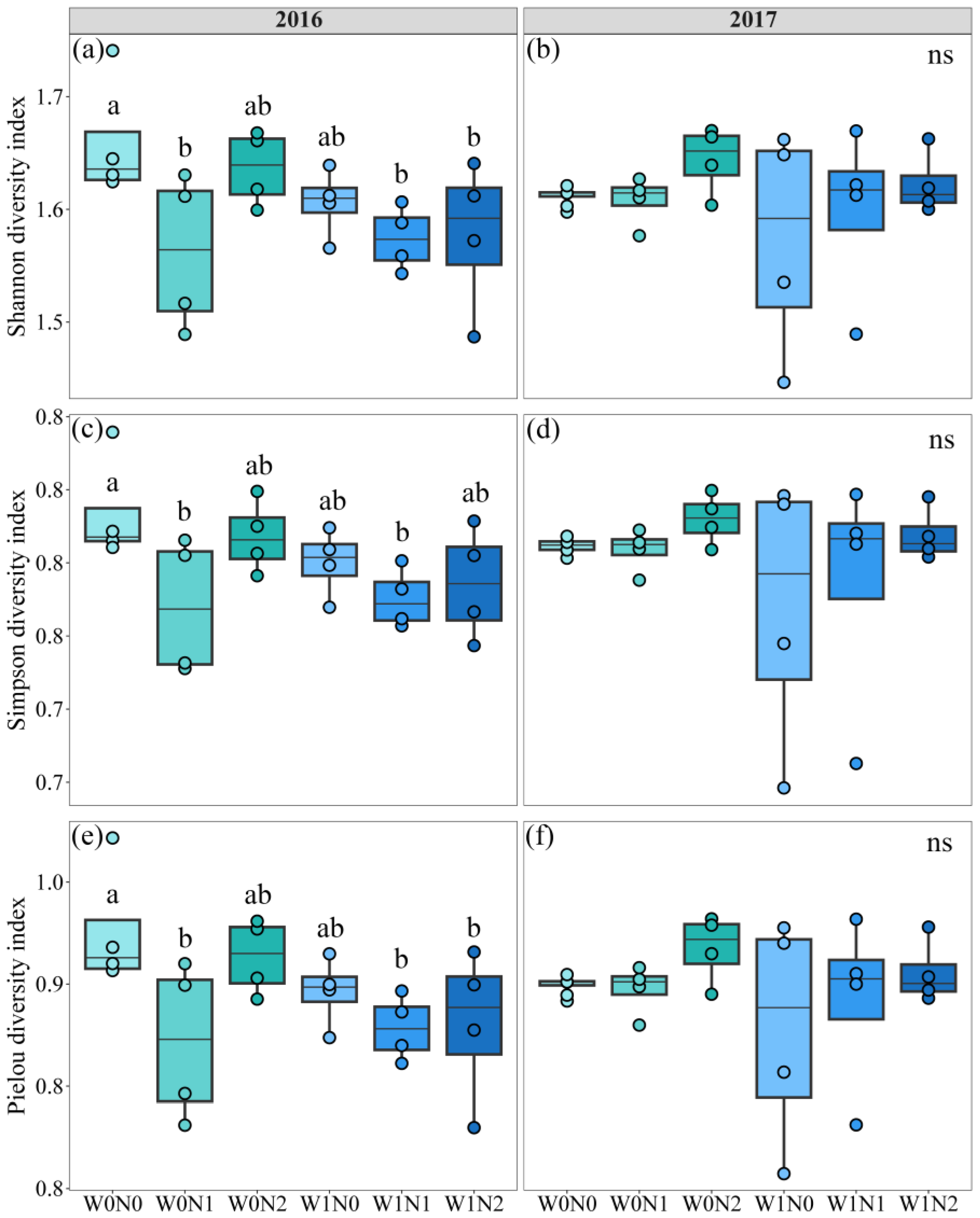
3.2. Effects of Increased Precipitation and N Deposition on Soil Microbial Community Diversity and Structure
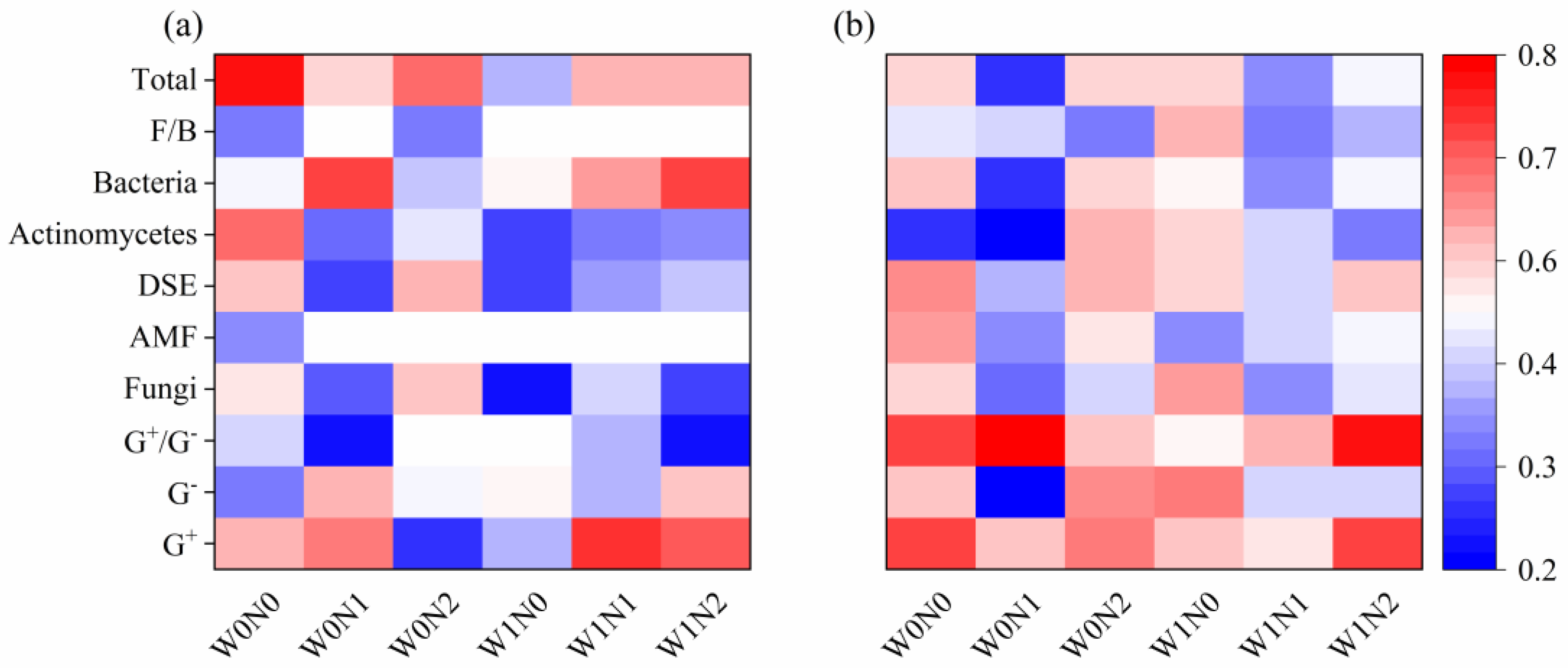
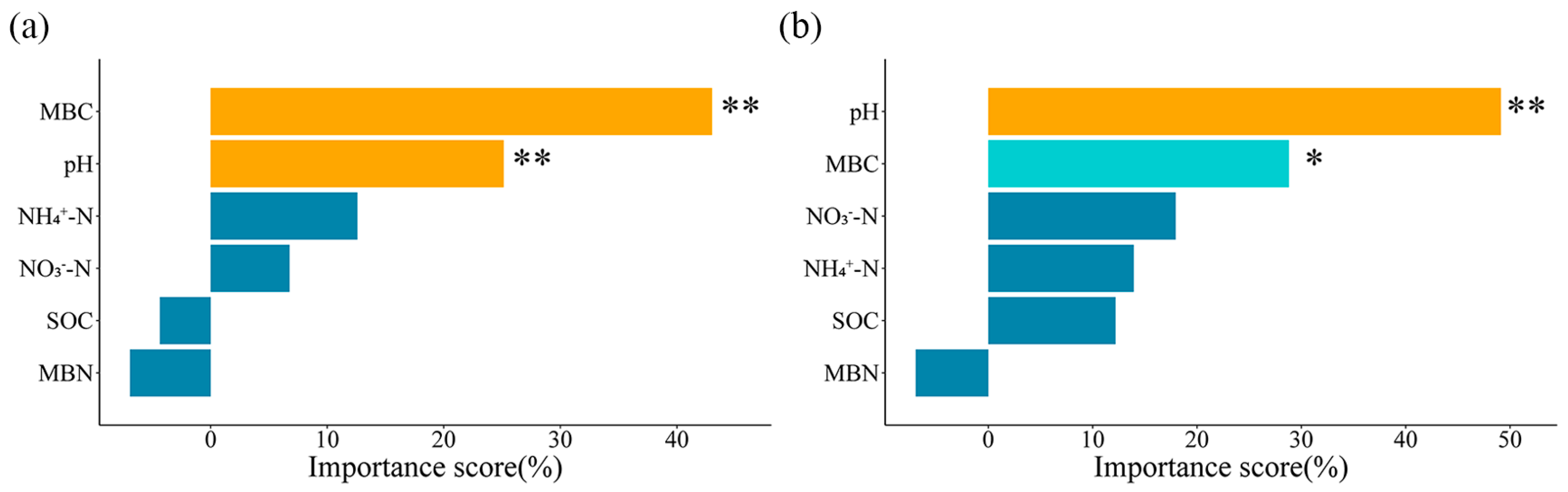
3.3. Relationships Between Soil Microbial Community and Soil Physicochemical Properties
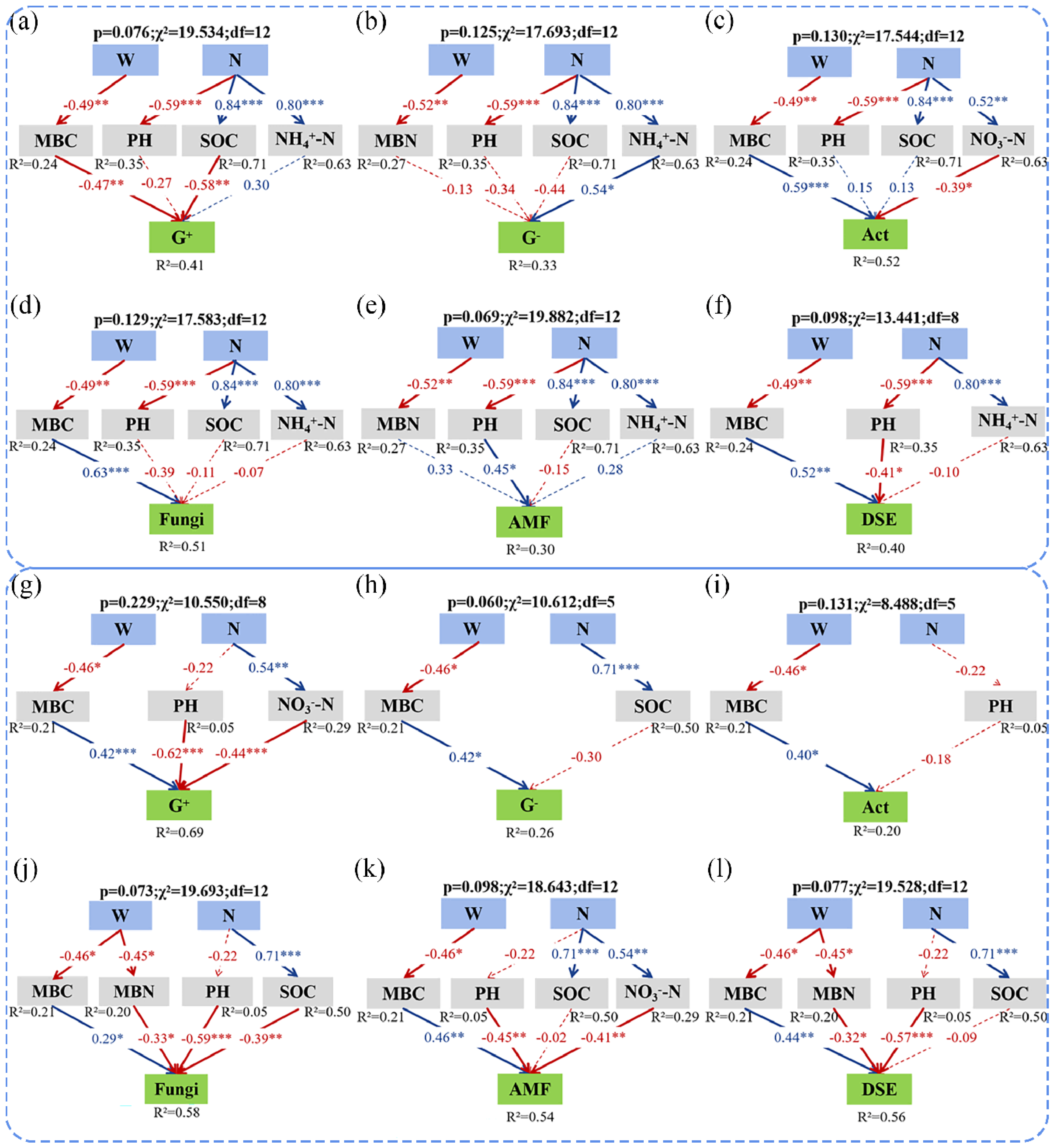
3.4. Direct and Indirect Effects of Soil Microbial Community Drivers
4. Discussion
4.1. Effects of Increased Precipitation on Soil Microbial Community
4.2. Effects of Increased N Deposition on Soil Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernando, T.M.; Biancari, L.; Chen, N.; Corrochano-Monsalve, M.; Jenerette, G.D.; Nelson, C.; Shilula, K.N.; Shpilkina, Y. Research Needs on the Biodiversity–Ecosystem Functioning Relationship in Drylands. Npj Biodivers. 2024, 3, 12. [Google Scholar] [CrossRef]
- Wang, X.; Geng, X.; Liu, B.; Cai, D.; Li, D.; Xiao, F.; Zhu, B.; Hua, T.; Lu, R.; Liu, F. Desert Ecosystems in China: Past, Present, and Future. Earth-Sci. Rev. 2022, 234, 104206. [Google Scholar] [CrossRef]
- Liu, L.; Ma, C.; Huo, S.; Xi, B.; He, Z.; Zhang, H.; Zhang, J.; Xia, X. Impacts of Climate Change and Land Use on the Development of Nutrient Criteria. J. Hydrol. 2018, 563, 533–542. [Google Scholar] [CrossRef]
- Brajesh, K.S.; Dawson, L.A.; Macdonald, C.A.; Buckland, S.M. Impact of Biotic and Abiotic Interaction on Soil Microbial Communities and Functions: A Field Study. Appl. Soil Ecol. 2009, 41, 239–248. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil Multifunctionality Is Affected by the Soil Environment and by Microbial Community Composition and Diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and Its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Yang, A.; Song, B.; Zhang, W.; Zhang, T.; Li, X.; Wang, H.; Zhu, D.; Zhao, J.; Fu, S. Chronic Enhanced Nitrogen Deposition and Elevated Precipitation Jointly Benefit Soil Microbial Community in a Temperate Forest. Soil Biol. Biochem. 2024, 193, 109397. [Google Scholar] [CrossRef]
- Zhai, C.; Han, L.; Xiong, C.; Ge, A.; Yue, X.; Li, Y.; Zhou, Z.; Feng, J.; Ru, J.; Song, J.; et al. Soil Microbial Diversity and Network Complexity Drive the Ecosystem Multifunctionality of Temperate Grasslands under Changing Precipitation. Sci. Total Environ. 2024, 906, 167217. [Google Scholar] [CrossRef]
