Abstract
Researchers have identified mercury as one of the most toxic environmental pollutants, with deleterious effects on human health and biota. Microorganisms play a key role in the accumulation, degradation, and neutralisation of mercury. Numerous bacteria, fungi, and microalgae possess the mer operon and its homologues, which contain genes responsible for the transport and detoxification of mercury compounds. Mercury-tolerant Microorganisms efficiently convert mercury into less toxic forms. Their tolerance characteristics position them as promising agents for the remediation of ecosystems altered by human activity. This review explores the mechanisms by which microorganisms resist mercury and their potential for biotechnological applications, including eco-friendly and cost-effective bioremediation of mercury-contaminated environments.
1. Introduction
According to WHO, mercury (Hg) is among the top ten most hazardous heavy metals (HM) and belongs to a group of six highly hazardous HMs, which include arsenic (As), cadmium (Cd), selenium (Se), lead (Pb), zinc (Zn), and mercury (Hg) itself. The annual intake of Hg compounds from anthropogenic sources near industrial facilities exceeds natural background levels several times [1].
A plethora of chemical and physical technologies, including adsorption, chemical precipitation, ion exchange, electrochemical treatment, membrane filtration, and reverse osmosis, are employed for the removal of metals from industrial effluents. Nevertheless, these processes are inefficient and economically disadvantageous compared to biological treatment methods [2,3,4]. Bioaccumulation and biosorption processes, based on the use of microorganisms and their enzymatic activity, are recognised as the most promising [5,6,7]. It is universally acknowledged that bioremediation is an effective and environmentally safe method for the remediation of environments contaminated with highly toxic HMs [8].
As with other transition metals, mercury is neither naturally degradable nor biodegradable, which makes its removal from the environment difficult. The restoration of Hg-contaminated ecosystems relies on processes that directly remove Hg, immobilise it, or transform it into harmless forms [9].
It is evident that mercury compounds, even in minute quantities, pose a threat to human health and life. These compounds have been shown to cause damage to the central nervous system, as well as to the respiratory and cardiovascular systems, and to induce DNA mutations, which can ultimately lead to the development of cancerous tumours. The toxicity of mercury is attributable to its capacity to bind to sulfhydryl or disulfide groups in proteins, resulting in the inactivation of enzymes. The clinical manifestations of mercury poisoning, also known as mercurialism, encompass a wide spectrum of symptoms, including irritability, memory impairment, depression, weight loss, fatigue, paranoia, hallucinations, and impaired concentration and attention [10,11,12,13,14]. Furthermore, the bioaccumulation and biomagnification of organic Hg compounds through food webs pose an increased risk to living organisms [15].
Hg readily replaces iron in blood components, such as haemoglobin and other iron-containing molecules, forming strong chemical bonds that may lead to various pathological disorders [16]. In domestic settings, the primary route of human exposure to Hg are through its vapours, while contaminated food and drinking water constitute less significant sources [17].
To mitigate the toxic effects of Hg on living organisms, governments of many countries worldwide have promulgated legislative acts regulating the extraction, treatment, and transport of this hazardous substance. The primary instruments in this regard are the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal [18] and the Minamata Convention on Mercury [19].
Anthropogenic emissions of mercury into the atmosphere have reached 2.500 tons per year and continue to increase steadily [20]. Notably, current Hg concentrations in the atmosphere are 5.5 to 7.6 times higher than natural background levels [21,22]. Mercury exists in three distinct forms in the environment: Elemental (Hg0), inorganic (Hg1+ and Hg2+), and organic (MeHg, CH3Hg+, and CH3HgCH3). Inorganic mercury is ubiquitous in the natural environment, predominantly present in the form of Hg2+. However, reliable data concerning the content of Hg in the form of Hg1+ in the environment are very limited due to the chemical properties of the substance [23]. In aquatic and anaerobic environments, inorganic mercury frequently undergoes methylation, resulting in the formation of the organic form, which is the most toxic to living organisms. Organic mercury exists in two main forms: Monomethyl mercury (MeHg, CH3Hg⁺) and dimethyl mercury (CH3HgCH3). Monomethyl mercury is the most toxic, acting as a potent neurotoxin [24,25]. Elemental mercury (Hg⁰), the least toxic form, can exist as both a liquid and a gas at room temperature and can evaporate from surface water and soil [26].
The primary objective of this review is to investigate the potential of employing microorganisms to inactivate mercury contamination.
2. Sources of Environmental Inputs and Geochemical Cycling of Mercury
The main sources of Hg entering the biosphere are divided into two categories: Natural and anthropogenic. Natural sources include the entire Earth’s crust, rocks, and the surface of the world’s oceans, all containing dispersed Hg compounds. A separate category includes Hg released into the atmosphere during volcanic eruptions [27,28].
Anthropogenic sources of Hg entering the environment can be categorised into three main classes: First, current emissions resulting from the mobilisation of Hg impurities and compounds in raw materials; second, emissions during technological and manufacturing processes; and third, historical emissions, where Hg is released through remobilisation [29]. Historical emissions refer to mercury that was released into the environment in the past and is now re-entering active biogeochemical cycles due to changing conditions (e.g., soil erosion, climate change, economic activities), leading to renewed contamination of the environment. The predominant source of anthropogenic Hg emissions worldwide is fossil fuel combustion [30,31]. The extraction of precious metals also plays a significant role; the mercury amalgamation method is still used in gold mining [27,32]. The predominant form of emissions is Hg0, accounting for more than 90% of the total mercury in the atmosphere [33]. Hg2+ constitutes the majority of mercury in soils, where it is bound to organic compounds, clay, and sulfides [27,34].
The majority of Hg found in the environment is present in the form of inorganic and organomercury compounds, with the exception of atmospheric mercury. The most prevalent compounds are HgCl2, HgOH, and HgS. In addition, CH3HgCl and CH3HgOH are the main organomercury compounds, which, together with others, occur in negligible amounts [35].
The geochemical cycle of mercury plays a key role in providing mercury forms that serve as substrates for microorganisms capable of methylating mercury and converting it into organic compounds such as methylmercury [36]. The cycle involves the anaerobic oxidation of mercury, followed by the incorporation of dissolved Hg²⁺ into the system, a process necessary for the formation of MeHg. The elimination of Hg0 during the reduction process leads to a restricted synthesis of MeHg, attributable to the exhaustion of inorganic mercury substrates [37]. The pathways of mercury reduction in the oxygen-free zones, where the methylation process occurs, have been mainly characterised in laboratory experiments and remain the most difficult to evaluate in the field [38].
3. Microorganisms as Primary Responders to Exposure to Metallic Eco-Pollutants
3.1. Diversity of Mercury-Tolerant Microorganisms
Microorganisms (fungi, microalgae, and bacteria) have great potential to remove metal contaminants by biosorption, bioaccumulation, and biotransformation [39,40,41]. The primary benefits of bioremediation methods include the reduction of secondary contamination, the generation of economic benefits, and the potential for application to heavily HM-contaminated sites [42].
