Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus from Different Retail Raw Meats in Shandong, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation, Identification, and Enumeration of S. aureus and MRSA
2.3. Antimicrobial Susceptibility Testing
2.4. Detection of Staphylococcal Enterotoxin Genes
2.5. MLST and Spa Typing
2.6. Statistical Analyses
3. Results
3.1. Prevalence of S. aureus and MRSA
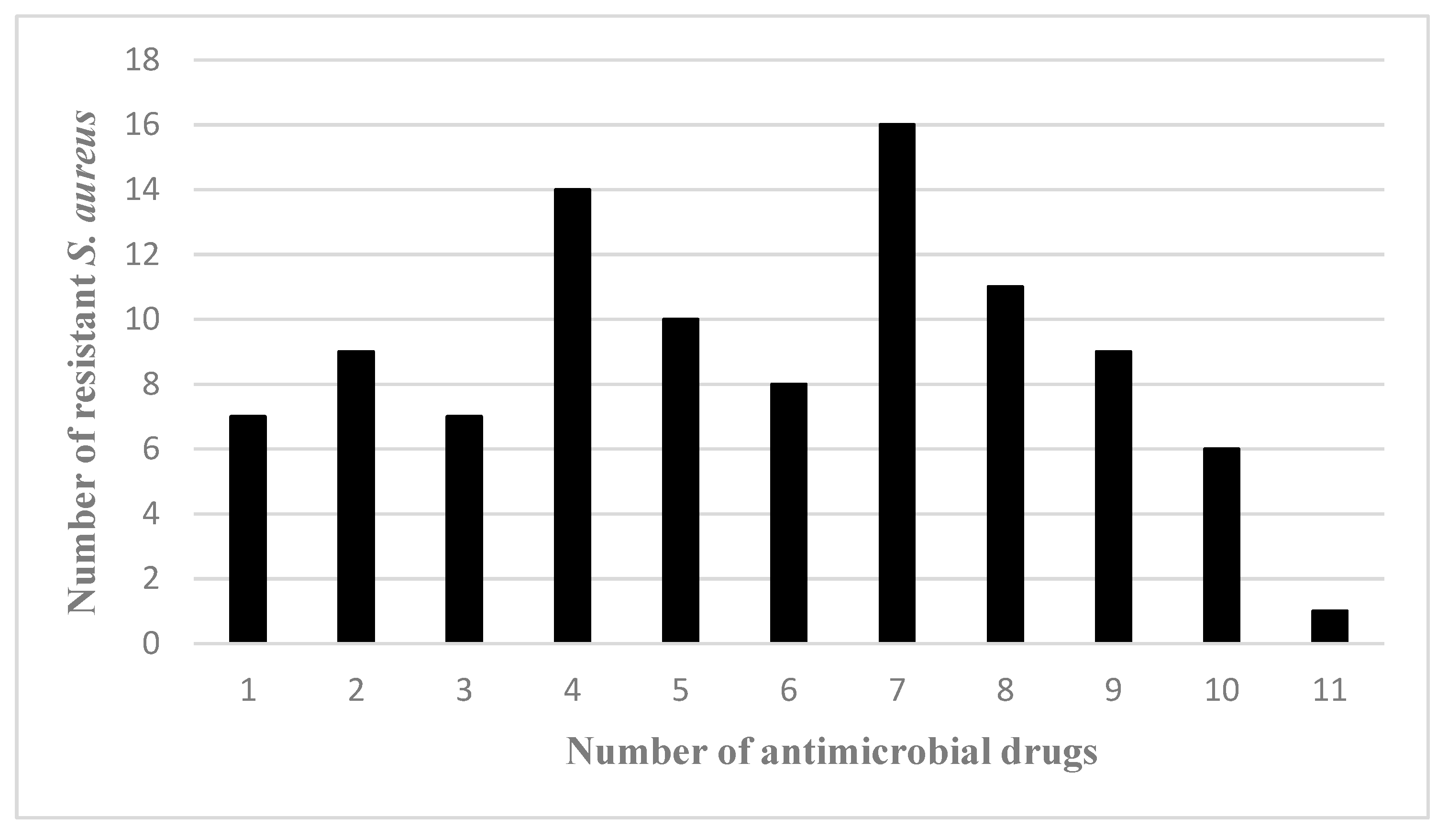
3.2. Antimicrobial Susceptibility Testing
3.3. Virulence Gene Distribution of S. aureus
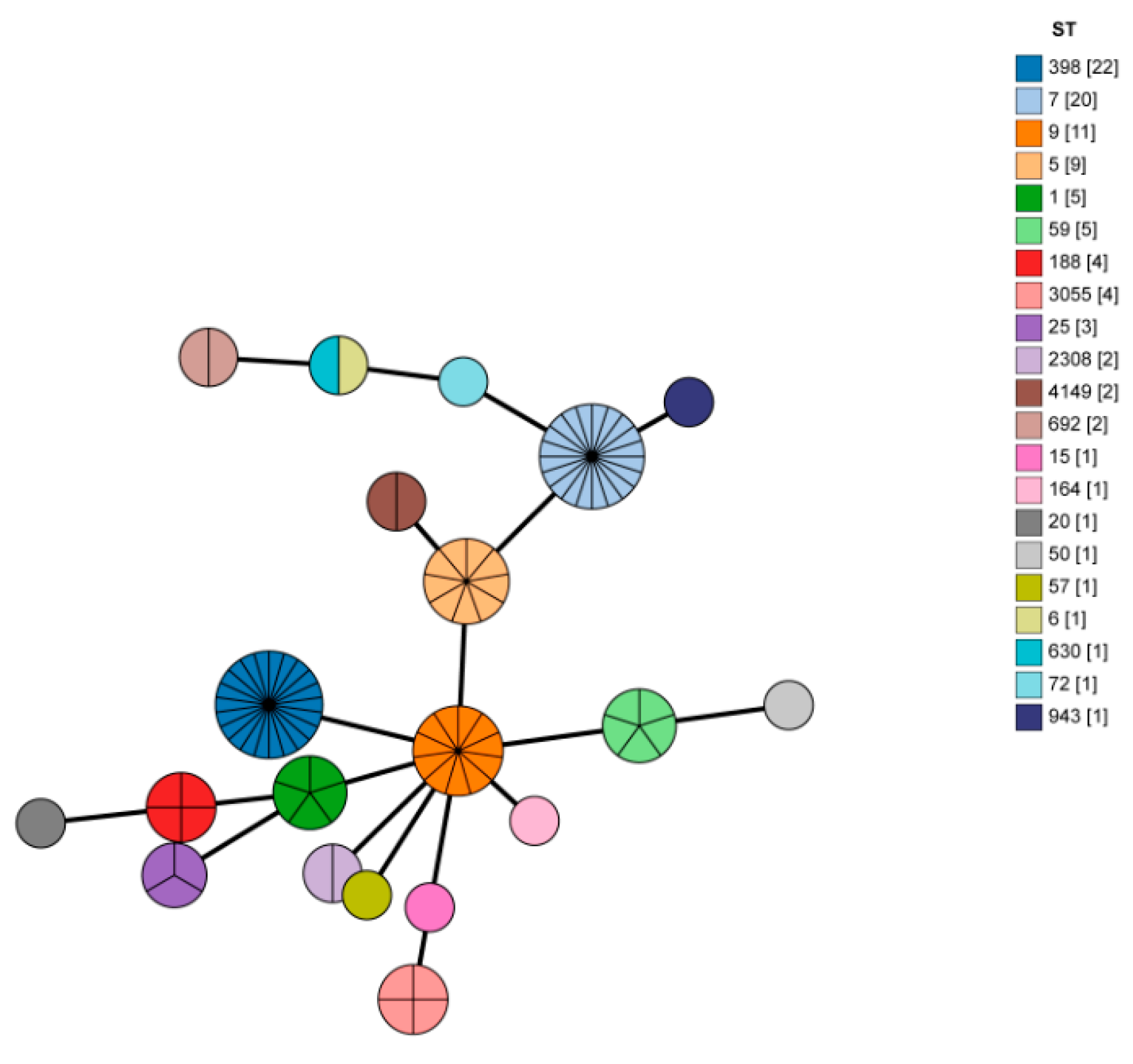
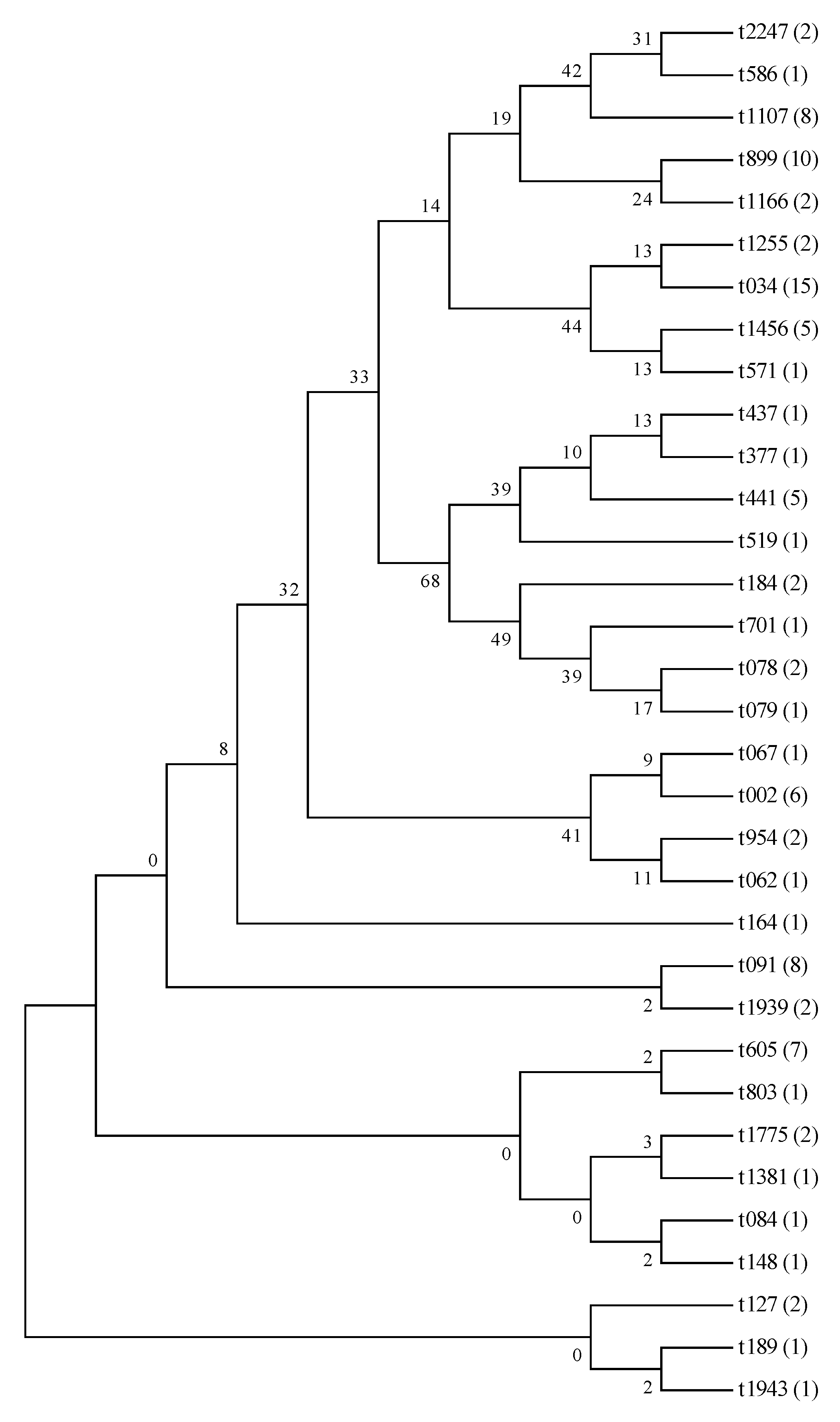
3.4. MLST and Spa Typing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velasco, V.; Vergara, J.; Bonilla, A.; Munoz, J.; Mallea, A.; Vallejos, D.; Quezada-Aguiluz, M.; Campos, J.; Rojas-Garcia, P. Prevalence and characterization of Staphylococcus aureus strains in the pork chain supply in Chile. Foodborne Pathog. Dis. 2018, 15, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Samir, M.; Khalid, I. Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int. J. Food Microbiol. 2021, 346, 109165. [Google Scholar]
- Savariraj, W.; Ravindran, N.; Kannan, P.; Paramasivam, R.; Senthilkumar, T.; Kumarasamy, P.; Rao, V. Prevalence, antimicrobial susceptibility and virulence genes of Staphylococcus aureus isolated from pork meat in retail outlets in India. J. Food Saf. 2018, 39, e12589. [Google Scholar] [CrossRef]
- Ou, C.; Shang, D.; Yang, J.; Chen, B.; Chang, J.; Jin, F.; Shi, C. Prevalence of multidrug-resistant Staphylococcus aureus isolates with strong biofilm formation ability among animal-based food in Shanghai. Food Control 2020, 112, 107106. [Google Scholar] [CrossRef]
- Pauly, N.; Wichmann-Schauer, H.; Ballhausen, B.; Torres Reyes, N.; Fetsch, A.; Tenhangen, B.A. Detection and quantification of methicillin-resistant Staphylococcus aureus in fresh broiler meat at retail in Germany. Int. J. Food Microbiol. 2019, 292, 8–12. [Google Scholar] [CrossRef]
- Zhang, P.; Miao, X.; Zhou, L.; Cui, B.; Zhang, J.; Xu, X.; Wu, C.; Peng, X.; Wang, X. Characterization of oxacillin-susceptible mecA-positive Staphylococcus aureus from food poisoning outbreaks and retail foods in China. Foodborne Pathog. Dis. 2020, 17, 728–734. [Google Scholar] [CrossRef]