- Li, Z.L.; Peng, Q.; Dong, Y.; Guo, Y. The Influence of Increased Precipitation and Nitrogen Deposition on the Litter Decomposition and Soil Microbial Community Structure in a Semiarid Grassland. Sci. Total Environ. 2022, 844, 157115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-T.; Liu, Y.-N.; Zhong, H.-X.; Chen, X.-W.; Sui, X. Effects of Simulated Nitrogen Deposition on the Soil Microbial Community Diversity of a Deyeuxia Angustifolia Wetland in the Sanjiang Plain, Northeastern China. Ann. Microbiol. 2022, 72, 11. [Google Scholar] [CrossRef]
- Zhao, C.; Sai, P.; Honghua, R.; Yakun, Z. Effects of Nitrogen Deposition on Soil Microbes. J. Nanjing For. Univ. 2015, 39, 149–155. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global Negative Effects of Nitrogen Deposition on Soil Microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Ma, S.; Fang, W.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X.; et al. Effects of Nitrogen Deposition on Soil Microbial Communities in Temperate and Subtropical Forests in China. Sci. Total Environ. 2017, 607–608, 1367–1375. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Effects of Forest Degradation on Microbial Communities and Soil Carbon Cycling: A Global Meta-Analysis. Glob. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Scott, L.C.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras-Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse Dynamics and Microbial Processes in Aridland Ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Wang, Z.; Na, R.; Koziol, L.; Schellenberg, M.P.; Li, X.; Ta, N.; Jin, K.; Wang, H. Response of Bacterial Communities and Plant-Mediated Soil Processes to Nitrogen Deposition and Precipitation in a Desert Steppe. Plant Soil 2020, 448, 277–297. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Pang, D.; Chen, Y.; Wu, M.; Zhang, Y.; He, W.; Li, X. Responses of Soil Microbial Community Structure under Litter to Changes in Precipitation and Nitrogen Addition in a Desert Steppe. Eur. J. Soil Biol. 2025, 124, 103696. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Guo, J.; Han, G.; Gutknecht, J.; Schmid, B.; Yu, L.; Liu, W.; Bi, J.; Wang, Z.; et al. Precipitation Modifies the Effects of Warming and Nitrogen Addition on Soil Microbial Communities in Northern Chinese Grasslands. Soil Biol. Biochem. 2015, 89, 12–23. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, K.; Wu, Q.; Cheng, X.; Li, Z.; Wang, Z.; Zhao, M.; Wilkes, A.; Bisselling, T.; Han, G.; et al. Seasonal Precipitation and Soil Microbial Community Influence Plant Growth Response to Warming and N Addition in a Desert Steppe. Plant Soil 2023, 482, 245–259. [Google Scholar] [CrossRef]