The cycling and transformation of HM in the environment are significantly influenced by microorganisms. In comparison to animals and plants, microorganisms demonstrate heightened sensitivity to metal stress [43,44].
It is well established that HMs, including Hg, are capable of inducing substantial morphological distortions in microbial cells, in addition to exerting an influence on the protein synthesis, lipid metabolism, respiration, and energy metabolism in microorganisms [45,46].
Intensive research into effective methods for preventing mercury contamination began in the 1950s, following the Minamata disaster in 1956. These measures became increasingly widespread as the consequences of the extensive use of fungicides and disinfectants containing organic mercury compounds became more apparent [47,48].
Researchers have shown a marked increase in interest in microorganisms that bioaccumulate mercury recently. Table 1 provides data on the source of isolation and taxonomic affiliation of some microorganisms with the ability to accumulate Hg.

Table 1.
Source of isolation and species composition of Hg-accumulating microorganisms.
The microorganisms studied so far play important roles in mercury cycling and have potential for bioremediation due to their detoxification abilities. However, these represent only a small fraction (Table 1) of the wide diversity of microorganisms investigated in this field. A broad range of microbial groups, differing in physiology and resistance mechanisms, are being studied to better understand how mercury resistance develops and functions across various environments. This diversity highlights the complexity of mercury contamination and the extensive scope of ongoing research aimed at harnessing microbial potential for environmental remediation.
3.2. Mechanisms of Tolerance of Microorganisms to Heavy Metals
In the field of microbiology, there is a growing understanding of the various strategies employed by microorganisms to increase resistance to toxic heavy metals. The most common mechanisms include [79]:
- The synthesis of organic acids and polysaccharides with chelating properties, capable of binding metal ions.
- The process of biosorption occurs at the level of cell walls of microorganisms.
- The intracellular accumulation of metals enables microorganisms to sequester toxic substances.
- The use of cysteine-rich buffer proteins, as these can bind to pollutants and reduce their toxicity.
- The sequestration in vacuoles is an effective method of storing mercury in an inactive metallic form.
- The chemical transformation of metals into less toxic forms.
These mechanisms enable microorganisms to survive in conditions of increased concentrations of toxic HMs, including Hg, and to adapt to extreme habitats. Exposure to low doses of negative factors or harmful substances can trigger the hormesis effect. This phenomenon is defined as the adaptive reactions of organisms to moderate environmental problems, thereby improving their functionality and/or increasing their ability to adapt to more serious influences in the future [80].
Microalgae. Microalgae are highly adaptable microorganisms capable of thriving in rapidly changing environmental conditions. Due to their rapid growth rates and biomass accumulation, microalgae are increasingly used in the development of methods for wastewater treatment targeting ecotoxicants [81].
Algae are characterised by several main mechanisms of tolerance to heavy metals (Table 2). The initial process is passive extracellular adsorption, or biosorption on the cell surface. The subsequent process is active intracellular accumulation, bioaccumulation, and biotransformation.
Microalgae have a high surface area-to-volume ratio, which facilitates HMs binding and accumulation. Passive processes occur through ion exchange, chelation, complexation, redox interactions, biomineralisation, and the deposition of insoluble complexes on their cell walls, extracellular polymeric substances, and intracellular compartments. The primary mechanisms encompass electrostatic, van der Waals, and hydrophobic interactions between the positively charged heavy metal cations and the negatively charged groups on the cell surface [82,83]. The process of biosorption is contingent upon a number of environmental factors, including temperature, solution pH, HM species, the presence of other ions in solution, and biomass [84]. The microorganism can actively take up metals through specific transport mechanisms. This process is accompanied by antioxidant reactions and changes in the redox state of cells [85].
The presence of HMs, including mercury, in aquatic environments can induce microalgae to produce elevated levels of reactive oxygen species (ROS). Excessive ROS generation in microalgal cells causes oxidative stress, leading to damage of vital cellular components such as proteins, lipids, and DNA. This oxidative damage disrupts the photosynthetic apparatus, reduces the activity of key enzymes involved in metabolism, and impairs cell division processes. As a result, the growth, photosynthetic efficiency, and overall viability of microalgae are negatively affected, which can alter their ecological roles and bioremediation potential [86,87].
Fungi. Microscopic fungi are widespread and ecologically important reductants and symbionts in soil and aquatic ecosystems, largely due to their diverse metabolic potential and versatile enzyme systems. In the context of mercury contamination, fungi play a significant role in mercury transformation and detoxification. Their intracellular and extracellular enzymes enable them to transform toxic mercury compounds into less harmful forms through processes such as methylation, demethylation, reduction, and biosorption. These mechanisms allow fungi to immobilize mercury, reduce its bioavailability, and mitigate its toxicity in the environment. Moreover, fungi can form symbiotic relationships with plants, enhancing phytoremediation efficiency by facilitating mercury uptake and tolerance. The ability of fungi to survive and function in mercury-contaminated environments makes them valuable agents for bioremediation strategies aimed at detoxifying mercury-polluted soils and waters [88,89].
One of the earliest cellular responses of microscopic fungi to HM is the generation of ROS and reactive nitrogen species (RNS), which lead to oxidative and/or nitrosative stress and disruption of the redox balance of the cell. Consequently, the antioxidant system, comprising compounds such as peroxidase, superoxide dismutase, and catalase, is activated [90].
The mechanisms of HM detoxification in fungi are categorised into two distinct types. The first involves the secretion of chemical compounds outside the cell to bind the metal in the extracellular space or on the cell wall, rendering it less toxic to the organism. The second type of detoxification is dominated by the chelation of toxic ions in the cytosol, which leads to inactivation and sequestration of heavy metals away from ongoing metabolic processes [91,92].
The bioaccumulation of HM by fungal cells is a complex process involving the interaction with organic acids and polymers, as well as their subsequent transportation to specific intracellular compartments. Cell wall ruptures resulting from metal exposure due to increased membrane permeability cause intracellular uptake and binding [90].
Bacteria. In bacteria, the principal mechanisms of protection and adaptation to HM are realised extracellularly due to alterations in pH and redox potential of the medium, mobilisation of phosphates, or production of polysaccharides, siderophores, and various antioxidant enzymes. Bacterial cells exhibit nonspecific resistance to HM and Hg ions through several known mechanisms. These mechanisms include (1) the presence of an extracellular barrier, (2) the active transport of metal ions out of the cell through ion channels, (3) the binding of biomolecules to the cell surface extracellularly, (4) the binding of biomolecules intracellularly, (5) the reduction of metal ions, and (6) the formation of a biofilm [79,93]. Concurrently, it is notable that a bacterial strain may possess multiple defence mechanisms in a concurrent manner.