- Pal, M.; Ketchakmadze, D.; Durglishvili, N.; Ketchakmadze, I. Staphylococcus aureus: A major pathogen of food poisoning. J. Nutr. Food Process. 2022, 5, 74. [Google Scholar] [CrossRef]
- Cheng, Q.; Kristine, C.; Evelyn, S.; Anthony, M. Enterotoxigenicity and genetic relatedness of Staphylococcus aureus in a commercial poultry plant and poultry farm. Int. J. Food Microbiol. 2022, 363, 109454. [Google Scholar]
- Hennekinne, J.A.; de Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhao, J.; Zhou, L.; Meng, J.; Wang, X. Characterization of Toxin Genes and Antimicrobial Susceptibility of Staphylococcus aureus from Retail Raw Chicken Meat. J. Food Prot. 2018, 81, 528–533. [Google Scholar] [CrossRef]
- Sanlibaba, P. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 2022, 361, 109461. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.P.; Queiroz, M.M.; Gualberto, M.L.; Nascimento, M.S. Klebsiella pneumonia carbapenemase (KPC), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus spp. (VRE) in the food production chain and biofilm formation on abiotic surfaces-sciencedirect. Curr. Opin. Food Sci. 2019, 26, 79–86. [Google Scholar] [CrossRef]
- Sivaraman, G.; Muneeb, K.; Sudha, S.; Shome, B.; Cole, J.; Holmes, M. Prevalence of virulent and biofilm forming ST88-IV-t2526 methicillin-resistant Staphylococcus aureus clones circulating in local retail fish markets in Assam, India. Food Control 2021, 127, 108098. [Google Scholar] [CrossRef]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and antimicrobial sensitivity pattern among patients-a multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef]
- Chung, H.; Kim, Y.; Kwon, J.; Im, H.; Ko, D.; Lee, J.; Choi, S.H. Molecular interaction between methicillin-resistant Staphylococcus aureus (MRSA) and chicken breast reveals enhancement of pathogenesis and toxicity for food-borne outbreak. Food Microbiol. 2021, 93, 103602. [Google Scholar] [CrossRef]
- Lee, G.; Lee, S.; Kim, S.; Park, J.; Kim, G.; Yang, S. Clonal distribution and antimicrobial resistance of methicillin-susceptible and-resistant Staphylococcus aureus strains isolated from broiler farms, slaughterhouses, and retail chicken meat. Poult. Sci. 2022, 101, 102070. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2023, 39, 3555–3577. [Google Scholar] [CrossRef]