- Ernest, D.O.; McBride, S.G.; Kupper, J.V.; Nelson, J.A.; McNear, D.H.; McCulley, R.L.; Barrett, J.E. Accurate Detection of Soil Microbial Community Responses to Environmental Change Requires the Use of Multiple Methods. Soil Biol. Biochem. 2022, 169, 108685. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Q.; Guo, Y.; Gao, G.; Wang, J. Simulation of Regional Temperature and Precipitation in the Past 50 Years and the Next 30 Years over China. Quat. Int. 2010, 212, 57–63. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, W.; Li, Q.; Han, M.; Tang, A.; Zhang, Y.; Luo, X.; Shen, J.; Wang, W.; Li, K.; et al. Changes of Nitrogen Deposition in China from 1980 to 2018. Environ. Int. 2020, 144, 106022. [Google Scholar] [CrossRef]
- James, N.G.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Rahman, A.; Natasha, N.S.; Hamid, N.W.A.; Nadarajah, K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- David, C.W.; Flemming, C.A.; Leung, K.T.; Sarah, J. Macnaughton. In Situ Microbial Ecology for Quantitative Appraisal, Monitoring, and Risk Assessment of Pollution Remediation in Soils, the Subsurface, the Rhizosphere and in Biofilms. J. Microbiol. Methods 1998, 32, 93–105. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, H.; Mao, Z.; Bao, X.; He, H.; Zhang, X.; Liang, C. Fungi Determine Increased Soil Organic Carbon More Than Bacteria through Their Necromass Inputs in Conservation Tillage Croplands. Soil Biol. Biochem. 2022, 167, 108587. [Google Scholar] [CrossRef]
- Anne, E.M. Ecological Diversity and Its Measurement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zhang, Y.; Zheng, N.; Wang, J.; Yao, H.; Qiu, Q.; Chapman, S.J. High Turnover Rate of Free Phospholipids in Soil Confirms the Classic Hypothesis of Plfa Methodology. Soil Biol. Biochem. 2019, 135, 323–330. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X. Microbes Studies of Tea Rhizosphere. Acta Ecol. Sin. 2004, 24, 1353–1357. [Google Scholar]
- Janet, K.J.; Hofmockel, K.S. Soil Microbiomes and Climate Change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Jeb, S.C.; Campbell, J.H.; Grizzle, H.; Acosta-Martìnez, V.; Zak, J.C. Soil Microbial Community Response to Drought and Precipitation Variability in the Chihuahuan Desert. Microb. Ecol. 2009, 57, 248–260. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, K.; Loik, M.E.; Sun, W. Differential Responses of Soil Bacteria and Fungi to Altered Precipitation in a Meadow Steppe. Geoderma 2021, 384, 114812. [Google Scholar] [CrossRef]