The role of bacterial extracellular polymeric substances (EPS), particularly lipopolysaccharides (LPS) and exopolysaccharides (EPSs), in conjunction with environmental response mechanisms and quorum sensing signals, has been demonstrated to be significant in stress survival, bacterial aggregation, and the progression of biofilm development and colonisation. As asserted by Nocelli et al. (2016), nitrogen-fixing species of Sinorhizobium sp. have the capacity to produce EPS, succinoglycan, and galactoglucan, which contribute to their survival under HgCl2 contamination. Furthermore, co-cultivation of EPSs-producing and metal-tolerant strains has been shown to have a protective effect on Hg-sensitive strains that do not produce EPSs [94].
It can be posited that EPS fulfils a protective function in relation to bacterial cells, acting as a barrier against external exposure to HM. These substances have the capacity to bind with toxic ions outside the cells, thereby mitigating the adverse effects of metal cations. Phytoplankton have been demonstrated to influence the chemical composition of trace amounts of Hg2+ in the natural environment through reactions and have been observed to directly absorb Hg2+ through complexation of EPS with Hg2+. In the cyanobacterium Microcystis aeruginosa, there is the potential for cell walls, capsules, and extracellular products (carbohydrates, proteins) to interact with Hg2+ ions, which contributes to an increase in cellular tolerance to Hg [95,96].

Table 2.
Mechanisms of resistance of microorganisms to HM and mercury.
Table 2.
Mechanisms of resistance of microorganisms to HM and mercury.
| Mechanisms of Resistance | Reference |
|---|---|
| Microalgae | |
| Metals are bound and sequestered by cell wall components (e.g., polysaccharides and proteins), and complexes are formed with HM on the cell surface | [97] |
| The expression of specific HM-binding peptides (phytochelatins and metallothioneins) results in the subsequent formation of peptide-HM complexes (phytochelatins and metallothioneins). For instance, phytochelatins form complexes with Hg2+. | [98] |
| Chelation of HM by organic acids, amino acids, and peptides with further formation of insoluble complexes | [99] |
| The synthesis of antioxidant enzymes in response to the presence of reactive oxygen species, including superoxide dismutase, peroxidase, and catalase | [100] |
| The synthesis and activation of non-enzymatic systems, including carotenoids, tocopherol, and glutathione | [101] |
| Overexpression of heat-shock proteins | [102] |
| Expression of genes encoding cation efflux proteins and HM ATPases, including mercury | [103,104] |
| Fungi | |
| The synthesis of chitin and chitosan with amino and hydroxyl groups for the purpose of biosorbing phenolic compounds, dyes, and heavy metals | [105] |
| Decrease in hyphae length and number of branches in response to metal stress. Changes in the distribution of fungal biomass within colonies | [106] |
| Excessive accumulation of hydrolase and oxidase; inhibition of colony growth | [107] |
| Changes in pigment synthesis | [108,109] |
| Synthesis of antioxidant enzymes in response to the presence of reactive oxygen species: Peroxidase, catalase, lignin peroxidase, manganese peroxidase | [110,111] |
| Increased production of HM-dependent ROS and RNS; modifications of RNA, DNA, and protein pools | [110] |
| Biosynthesis of organic acids in response to the presence of HM in the medium, e.g., oxalic, citric, succinic, malic, acetic, and gluconic acids for subsequent binding to HM ions | [112,113] |
| The synthesis of siderophores, which form complexes with HMs such as Cd, copper, Pb, Zn, nickel, and As, prevents these metals from being taken up by the cell | [114,115] |
| The interaction of HM ions with functional groups on the cell surface, including hydroxyl, amide, carboxyl, and phosphate groups | [116] |
| Synthesis of transporters of the ATP-binding cassette (ABC) family involved in intracellular HM transport | [117] |
| Synthesis of specific metal-binding proteins: Glutathione, phytochelatins, and metallothioneins | [118,119,120] |
| Active synthesis of transport proteins in the presence of excessive HM concentration in the medium, transport of HM ions from cytosol to vacuole to prevent toxicity | [121] |
| Bacteria | |
| Metal-tolerant strains precipitate HM ions (Pb2+, Hg2+, Cd2+, etc.) in the form of sulphide granules on the outer surface of cells | [5,122,123] |
| Use of HM ions as thermal acceptors in energy metabolism during anaerobic respiration: Reduction of Fe3+ to Fe2+, reduction of Cr6+ to Cr3+ | [124] |
| Synthesis of surfactants of biological origin | [123] |
| Biofilm formation in response to metal stress. Synthesis of polymeric compounds to bind metal ions and prevent their entry into the complex and cells | [125] |
| Regulation of cell wall fluidity and permeability in response to changes in external conditions depending on the culture medium, the presence of toxic compounds in the medium and various stresses | [8,126] |
| Bioaccumulation of HM inside the cell through channel systems | [127] |
| Synthesis of siderophores—low molecular weight chelating compounds | [128,129] |
It is evident that soil microorganisms demonstrate a high level of tolerance to HM. This tolerance is likely the result of evolutionary adaptation to contaminated environments.
3.3. Mechanisms of Bacterial Cell Resistance to Mercury
To date, research has focused on the adaptation mechanisms exhibited by bacteria in response to Hg exposure. Hg-tolerant bacteria are responsible for three major biological transformations of Hg: (1) Reduction of Hg2+ to metallic Hg0, (2) methylation of Hg2+ to MeHg, and (3) demethylation of MeHg to CH4 and Hg2+ with further volatilization of Hg0 [130].
3.3.1. Nonspecific Resistance
The cellular mechanisms of resistance to Hg most often include (Figure 1) a decrease in the uptake of mercury ions from the medium, transport of mercury ions out of the cell, extracellular sequestration, and bioaccumulation [29]. The presence of unique physiological properties in certain groups of mercury-resistant bacteria increases their bioremediation potential.
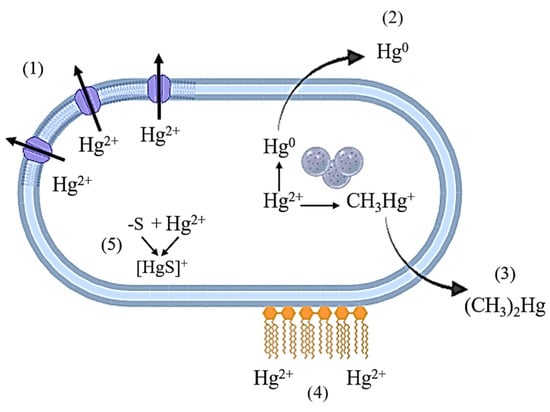
Figure 1.
Mechanisms of resistance of bacterial cells to mercury compounds. 1—transport of Hg2+ ions from the cell through ion channels; 2—reduction to elemental Hg0; 3—methylation to volatile compounds; 4—extracellular binding to biomolecules on the cell surface; 5—formation of insoluble compounds with subsequent deposition in the cell.