- Schoenmakers, K. How China is getting its farmers to kick their antibiotics habit. Nature 2022, 586, 60–62. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Hu, M.; Zhang, Y.; Li, L.; Zhang, Q.; Yuan, X.; Wang, W.; Liu, Y. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from bulk tank milk in Shandong dairy farms. Food Control 2021, 121, 107836. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 33rd ed.; Clinical and Laboratory Standards Institute: Berwyn, IL, USA, 2023. [Google Scholar]
- Van Duijkeren, E.; Ikawaty, R.; Broekhuizen-Stins, M.J.; Jansen, M.D.; Spalburg, E.C.; de Neeling, A.J.; Allaart, J.; Nes, A.; Wagenaar, J.; Fluit, A.C. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet. Microbiol. 2008, 126, 383–389. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, F.; Zhang, J.; Lei, T.; Chen, M.; Ding, Y.; Xue, L. Prevalence and characterization of Staphylococcus aureus isolated from retail vegetables in China. Front. Microbiol. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Chouaib, N.E.H.; Benhamed, N.; Kaas, R.S.; Otani, S.; Benyettou, I.; Bekki, A.; Hansen, E.B. Analysis of genetic signatures of virulence and resistance in foodborne Staphylococcus aureus isolates from Algeria. LWT 2024, 209, 116754. [Google Scholar] [CrossRef]
- Ramadan, H.A.; El-Baz, A.M.; Goda, R.M.; El-Sokkary, M.M.A.; El-Morsi, R.M. Molecular characterization of enterotoxin genes in methicillin-resistant S. aureus isolated from food poisoning outbreaks in Egypt. J. Health Popul. Nutr. 2023, 42, 86. [Google Scholar] [CrossRef] [PubMed]
- Titouche, Y.; Houali, K.; Ruiz-Ripa, L.; Vingadassalon, N.; Nia, Y.; Fatihi, A.; Cauquil, A.; Bouchez, P.; Bouhier, L.; Torres, C.; et al. Enterotoxin genes and antimicrobial resistance in Staphylococcus aureus isolated from food products in Algeria. J. Appl. Microbiol. 2020, 129, 1043–1052. [Google Scholar] [CrossRef]
- Seow, W.; Mahyudin, N.; Syafinaz, A.; Son, R.; Noor, A. Antimicrobial resistance of Staphylococcus aureus among cooked food and food handlers associated with their occupational information in Klang Valley, Malaysia. Food Control 2021, 124, 107872. [Google Scholar] [CrossRef]