- Zhang, B.; Penton, C.R.; Xue, C.; Quensen, J.F.; Roley, S.S.; Guo, J.; Garoutte, A.; Zheng, T.; Tiedje, J.M. Soil Depth and Crop Determinants of Bacterial Communities under Ten Biofuel Cropping Systems. Soil Biol. Biochem. 2017, 112, 140–152. [Google Scholar] [CrossRef]
- Mao, H.; Whalen, J.K.; Zhang, Z.; Sheng, X.; Hu, G.; Chen, B.; Ma, M. Arbuscular Mycorrhizal Fungi Associated with Alpine Meadow Multifunctionality in a Warmer Climate with Variable Precipitation. Soil Biol. Biochem. 2024, 198, 109555. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Su, Y.G. Effects of Increasing Precipitation on Soil Microbial Community Composition and Soil Respiration in a Temperate Desert, Northwestern China. Soil Biol. Biochem. 2015, 83, 52–56. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.-R.; An, S.-S.; Jiao, F.; Huang, Q. Response of Soil Bacterial Community Structure to Precipitation Change in Grassland of Loess Plateau. Huan Jing Ke Xue Huanjing Kexue 2020, 41, 4284–4293. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.; Lu, X.; Fu, L.; Liu, Y. Responses of Soil Bacterial Communities to Precipitation Change in the Semi-Arid Alpine Grassland of Northern Tibet. Front. Plant Sci. 2022, 13, 1036369. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Fuchslueger, L.; Schnecker, J.; Metze, D.; Nelson, D.B.; Kahmen, A.; Watzka, M.; Pötsch, E.M.; Schaumberger, A.; Bahn, M.; et al. Soil Fungi Remain Active and Invest in Storage Compounds During Drought Independent of Future Climate Conditions. Nat. Commun. 2024, 15, 10410. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Zarandi, M.; Etesami, H.; Glick, B.R. Fostering Plant Resilience to Drought with Actinobacteria: Unveiling Perennial Allies in Drought Stress Tolerance. Plant Stress 2023, 10, 100242. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, H.; Li, J.; Wang, H.; Liu, S.; Liu, X. Reduced Precipitation Neutralizes the Positive Impact of Soil Warming on Soil Microbial Community in a Temperate Oak Forest. Sci. Total Environ. 2022, 806, 150957. [Google Scholar] [CrossRef]
- Li, Y.; Wei, S.; Wang, H.; Zhang, E.; Duan, X. Responses of Soil Microbial Biomass Carbon and Microbial Entropy to Soil Properties in Typical Sloping Croplands of China under Erosion Conditions. Eur. J. Soil Biol. 2024, 122, 103660. [Google Scholar] [CrossRef]