The antioxidant system of the organism, especially in bacteria lacking the Hg resistance operon, is also involved in reducing the toxic effects of Hg on bacterial cells [131,132]. Gram-negative anaerobic bacteria Shewanella oneidensis demonstrate heightened sensitivity to Hg2+ ions under aerobic conditions compared to those involving fumarate reduction. The sensitivity of aerobic cells to mercury is attributable to metallic damage to the cell membrane rather than to damage of intracellular components. In this instance, mercury enters the cell and is deactivated by binding to glutathione. Under aerobic conditions, less mercury enters the cell, glutathione content is not reduced, and lipids are damaged [132]. This supports the notion that the components most sensitive to mercury may not be intracellular targets but rather macromolecules located on the cell membrane.
Bioremediation, in the context of heavy metal exposure to microbial cells, involves utilizing the ability of microorganisms to survive, transform, and neutralize toxic metals to remediate contaminated ecosystems. The process of bioremediation occurs through various mechanisms, including:
- Redox processes and alkylation. The process of bacterial methylation of Hg is contingent upon a number of environmental factors that affect metal bioavailability and microbial community structure. These factors include temperature, pH, redox potential, availability of nutrients and electron acceptors, as well as the presence of ligands and adsorbing surfaces [133]. In numerous systems, sulfate-reducing bacteria [66,67,134,135,136] and iron-reducing bacteria [137,138] have been observed to act as microbiological methylators of mercury. The biochemical mechanism of mercury methylation by these bacteria involves two pathways: One acetyl-CoA–dependent and one independent [139].
- Passive adsorption. The adsorption process is not contingent upon the metabolism of the bacterial cell. The metals are located on the cell surface as a consequence of electrostatic interactions, van der Waals forces, covalent binding, changes in the redox potential, or a combination of these processes. The mechanism in question has been explained by a number of processes, including precipitation and surface complexation, ion exchange as the sole dominant role, and physical adsorption [140]. According to the findings of François et al. (2012) [141], in the presence of HgCl2, inactivated bacterial biomass has been shown to remove between 40.0 and 120.0 mg Hg per g biomass dry weight while concurrently forming extracellular Hg deposits.
As previously referenced, bacteria have the capacity to produce EPS that prevents the toxic effects of Hg2+ on the cell [94,95,96]. Furthermore, the uptake of mercury depends on the characteristics of extracellular compounds that bind Hg2+ in the external environment. In this instance, some substances promote the uptake of mercury ions into the cell and subsequent methylation, while others inhibit both processes [142].
3.3.2. Specific Resistance: mer Operon Genes
Research has identified a predominance of mercury resistance at the molecular level in bacteria [143]. The mer operon is the most extensively studied mechanism responsible for mercury inactivation in bacteria. It originally evolved in geothermal bacteria. Recent studies have indicated the presence of the operon in a variety of bacterial samples, including those derived from aerobic, anaerobic, aquatic, and soil environments [144]. The merB operon gene plays a pivotal role in the conversion, transport, and detoxification of highly toxic organic compounds like Hg. merA, in conjunction with a set of genes encoding membrane transporters, which are typically closely related in the operon, contributes to the deactivation of inorganic compounds [145]. Central to the mer operon is the merA gene, which encodes a flavin-dependent NAD(P)-disulfide oxidoreductase (MerA). This enzyme catalyses the conversion of Hg2+ to Hg0, with the resulting product subsequently diffusing out of the cell [146,147]. The current process is distinct from the majority of other HM detoxification systems in bacteria, which primarily utilise ATPase-based transport systems to facilitate the movement of metal ions across cell membranes or to sequester toxic ions in extracellular structures [148].
MerA confers a narrow spectrum of resistance to inorganic mercury only. MerB, an organomercury lyase encoded by the merB gene, provides a broader spectrum of resistance to mercury. This enzyme catalyses the cleavage of the C–Hg bond in organomercury compounds, thereby enabling the subsequent reduction of Hg2+. The operon under consideration also contains several coding genes that are responsible for the periplasmic protein MerP and inner membrane-spanning proteins MerC, MerE, MerF, MerG, and MerT (Figure 2) [143]. The overall gene expression is regulated by MerR and MerD proteins, which function as a promoter and co-repressor of transcription in the presence and absence of Hg²⁺, respectively [144,145,146,147,148,149,150].
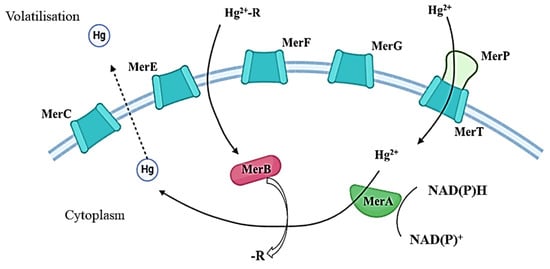
Figure 2.
Main pathways of Hg conversion and transport by Mer family proteins. Hg2+-R—organic compounds; Hg2+—inorganic compounds.
In contrast to many aerobic microorganisms, which recover mercury via a specific enzymatic pathway encoded by the mer operon [151], Hg recovery in anaerobic microorganisms occurs through metabolic pathways associated with anaerobic respiration, fermentation, and anoxygenic photosynthesis [152]. Previously [153,154], before the emergence of contemporary methodologies for identifying methylmercury compounds in various media, a diverse array of aerobic and anaerobic Gram-positive and Gram-negative bacteria, as well as fungi capable of producing this compound, were documented. Recent studies utilising pure cultures have documented the capacity to synthesise methylmercury from inorganic compounds exclusively in sulfate-reducing bacteria (predominantly Desulfovibrio spp. and Desulfobulbus spp.) [135,136,155] and iron-reducing bacteria (predominantly Geobacter spp. and Shewanella spp.) [137,138,156].
Mercury resistance genes encode proteins involved in the uptake and regulation of mercury, as well as the enzymatic reduction of toxic mercury ions (Hg2+) to less toxic elemental mercury (Hg0). These processes facilitate mercury detoxification and influence its mobility and bioavailability in the environment. These enzymes facilitate the accelerated conversion of Hg2+ and, occasionally, MeHg into elemental metallic mercury (Hg0). In 2012, the initial database comprising publicly accessible genomes was assembled. This database encompasses 272 mer operons derived from the genomes of 246 distinct bacterial and archaeal isolates [143,144,157]. Research into mer genes continues, and new data have emerged on mer operons in nitrogen-fixing rhizobia. These discoveries have important implications not only for soil bioremediation but also for host plants growing in mercury-contaminated soils [158].