- Ghoreyshizadeh, E.; Manouchehrifar, M.; Ramazanzadeh, M.A.M. Occurrence and Characteristics of Toxigenic Staphylococcus aureus in Retail Foods in Iran. Foodborne Pathog. Dis. 2024, 21, 331–338. [Google Scholar] [CrossRef]
- Idrees, M.M.; Saeed, K.; Shahid, M.A.; Akhtar, M.; Qammar, K.; Hassan, J.; Khaliq, T.; Saeed, A. Prevalence of mecA-and mecC-associated methicillin-resistant Staphylococcus aureus in clinical specimens, Punjab, Pakistan. Biomedicines 2023, 11, 878. [Google Scholar] [CrossRef]
- Buyukcangaz, E.; Velasco, V.; Sherwood, J.S.; Stepan, R.M.; Koslofsky, R.J.; Logue, C.M. Molecular typing of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) isolated from animals and retail meat in North Dakota, United States. Foodborne Pathog. Dis. 2013, 10, 608–617. [Google Scholar] [CrossRef]
- Ge, B.; Mukherjee, S.; Hsu, C.H.; Davis, J.A.; Tran, T.T.T.; Yang, Q.; Abbott, J.; Ayers, S.; Young, S.; Crarey, E.; et al. MRSA and multidrug-resistant Staphylococcus aureus in U.S. retail meats, 2010–2011. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, L.; Zhang, J.; Qi, Y.; Huang, B.; She, Z. Prevalence, Antibiotic Resistance, and Molecular Typing of Staphylococcus aureus Isolated from Ready-to-Eat Foods in Guangdong, South China. Foodborne Pathog. Dis. 2025, 22, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Fri, J.; Njom, H.A.; Ateba, C.A.; Ndip, R.N. Antibiotic resistance and virulence gene characteristics of methicillin-resistant Staphylococcus aureus (MRSA) isolated from healthy edible marine fish. Int. J. Microbiol. 2020, 2020, 9803903. [Google Scholar] [CrossRef]
- Adzitey, F.; Ekli, R.; Aduah, M. Incidence and antibiotic susceptibility of Staphylococcus aureus isolated from ready-to-eat meats in the environs of Bolgatanga municipality of Ghana. Cogent Environ. Sci. 2020, 6, 1791463. [Google Scholar] [CrossRef]
- Florianova, M.; Korena, K.; Juricova, H. Whole-genome analysis of methicillin-resistant and methicillin-sensitive Staphylococcus aureus in dry-fermented salami. LWT 2022, 170, 114042. [Google Scholar] [CrossRef]