- Sun, X.; Sun, H.; Zhang, Q.; Zhu, B.; Dai, H.; Zeng, Q.; Chen, J.; Chen, W.; Chen, Y. Soil Particulate Organic Carbon Regulates Microbial Carbon Use Efficiency in Subtropical Forests under Nitrogen Addition in Different Seasons. Appl. Soil Ecol. 2024, 203, 105680. [Google Scholar] [CrossRef]
- Feng, J.; Shi, C.; Hafiz, A.H.; Liu, Y.-B.; Liu, T.-P.; Li, Y.-H.; Liu, J.-F.; Wang, L.-C. Effects of Mulching and Slow-Release Fertilizer Application Reduction on Soil Microbial Community Structure in Rapeseed Field under Two Different Rainfall Conditions. Huan Jing Ke Xue Huanjing Kexue 2022, 43, 4322–4332. [Google Scholar] [CrossRef]
- Uffe, N.N.; Ball, B.A. Impacts of Altered Precipitation Regimes on Soil Communities and Biogeochemistry in Arid and Semi-Arid Ecosystems. Glob. Chang. Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of Nitrogen Enrichment on Belowground Communities in Grassland: Relative Role of Soil Nitrogen Availability Vs. Soil Acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Walaa, K.M.; Abu-Izneid, T.; Salah-Tantawy, A. High-Throughput Sequencing Reveals the Structure and Metabolic Resilience of Desert Microbiome Confronting Climate Change. Front. Plant Sci. 2024, 15, 1294173. [Google Scholar] [CrossRef]
- Hu, G.; Wu, S.; Zhou, X.; Ruan, A. Unveiling Soil Microbial Community Dynamics in Desertification: A Case Study from the Tianshan Mountains, Xinjiang. Ecol. Indic. 2024, 166, 112342. [Google Scholar] [CrossRef]
- Wang, M.; Frey, B.; Li, D.; Liu, X.; Chen, C.; Liu, Y.; Zhang, R.; Sui, X.; Li, M.-H. Effects of Organic Nitrogen Addition on Soil Microbial Community Assembly Patterns in the Sanjiang Plain Wetlands, Northeastern China. Appl. Soil Ecol. 2024, 204, 105685. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally Nitrogen Addition Alters Soil Microbial Community Structure, but Has Minor Effects on Soil Microbial Diversity and Richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Xiang, D.; Veresoglou, S.D.; Rillig, M.C.; Xu, T.; Li, H.; Hao, Z.; Chen, B. Relative Importance of Individual Climatic Drivers Shaping Arbuscular Mycorrhizal Fungal Communities. Microb. Ecol. 2016, 72, 418–427. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Gao, Y.; Xing, F.; Sun, S.; Zhang, T.; Zhu, X.; Chen, C.; Li, Z. Responses of Soil Extracellular Enzyme Activities and Microbial Community Properties to Interaction between Nitrogen Addition and Increased Precipitation in a Semi-Arid Grassland Ecosystem. Sci. Total Environ. 2020, 703, 134691. [Google Scholar] [CrossRef]