Phylogenetic analysis of merA homologues was conducted by Barkay et al. in 2010 [151] and demonstrated that the earliest branching of the merA gene originated from thermophilic bacteria (Aquificales), whereas in archaea it was acquired by lateral gene transfer. The complexity of the mer operon structure, attributable to the presence of other genes related to merA in the operon structure, increased over time during the process of additional genetic information being recruited. The transformation resulted in an improved Hg detoxification apparatus with increased efficiency and function by enhancing the spectrum of mercury organic compound detoxification involving the merB gene [143].
mer resistance genes can be localised on mobile genetic elements [130]. The strain Pseudomonas sp. K-62 harbours plasmids pMR26 and pMR68, which provide broad-spectrum resistance to mercury. These plasmids contain three mer gene clusters that confer bacterial resistance to mercury ions and organomercury compounds, encoding the mercury transport system and organomercury lyase [159]. Strain K-62 was isolated from soil contaminated with phenylmercury, exhibiting a tolerance level of 450.0 mg/kg HgCl2, 120.0 mg/kg phenylmercury acetate, and 20.0 mg/kg methylmercury phosphate [48].
One group of microorganisms showing great potential for the purpose of bioremediation of contaminated territories is that of Actinomycetes. Actinomycetes have been shown to possess the capacity for the biodegradation of a wide range of organic and inorganic xenobiotics, as well as a high level of biosorption and bioaccumulation of HM ions. They are identified as playing a dominant role in the processes of natural self-purification in open ecosystems, exhibiting ecological plasticity, and lacking pronounced pathogenic properties [41,76,77,159,160].
It is known that some actinomycete strains tolerant to other HMs form protein complexes that encourage further binding of HMs. These also have ATP-dependent channels that ensure the outflow of toxic HM ions from the cell [76,77,161,162].
A bioinformatic search of the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 17 December 2024) was conducted using keywords associated with mercury resistance (e.g., “mercury resistance genes”, “mer operon”, “actinomycetes”, “Hg resistance”) to identify the most prevalent coding sequences that may underlie the resistance of actinomycetes to elevated concentrations of Hg ions in the growth medium. This tolerance is attributed to the conversion of toxic forms to metallic Hg, which is subsequently expelled from the cell. Information regarding encoded proteins can be found on mobile genetic elements and in the chromosome (Table 3).

Table 3.
The most prevalent mercury resistance genes in Actinomycetes.
A bioinformatic search of the NCBI Protein database (https://www.ncbi.nlm.nih.gov/protein/, accessed on 24 December 2024) allows us to identify the most prevalent proteins in Actinomycetes representatives. The presence of these proteins is indicative of various forms of resistance, the presence of transport systems, and the increasing accumulation of bacterial cells in Hg(II) ions (Table 4).

Table 4.
The most prevalent proteins that determine Hg resistance in Actinomycetes.
As can be observed, the vast majority of Hg resistance proteins and genes are associated with the mer operon, which is consistent with information from the literature.
4. Methods of Hg Removal from Pollution Media
4.1. Physicochemical Methods
Various chemical methods for the removal of mercury from aqueous solutions have been employed experimentally in research laboratories and industrial settings [163,164,165,166]. However, the application of such methodologies for soil and water purification in natural environments is hindered by several challenges. Primarily, these methods require substantial quantities of chemical reagents, which may lead to secondary contamination of environmental sites. Numerous experimental studies have been conducted on the removal of mercury from aqueous solutions. These include methods such as coagulation, filtration, adsorption by activated carbon, ion exchange, chemical reduction, precipitation, and reverse osmosis [27,75,167]. Physicochemical methods have their advantages and disadvantages (Table 5). The most frequent undesirable consequences are the costliness of the methodology, long-time expenditures, and formation of secondary pollutants. Therefore, there is an urgent need to develop advanced, safe, cost-effective, and efficient technologies for Hg neutralisation.

Table 5.
Advantages and disadvantages of physicochemical methods of Hg removal from aqueous solutions.
4.2. Biological Objects Perspective for Hg Removal from Contaminated Media
Microalgae. Microalgae of various species are able to thrive in industrial wastewater, agricultural wastewater, and domestic wastewater that contain elevated concentrations of inorganic and organic substances. As demonstrated in [172], common HMs in wastewater have a significant detrimental effect on the viability of microalgae. The toxicity of HMs to most microalgae species varies significantly; the relative toxicity series is as follows: Hg > Cd > Cu > Zn > Pb > Co > Cr [173,174].
In the development of methodologies for the treatment of wastewater from HM, the green algae Chlorella are frequently utilised (Table 1). In the context of employing C. vulgaris, the efficacy of Hg removal at an initial concentration of 10.0 µg/L Hg in the medium is observed to reach 95% when utilising genetically modified cells, in comparison to the performance of native cells [51]. The use of Chlorella as a dietary supplement leads to a significant increase in the adsorption of mercury from the human body. In this regard, Chlorella has been shown to exhibit considerable potential for metal adsorption, making it a promising candidate for mitigating the toxic effects of mercury compounds [175]. The effective use of microalgae and bacterial consortia has demonstrated significant potential for mercury remediation. For instance, Chlamydomonas has been shown to remove up to 75% of the mercury initially present in solution [176]. The detoxification of wastewater using Chlamydomonas in combination with bacterial consortia represents a promising strategy for reducing environmental pollutant levels [177,178].
The blue-green alga Limnothrix planctonica can transform up to 60.0 µg/kg Hg2+ into a form with special properties—β-HgS. Such transformations have also been observed in the green alga Selenastrum minutum [54]. Under aerobic conditions, Hg2+ is predominantly transformed into β-HgS, a process that occurs in both prokaryotic and eukaryotic algae. This has significant implications for the cycling of Hg compounds in the aquatic environment [54].
Fungi. Recent studies (Table 1) have reported on certain species of fungi that contribute to mercury biovolatilisation [60]. This finding suggests that they may play a role in the biogeochemical cycle of Hg and that they have the potential to remediate contaminated soil using biological means.
The precise mechanisms underlying the transformation of mercury by fungi, as well as their resistance and tolerance to Hg, remain to be fully elucidated. This limitation hampers the potential for the widespread application of fungi in the bioremediation of mercury-contaminated environments. In laboratory experiments, certain species of microscopic fungi have been observed to possess the capacity to accumulate and transform toxic forms of mercury. Examples of such species include Penicillium sp., Aspergillus sp., Rhizopus sp., and Trichoderma sp. [179]. The strain Penicillium spp. DC-F11 was found to have the potential to reduce phytotoxicity of Hg2+ and total and exchangeable Hg in soil up to 26% at an initial Hg concentration of 100.0 mg/kg dry weight [60]. As demonstrated in studies [180,181,182], fungi have the capacity to reduce Hg2+ toxicity through extracellular sequestration by adsorption and deposition. Primary adaptive intracellular responses to Hg2+ exposure have been identified that include merA and merB homologues [183], metabolism of thiol compounds [184], defence against oxidative stress, and metabolism for damage repair [185]. Apparently, fungal resistance to Hg is a consequence of a multisystem process.