- Xing, X.; Li, G.; Zhang, W.; Wang, X.; Xia, X.; Yang, B.; Meng, J. Prevalence, antimicrobial susceptibility, and enterotoxin gene detection of Staphylococcus aureus isolates in ready-to-eat foods in Shaanxi, People’s Republic of China. J. Food Prot. 2014, 77, 331–334. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Salmain, M.; Boujday, S. Gold colloid-nanostructured surfaces for enhanced piezoelectric immunosensing of staphylococcal enterotoxin A. Sens. Actuators B Chem. 2018, 255, 1604–1613. [Google Scholar] [CrossRef]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; FabresKlein, M.H.; Mendes, T.A.D.; Ribon, A.O.B. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Grispoldi, L.; Massetti, L.; Sechi, P.; Iulietto, M.F.; Ceccarelli, M.; Karama, M. Characterization of enterotoxin-producing Staphylococcus aureus isolated from mastitis cows. J. Dairy Sci. 2019, 102, 1059–1065. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Cao, Y.; Yan, W.; Niu, X.; Zhou, L.; Chen, J.; Sun, Y.; Li, C.; Zhang, X.; et al. The Distribution of 18 enterotoxins and enterotoxin-like genes in Staphylococcus aureus strains from different sources in East China. Foodborne Pathog. Dis. 2016, 13, 171–176. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, X.; Wang, J.; Hua, D.; You, Y.; Wu, Q.; Ji, Q.; Zhang, J.; Li, L.; Hu, Y.; et al. A food poisoning caused by ST7 Staphylococcal aureus harboring sea gene in Hainan province, China. Front. Microbiol. 2023, 14, 1110720. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Liu, C.M.; Larsen, J.; Soldanova, K.; Aziz, M.; Contente-Cuomo, T.; Petersen, A.; Vandendriessche, S.; Jimenez, J.; Mammina, C.; et al. Rapid differentiation between livestock-associated and livestock independent Staphylococcus aureus CC398 clades. PLoS ONE 2013, 8, e79645. [Google Scholar] [CrossRef]
- Pomorska, K.; Jakubu, V.; Malisova, L.; Fridrichova, M.; Musilek, M.; Zemlickova, H. Antibiotic resistance, spa typing and clonal analysis of methicillin-resistant Staphylococcus aureus (MRSA) isolates from blood of patients hospitalized in the Czech Republic. Antibiotics 2021, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, M.; Zhang, Q.; Li, L.; Zhang, Y.; Luo, Y.; Liu, Y. Whole-Genome Epidemiology and Characterization of MethicillinSusceptible Staphylococcus aureus ST398 from Retail Pork and Bulk Tank Milk in Shandong, China. Front. Microbiol. 