- Ullah, A.; Akbar, A.; Luo, Q.; Khan, A.H.; Manghwar, H.; Shaban, M.; Yang, X. Microbiome Diversity in Cotton Rhizosphere under Normal and Drought Conditions. Microb. Ecol. 2019, 77, 429–439. [Google Scholar] [CrossRef]
- Li, Y.; Zou, N.; Liang, X.; Zhou, X.; Guo, S.; Wang, Y.; Qin, X.; Tian, Y.; Lin, J. Effects of Nitrogen Input on Soil Bacterial Community Structure and Soil Nitrogen Cycling in the Rhizosphere Soil of Lycium barbarum L. Front. Microbiol. 2023, 13, 1070817. [Google Scholar] [CrossRef]
- Lux, J.; Xie, Z.; Sun, X.; Wu, D.; Scheu, S. Changes in Microbial Community Structure and Functioning with Elevation Are Linked to Local Soil Characteristics as Well as Climatic Variables. Ecol. Evol. 2022, 12, e9632. [Google Scholar] [CrossRef]
- Fan, M.; Li, J.; Tang, Z.; Shangguan, Z. Soil Bacterial Community Succession During Desertification in a Desert Steppe Ecosystem. Land Degrad. Dev. 2020, 31, 1662–1674. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Lai, K.; McNee, M.; Flower, K.; Singh, B.P.; Minkey, D.; et al. Soil Aggregation and Associated Microbial Communities Modify the Impact of Agricultural Management on Carbon Content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef]
- Chen, X.; Hao, B.; Jing, X.; He, J.-S.; Ma, W.; Zhu, B. Minor Responses of Soil Microbial Biomass, Community Structure and Enzyme Activities to Nitrogen and Phosphorus Addition in Three Grassland Ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.; Zhang, X.; Kuang, J.; Ling, N.; et al. Critical Transition of Soil Bacterial Diversity and Composition Triggered by Nitrogen Enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of Nitrogen Enrichment on Soil Microbial Characteristics: From Biomass to Enzyme Activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.-W.; Wang, Y.; Shelton, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of Soil Glycosidase Activity and Soil Respiration by Nitrogen Addition. Glob. Chang. Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Lan, Z.; Hu, S.; Bai, Y. Soil Acidification Exerts a Greater Control on Soil Respiration Than Soil Nitrogen Availability in Grasslands Subjected to Long-Term Nitrogen Enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.H.E.; Wan, S. Predominant Role of Water in Regulating Soil and Microbial Respiration and Their Responses to Climate Change in a Semiarid Grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Zheng, C.; Suter, H.; Huang, Z. Different Responses of Soil Bacterial and Fungal Communities to Nitrogen Deposition in a Subtropical Forest. Sci. Total Environ. 2021, 755, 142449. [Google Scholar] [CrossRef]
- Paterson, E.; Sim, A.; Osborne, S.M.; Murray, P.J. Long-Term Exclusion of Plant-Inputs to Soil Reduces the Functional Capacity of Microbial Communities to Mineralise Recalcitrant Root-Derived Carbon Sources. Soil Biol. Biochem. 2011, 43, 1873–1880. [Google Scholar] [CrossRef]
- Cui, Y.; Peng, S.; Delgado-Baquerizo, M.; Rillig, M.C.; Terrer, C.; Zhu, B.; Jing, X.; Chen, J.; Li, J.; Feng, J.; et al. Microbial Communities in Terrestrial Surface Soils Are Not Widely Limited by Carbon. Glob. Chang. Biol. 2023, 29, 4412–4429. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Yang, L.; Shi, H.; Li, L.; Bai, W.; Zhao, Y. Soil Acidification under Long-Term N Addition Decreases the Diversity of Soil Bacteria and Fungi and Changes Their Community Composition in a Semiarid Grassland. Microb. Ecol. 2023, 85, 221–231. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Liu, C.; He, Z.; Chen, Y.; Xu, Y.; Tang, W.; Chen, L. Effects of Nitrogen Deposition on the Rhizosphere Nitrogen-Fixing Bacterial Community Structure and Assembly Mechanisms in Camellia Oleifera Plantations. Front. Microbiol. 2024, 15, 1414724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, L.; Zhang, H.; Deng, Y.; Hu, B.; Wang, W. Distinct Roles of Bacteria and Fungi in Mediating Soil Extracellular Enzymes under Long-Term Nitrogen Deposition in Temperate Plantations. For. Ecol. Manag. 2023, 529, 120658. [Google Scholar] [CrossRef]
- Kelly, S.R.; Craine, J.M.; Fierer, N. Nitrogen Fertilization Inhibits Soil Microbial Respiration Regardless of the Form of Nitrogen Applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Habekost, M.; Eisenhauer, N.; Scheu, S.; Steinbeiss, S.; Weigelt, A.; Gleixner, G. Seasonal Changes in the Soil Microbial Community in a Grassland Plant Diversity Gradient Four Years after Establishment. Soil Biol. Biochem. 2008, 40, 2588–2595. [Google Scholar] [CrossRef]

| Year | Treatment | pH | SOC | NO3−-N | NH4+-N | MBC | MBN |
|---|---|---|---|---|---|---|---|
| (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |||