Yeasts have demonstrated significant potential for bioremediation. Due to their high degree of self-aggregation and ability to separate from aqueous media, they can be effectively applied in the biological treatment of Hg-contaminated water. As with other bioremediation agents, different yeast species exhibit varying degrees of resistance to toxicants. A comparison of two yeast species revealed that Candida albicans is more tolerant to mercury and can survive at 0.75 µg of HgCl2. It also produces significantly higher amounts of methylmercury (13.0 ng/mL) than Saccharomyces cerevisiae (6.0 ng/mL) [58].
Fungi are capable of intracellular accumulation and deposition of Hg at the cell growth stage. For instance, the Hg-tolerant yeast Yarrowia spp. has been observed to accumulate up to 50% Hg from liquid culture medium at an initial content of 870.0 µg. The majority of the adsorbed Hg is found in the cell wall and spheroplasts. As demonstrated in [63], while there is evidence of trace quantities of Hg being found in the mitochondria, the majority of Hg is found in the nuclei and other organelles of the cell. The aforementioned evidence indicates mercury bioaccumulation.
Bacteria. The ability of bacteria to withstand the toxic effects of Hg forms the basis for bacterial remediation of contaminated environments. In order to survive under conditions of mercury stress, microorganisms have developed a large number of adaptations for Hg detoxification [43,144,186]. As previously stated, bacteria and archaea employ the operon to enzymatically reduce Hg2+ or MeHg to the volatile, less toxic form, Hg0. The development of carriers and consortiums based on Hg-volatilising bacteria is a consequence of the high efficiency of the system [187]. In this process, Hg is removed after reduction to Hg0 by evaporation and collection on porous adsorbents [188].
The development of an effective bioremediation approach can be facilitated by leveraging the recovery mechanism activated by the merA gene. It is important to note that other detoxification methods also exist, namely biosorption and bioaccumulation. Mercury can be both accumulated and taken up by living bacterial cells as well as by dead bacterial biomass [141].
A wide range of Gram-positive and Gram-negative bacteria (Table 1) have a unique system of tolerance and subsequent conversion of toxic forms of Hg to non-toxic forms. It has been demonstrated that their abundance in the medium can increase with increasing Hg levels [186,189].
As well as the mer system, other mechanisms of Hg detoxification have been described. For example, some substances can limit Hg2+ entry into cells [95,190]. The binding of Hg2+ to the endoplasmic reticulum has been found to provide a low level of bacterial tolerance to this type of stress. It was also determined that thiol compounds and the antioxidant system are involved in the mechanism of resistance exhibited by bacterial cells to Hg [191]. Different bacteria can be used in the bioremediation of mercury. Escherichia coli expressing adsorption proteins can remove mercury once the concentration of mercury in a medium reaches a certain level. Subsequent to the adsorption processes, it is possible to remove bacterial cells with adsorbed Hg from the medium by employing various immobilisation strategies [192].
In the development of mercury bioremediation methods, the use of sulfate-reducing bacteria highly adapted to combined exposure to mercury and other heavy metals is promising. As demonstrated in [193], the inorganic reduction of sulfate to H2S by these bacteria can lead to a reduction in the toxicity of heavy metal ions. This, in turn, can lead to a change in the valence of toxic and soluble metals, thereby rendering them immobile and biologically inactive. Other species of sulfate-reducing bacteria have been found to be capable of methylation and demethylation of mercury. The genus Desulfovibrio is distinguished by its capacity to synthesise MeHg. As demonstrated in [67], the level of production varies between different species within the range of 0.14 to 7.30 nM/L−1.
The isolation of Hg-tolerant bacterial strains from soils and water bodies contaminated with HM is of particular concern. α-Proteobacteria demonstrate elevated resistance to Hg, with a minimum inhibitory concentration of 33.5 mg/L. The detection of mercury volatiles, in conjunction with the presence of mercury reductase enzyme, provided evidence that Proteobacteria have the capacity to absorb Hg [73].
Bacillus cereus and Drepanocladus revolvens species have a high capacity to adsorb Hg and bioaccumulate up to 67.0 mg mercury/g dry biomass under aqueous conditions [64]. Some aerobic bacteria and archaea, for example, B. cereus and Pseudomonas putida, have been observed to reduce soluble Hg⁺ ions to elemental Hg0 using the MerA protein [194]. At the same time, an operon present on the VS1 plasmid confers resistance to 20.0 μM Hg2+ to P. aeruginosa, which further promotes reduction processes to metallic Hg [74]. P. putida V1 has the ability to convert HgCl2 to gaseous Hg0 and degrade MeHg, thimerosal, and phenylmercury acetate [75]. Furthermore, the capacity of certain bacterial strains to demethylate methylmercury has been demonstrated. This process significantly reduces the risk of bioaccumulation at subsequent parts of the food chain [195].
Cyanobacteria are also involved in Hg2+ biotransformation processes. Analyses have revealed the presence of free Hg2+ ions and their complexes, as well as metacinnabar (β-HgS), which constitutes the main biotransformed mercury pool associated with bacterial cells. Such products have been detected during transformation in species such as Limnothrix planctonica (Lemm.), Synechococcus leopoldiensis (Racib.) Komárek, and Phormidium limnetica (Lemm.). In the initial phases of Hg exposure, there is a rapid synthesis of β-HgS and Hg0 among the representatives. The available data suggest that cyanobacteria located at the water-air interface are capable of converting substantial quantities of Hg2+ into β-HgS within the environment [71].
Furthermore, the presence of aerobic bacteria has been demonstrated to facilitate the detoxification of both inorganic Hg and methylmercury compounds. For instance, the soil aerobic nitrogen-fixing bacterium Xanthobacter autotrophicus exhibits a tolerance to 0.04 mg/L−1 Hg2+ and 0.01 mg/L−1 MeHg. The bacterium has been shown to be capable of removing mercury compounds from the medium through a process of reduction and volatilization of Hg0 [78].
Toxic metal exposure can harm microbial communities in water and soil, hinder the remediation of contaminated environments, and promote the proliferation of antibiotic-resistant pathogens [196,197]. In this regard, the study of mercury tolerance in the main antibiotic producers is of extreme relevance. Among the Streptomyces representatives, strains exhibiting resistance to HgCl2 at a concentration of 1.0 mM and phenylmercury acetate have been identified [198,199]. The study, along with others in the field, corroborates the emergence of tolerance as a consequence of the prolonged impact of HM on soil microbiota, in conjunction with the selection of resistance to antibiotics [200,201].
It is imperative to study representatives of deep-sea bacteria due to the fact that the process of Hg methylation occurs under oxygen-free conditions at the bottom of water bodies. For instance, strains of P. stutzeri, Bacillus sp., and Pseudoalteromonas sp. isolated from the central Indian Ocean have increased tolerance to mercury compounds up to 100.0 mg/L as HgCl2 and a high potential for Hg removal from the medium. In the context of a laboratory setting, the isolates have been observed to demonstrate the capacity to eliminate up to 80% of HgCl2 from a culture fluid sample with an initial HgCl2 content of 50.0 mg/L Hg2+ [65].