2021, 12, 764105. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.J. Detection and phylogeny of Staphylococcus aureus sequence type 398 in Taiwan. J. Biomed. Sci. 2020, 27, 15. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, L.; Zhao, N.; Lv, H.; Liu, Y.; He, L.; Liu, Q. Increasing prevalence of hypervirulent ST5 methicillin susceptible Staphylococcus aureus subtype poses a serious clinical threat. Emerg. Microbes Infect. 2021, 10, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, S.; Lei, T.; Wu, Q.; Zhang, J.; Huang, J.; Dai, J.; Chen, M.; Ding, Y.; Wang, J.; et al. Presence and characterization of methicillin-resistant Staphylococcus aureus co-carrying the multidrug resistance genes cfr and Isa (E) in retail food in China. Int. J. Food Microbiol. 2022, 363, 109512. [Google Scholar] [CrossRef]
- Patel, K.; Godden, S.M.; Royster, E.E.; Crooker, B.A.; Johnson, T.J.; Smith, E.A.; Sreevatsan, S. Prevalence, antibiotic resistance, virulence and genetic diversity of Staphylococcus aureus isolated from bulk tank milk samples of U.S. dairy herds. BMC Genom. 2021, 22, 367. [Google Scholar] [CrossRef]
- Gelbíová, T.; Brodíková, K.; Karpíková, R. Livestock-associated methicillin-resistant Staphylococcus aureus in Czech retailed ready-to-eat meat products. Int. J. Food Microbiol. 2022, 374, 109727. [Google Scholar] [CrossRef]
- Schnitt, A.; Lienen, T.; Wichmann-Schauer, H.; Cuny, C.; Tenhagen, B.A. The occurrence and distribution of livestock-associated methicillin-resistant Staphylococcus aureus ST398 on German dairy farms. J. Dairy Sci. 2020, 103, 11806–11819. [Google Scholar] [CrossRef]
- Luo, K.; Shao, F.; Kamara, K.N.; Chen, S.; Zhang, R.; Duan, G.; Yang, H. Molecular characteristics of antimicrobial resistance and virulence determinants of Staphylococcus aureus isolates derived from clinical infection and food. J. Clin. Lab. Anal. 2018, 32, e22456. [Google Scholar] [CrossRef] [PubMed]
| Sample Type | No of Samples | No S. aureus (%) | No MRSA (%) |
|---|---|---|---|
| Pork | 158 | 37 (23.4) | 6 (3.8) |
| Chicken | 110 | 15 (13.6) | 2 (1.8) |
| Beef | 55 | 10 (18.2) | 1 (1.8) |
| Mutton | 54 | 8 (14.8) | 1 (1.9) |
| Duck | 65 | 17 (26.2) | 1 (1.5) |