| 2016 | W0N0 | 8.75 ± 0.12 a | 2.14 ± 0.08 bc | 4.40 ± 0.31 d | 1.57 ± 0.35 c | 168.33 ± 30.14 bc | 66.43 ± 3.90 bc |
| W0N1 | 8.55 ± 0.02 ab | 2.37 ± 0.09 b | 6.19 ± 0.12 bc | 6.99 ± 1.79 ab | 181.37 ± 18.10 ab | 68.54 ± 2.37 b | |
| W0N2 | 8.47 ± 0.06 b | 2.70 ± 0.08 a | 8.91 ± 0.28 a | 8.66 ± 1.47 a | 223.87 ± 6.20 a | 76.62 ± 1.56 a | |
| W1N0 | 8.73 ± 0.10 ab | 2.06 ± 0.03 c | 6.37 ± 0.29 b | 3.01 ± 0.55 c | 160.03 ± 11.84 bc | 65.72 ± 1.64 bc | |
| W1N1 | 8.65 ± 0.11 ab | 2.19 ± 0.04 bc | 6.89 ± 0.29 b | 4.04 ± 0.74 bc | 164.77 ± 7.51 bc | 66.08 ± 1.56 bc | |
| W1N2 | 8.49 ± 0.04 ab | 2.76 ± 0.13 a | 5.54 ± 0.23 c | 9.94 ± 1.04 a | 121.55 ± 19.20 c | 60.17 ± 1.45 c | |
| 2017 | W0N0 | 8.70 ± 0.11 a | 2.09 ± 0.06 bc | 4.29 ± 0.21 d | 1.52 ± 0.42 c | 161.20 ± 31.57 bc | 63.10 ± 3.08 bc |
| W0N1 | 8.57 ± 0.02 a | 2.41 ± 0.10 ab | 6.04 ± 0.16 bc | 6.93 ± 1.71 ab | 183.26 ± 21.83a b | 67.11 ± 3.15 b | |
| W0N2 | 8.50 ± 0.11 a | 2.56 ± 0.17 a | 8.79 ± 0.45 a | 8.20 ± 1.75 a | 227.28 ± 7.37 a | 74.43 ± 1.42 a | |
| W1N0 | 8.53 ± 0.11 a | 2.03 ± 0.05 c | 6.14 ± 0.26 bc | 2.76 ± 0.68 c | 163.97 ± 11.20 bc | 64.26 ± 1.61 bc | |
| W1N1 | 8.75 ± 0.17 a | 2.14 ± 0.04 bc | 6.61 ± 0.34 b | 3.68 ± 0.90 bc | 162.88 ± 8.63 bc | 64.46 ± 1.44 bc | |
| W1N2 | 8.50 ± 0.01 a | 2.57 ± 0.15 a | 5.46 ± 0.29 c | 8.96 ± 1.64 a | 119.28 ± 16.97 c | 59.65 ± 2.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Ji, Z.; Wang, H.; Duan, W.; Cao, W.; Li, W.; Jia, Y. Interactive Effects of Precipitation and Nitrogen on Soil Microbial Communities in a Desert Ecosystem. Microorganisms 2025, 13, 1393. https://doi.org/10.3390/microorganisms13061393
Dong Q, Ji Z, Wang H, Duan W, Cao W, Li W, Jia Y. Interactive Effects of Precipitation and Nitrogen on Soil Microbial Communities in a Desert Ecosystem. Microorganisms. 2025; 13(6):1393. https://doi.org/10.3390/microorganisms13061393
Chicago/Turabian StyleDong, Qianqian, Zhanquan Ji, Hui Wang, Wan Duan, Wenli Cao, Wenshuo Li, and Yangyang Jia. 2025. "Interactive Effects of Precipitation and Nitrogen on Soil Microbial Communities in a Desert Ecosystem" Microorganisms 13, no. 6: 1393. https://doi.org/10.3390/microorganisms13061393
APA StyleDong, Q., Ji, Z., Wang, H., Duan, W., Cao, W., Li, W., & Jia, Y. (2025). Interactive Effects of Precipitation and Nitrogen on Soil Microbial Communities in a Desert Ecosystem. Microorganisms, 13(6), 1393. https://doi.org/10.3390/microorganisms13061393