Actinomycetes of the genus Rhodococcus represent a group of microorganisms with considerable potential for remediation of contaminated areas affected by mercury pollution. As demonstrated in [76], tolerance to mercury in R. erythropolis BD2 is attributable to the location of mercury resistance genes on the plasmid that carries the mer operon [202]. The loss of this plasmid consequently results in the loss of bacterial resistance to Hg ions. Another tolerant strain, R. qingshengii RL1, has been identified relatively recently [77]. R. qingshengii RL1 has been shown to be capable of survival at an Hg concentration of 1.0 mM in nutrient medium [77]. The presence of mercury resistance genes has been identified in this strain, including the coding sequences of the MerR transcription regulator located in the chromosome and a unique alkyl-mercury lyase involved in the degradation of toxic organomercury compounds [77,203].
Some bacterial strains exhibit characteristics of polyresistance to common toxic metals, including cadmium, lead, mercury, and arsenic. The E. coli K-12 strain, which produces the periplasmic protein ZinT, has been demonstrated to bind Ni, Zn, Cd, and Hg and to convert them into less toxic forms [204,205]. In this study, bacterial isolates of Enterobacteriaceae were obtained from the water of Bo Ismail Bay in Algeria. These isolates were found to demonstrate tolerance to several heavy metals, including Zn, Cu, Pb, Hg, and Cd. Concurrently with HM resistance, strains demonstrate tolerance to amoxicillin, imipenem, cefotaxime, tetracycline, gentamicin, ciprofloxacin, and trimethoprim-sulfamethoxazole, thereby substantiating the combined resistance of bacteria to diverse toxicants [69]. Nonetheless, the presence of multiple HM ions in the medium gives rise to a competitive interaction with reactive groups on the cell surface [206]. The uptake of Hg can be significantly (more than 50%) reduced in the presence of competing HMs, such as Zn, Cd, and Ni [207]. Research indicates that Hg-methylating bacteria possess the ability to generate methyl lead and methyl cadmium, which are classified as highly toxic organic compounds [208].
These are not all the known representatives of microorganisms that could potentially be used in the development of eco-innovative methods for the remediation and detoxification of mercury wastes from industrial and coastal effluents, as well as from the natural environment. Further research is required to identify the precise molecular mechanisms underlying enhanced mercury tolerance and to optimise growth conditions to maximise the predicted mercury removal potential.
5. Conclusions
There is a long-standing and profoundly serious concern regarding the presence of mercury compounds within open ecosystems. In the aftermath of large-scale environmental disasters, mercury pollution has emerged as a significant threat to living organisms. The heightened toxicity of this pollutant poses a grave and enduring risk to human health and the integrity of the biosphere. The presence of both organic and inorganic Hg compounds in the environment, even in trace amounts, creates the risk of chronic toxic effects on living organisms and threatens the stability of natural ecosystems. It is evident that traditional physical and chemical methods for combating this toxic substance are ineffective and environmentally unsafe. Consequently, researchers are actively developing biotechnological approaches. These approaches utilise active microbial strains or consortia to enhance the detoxification and degradation of pollutants in the environment. These microorganisms possess the capacity to effectively detoxify and inactivate ecopollutants in aquatic and terrestrial ecosystems. An increasing body of research focuses on the fundamental study of mercury’s bioavailability and toxic effects on natural microbial communities. These communities play a primary role in responding to pollutants and accumulating hazardous substances. A comprehensive investigation into the mechanisms of detoxification, biodegradation, and bioremediation of Hg by new strains of bioaccumulators, accompanied by detailed physiological and biochemical characterisation, will facilitate the development of innovative technical solutions for the remediation of contaminated sites. This research will also contribute to the development of environmentally safe biotechnological approaches for the neutralisation and utilisation of hazardous ecotoxicants in the near future. Looking ahead, it is important to prioritise research on the genetic and metabolic enhancement of mercury-resistant microorganisms to improve their detoxification potential. Further studies on microbial community dynamics, horizontal gene transfer, and the development of molecular tools for real-time monitoring will deepen understanding of mercury resistance and transformation. Integration of comprehensive analyses and pilot-scale field studies will be essential to validate and implement effective bioremediation strategies. Combining microbial bioremediation with phytoremediation and physicochemical methods may offer more comprehensive and sustainable solutions for mercury pollution.
Author Contributions
Conceptualization, A.A.G., L.V.L. and I.B.I.; investigation, A.A.G.; data curation, L.V.L.; writing—original draft preparation, A.A.G. and L.V.L.; writing—review and editing, A.A.G., L.V.L. and I.B.I.; project administration, I.B.I.; funding acquisition, I.B.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation [124020500028-4].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WHO | World Health Organization |
| HM | Heavy metal |
| Hg | Mercury |
| As | Arsenic |
| Cd | Cadmium |
| Pb | Lead |
| Zn | Zinc |
| DNA | Deoxyribonucleic acid |
| MeHg | Organomercury/monomethyl |
| ROS | Reactive oxygen species |
| EPS | Extracellular polymeric substances |
| LPS | Particularly lipopolysaccharides |
| EPSs | Exopolysaccharides |
| RNS | Reactive nitrogen species |
| RNA | Ribonucleic acid |
| NCBI | National Center for Biotechnology Information |
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. (JMESS) 2015, 1, 12–18. [Google Scholar]
- Morin-Crini, N.; Crini, G. Eaux Industrielles Contaminées; Presses universitaires de Franche-Comté: Besançon, France, 2017; Chapitre I; pp. 19–39. [Google Scholar] [CrossRef]
- El Baz, S.; Baz, M.; Barakate, M.; Hassani, L.; El Gharmali, A.; Imziln, B. Resistance to and accumulation of heavy metals by actinobacteria isolated from abandoned mining areas. Sci. World J. 2015, 1, 34–48. [Google Scholar] [CrossRef]
- Kumari, S.; Amit; Jamwal, R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Rani, L.; Srivastav, A.L.; Kaushal, J. Bioremediation: An effective approach of mercury removal from the aqueous solutions. Chemosphere 2021, 280, 130654. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Hussein, S.H.; Ruiz, O.N.; Terry, N.; Daniell, H. Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: Enhanced root uptake, translocation to shoots, and volatilization. Environ. Sci. Technol. 2007, 41, 8439–8446. [Google Scholar] [CrossRef]
- Azevedo, B.F.; Furieri, L.B.; Peçanha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simões, M.R.; Fiorim, J.; de Batista, P.R.; Fioresi, M.; Rossoni, L.; et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef]
- Bravo, A.G.; Cosio, C.; Amouroux, D.; Zopfi, J.; Chevalley, P.-A.; Spangenberg, J.E.; Ungureanu, V.-G.; Dominik, J. Extremely elevated methyl mercury levels in water, sediment and organisms in a Romanian reservoir affected by release of mercury from a chloralkali plant. Water Res. 2014, 49, 391–405. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio. 2018, 47, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Eizadi-Mood, N.; Hassanian-Moghaddam, H.; Etemad, L.; Moshiri, M.; Vahabzadeh, M.; Sadeghi, M. Recent advances in the clinical management of intoxication by five heavy metals: Mercury, lead, chromium, cadmium and arsenic. Heliyon 2025, 11, e42696. [Google Scholar] [CrossRef] [PubMed]
- Outridge, P.M.; Mason, R.P.; Wang, F.; Guerrero, S.; Heimbürger-Boavida, L.E. Updated Global and Oceanic Mercury Budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 2018, 52, 11466–11477. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Matulik, A.G.; Kerstetter, D.W.; Hammerschlag, N.; Divol, T.; Hammerschmidt, C.R.; Evers, D.C. Bioaccumulation and biomagnification of mercury and methylmercury in four sympatric coastal sharks in a protected subtropical lagoon. Mar. Pollut. Bull. 2017, 116, 357–364. [Google Scholar] [CrossRef]
- Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal; United Nations Environment Programme: Geneva, Switzerland, 1989; Available online: https://www.basel.int/Portals/4/Basel%20Convention/docs/text/BaselConventionText-r.pdf (accessed on 27 May 2025).