| Total | 442 | 87 (19.7) | 11 (2.5) |
| Antimicrobial Class | Antimicrobial Agent | No. of S. aureus (%) | Significance Group 1 |
|---|---|---|---|
| β-Lactams | Penicillin | 94 (95.9) | a |
| Amoxicillin/clavulanic acid | 17 (17.3) | b | |
| Ceftiofur | 9 (9.2) | jkl | |
| Cefotaxime | 2 (2.0) | klm | |
| Oxacillin | 10 (10.2) | ijk | |
| Ampicillin | 81 (82.7) | b | |
| Macrolides | Erythromycin | 61 (62.2) | cd |
| Lincomycin | Clindamycin | 52 (53.1) | cde |
| Pirlimycin | 45 (45.9) | efg | |
| Quinolones | Ciprofloxacin | 25 (25.5) | fgh |
| Sulfonamides | Sulfamethoxazole | 47 (48.0) | def |
| Trimethoprim/sulfamethoxazole | 11 (11.2) | jkl | |
| Glycopeptide | Vancomycin | 0 | m |
| Tetracyclines | Doxycycline | 3 (3.1) | lm |
| Tetracycline | 51 (52.0) | c | |
| Chloramphenicol | Florfenicol | 11 (11.2) | jkl |
| Rifampicin | Rifampicin | 3 (3.1) | lm |
| Aminoglycosides | Gentamicin | 30 (30.6) | fgh |
| Genes | Pork (n = 43) | Chicken (n = 17) | Beef (n = 11) | Mutton (n = 9) | Duck (n = 18) | Total (n = 98) | Percentage (%) |
|---|---|---|---|---|---|---|---|
| sea | 4 | 1 | 0 | 0 | 0 | 5 | 5.1 |
| seb | 4 | 2 | 0 | 1 | 0 | 7 | 7.1 |
| sec | 4 | 2 | 0 | 1 | 1 | 8 | 8.2 |
| sed | 3 | 4 | 0 | 1 | 2 | 10 | 10.2 |
| see | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Hou, B.; Ju, Z.; Wang, W. Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus from Different Retail Raw Meats in Shandong, China. Microorganisms 2025, 13, 1361. https://doi.org/10.3390/microorganisms13061361
Zhao X, Hou B, Ju Z, Wang W. Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus from Different Retail Raw Meats in Shandong, China. Microorganisms. 2025; 13(6):1361. https://doi.org/10.3390/microorganisms13061361
Chicago/Turabian StyleZhao, Xiaonan, Bingyu Hou, Zijing Ju, and Wenbo Wang. 2025. "Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus from Different Retail Raw Meats in Shandong, China" Microorganisms 13, no. 6: 1361. https://doi.org/10.3390/microorganisms13061361
APA StyleZhao, X., Hou, B., Ju, Z., & Wang, W. (2025). Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus from Different Retail Raw Meats in Shandong, China. Microorganisms, 13(6), 1361. https://doi.org/10.3390/microorganisms13061361