- Minamata Convention on Mercury; United Nations Environment Programme: Geneva, Switzerland, 2013; Available online: https://minamataconvention.org/en/resources/minamata-convention-mercury-text-and-annexes (accessed on 27 May 2025).
- Yang, Z.; Li, H.; Yang, Q.; Qu, W.; Zhao, J.; Feng, Y.; Hu, Y.; Yang, J.; Shih, K. Development of selenized magnetite (Fe3O4−xSey) as an efficient and recyclable trap for elemental mercury sequestration from coal combustion flue gas. Chem. Eng. J. 2020, 394, 125022. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Streets, D.G.; Sunderland, E.M. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Glob. Biogeochem. Cycles 2013, 27, 410–421. [Google Scholar] [CrossRef]
- AMAP/UN Environment. Technical Background Report to the Global Mercury Assessment 2018; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2019. [Google Scholar]
- Ramesh, G.; Radhakrishnan, T. A universal sensor for mercury (Hg, Hg(I), Hg(II)) based on silver nanoparticle-embedded polymer thin film. ACS Appl. Mater. Interfaces 2011, 3, 988–994. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–75. [Google Scholar] [CrossRef]
- You, C.-H.; Kim, B.-G.; Jo, E.-M.; Kim, G.-Y.; Yu, B.-C.; Hong, M.-G.; Kim, D.-S.; Hong, Y.-S. The relationship between the fish consumption and blood total/methyl-mercury concentration of costal area in Korea. Neurotoxicology 2012, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Chávez, R.D.; Ali, A.; Cabaniss, S.E. Mercury in natural waters: A mini-review. Environ. Forensics 2011, 12, 14–18. [Google Scholar] [CrossRef]
- Xu, J.; Bravo, A.G.; Lagerkvis, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Zhang, G.; Chen, X.; Zhao, Q.; Wang, W.; Bian, H.; Li, Z.; Wang, D. Measurement and scaling of mercury on soil and air in a historical artisanal gold mining area in northeastern China. Chin. Geogr. Sci. 2019, 29, 245–257. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Chatterjee, S.; Rath, S.; Dash, H.R.; Das, S. Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation. J. Hazard. Mater. 2022, 423 Pt A, 126985. [Google Scholar] [CrossRef]
- Sherman, L.; Blum, J.D.; Keeler, G.J.; Demers, J.D.; Dvonch, J.T. Investigation of local mercury deposition from a coal-fired power plant using mercury isotopes. Environ. Sci. Technol. 2012, 46, 382–390. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Haroon, M.; Hayyat, M. Assessing the dual impact of gold mining on local communities: Socio-economic benefits and environmental challenges. Resour. Policy 2025, 103, 105559. [Google Scholar] [CrossRef]
- Gonzalez-Raymat, H.; Liu, G.; Liriano, C.; Li, Y.; Yin, Y.; Shi, J.; Jiang, G.; Cai, Y. Elemental mercury: Its unique properties affect its behavior and fate in the environment. Environ. Pollut. 2017, 229, 69–86. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The causes and effects of mercury and methylmercury contamination in the marine environment: A review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- De, J.; Dash, H.R.; Das, S. Mercury pollution and bioremediation—A case study on biosorption by a mercury-resistant marine bacterium. Microb. Biodegrad. Bioremediation 2014, 137–166. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Lean, D.; Loseto, L.; Carignan, R. Effect of dissolved organic carbon on the photoproduction of dissolved gaseous mercury in lakes: Potential impacts of forestry. Environ. Sci. Technol. 2004, 38, 2664–2672. [Google Scholar] [CrossRef]
- Whalin, L.; Kim, E.H.; Mason, R. Factors influencing the oxidation, reduction, methylation and demethylation of mercury species in coastal waters. Mar. Chem. 2007, 107, 278–294. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sanghi, R.; Mudhoo, A. Green practices to save our precious “Water Resource”. In Advances in Water Treatment and Pollution Prevention; Sharma, S.K., Sanghi, R., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 1–36. [Google Scholar] [CrossRef]
- Mortula, M.; Ahmad, A. Leaching of antimony from bottle water. ACSEE 2013, 85–88. [Google Scholar]
- Litvinenko, L.V. Ability of the Dietzia, Gordonia and Rhodococcus actinobacteria to accumulate nickel ions. Microbiology 2019, 88, 191–199. [Google Scholar] [CrossRef]
- Yadav, P.; Bora, J.; Gupta, A.; Raina, G.; Nigam, S.; Chaturvedi, N.; Kumar, A.; Singh, R.P.; Singh, S.K.; Verma, H. Microbial bioremediation of heavy metal approaches and advancement. Adv. Chem. Pollut. Environ. Manag. Prot. 2025, 12, 473–488. [Google Scholar] [CrossRef]
- Oyetibo, G.O.; Miyauchi, K.; Huang, Y.; Chien, M.-F.; Ilori, M.O.; Amund, O.O.; Endo, G. Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutions. Int. Biodeterior. Biodegrad. 2016, 119, 615–620. [Google Scholar] [CrossRef]
- Pátek, M.; Grulich, M.; Nešvera, J. Stress response in Rhodococcus strains. Biotechnol. Adv. 2021, 53, 107698. [Google Scholar] [CrossRef]
- Gauthier, I.; McGugin, R.W.; Richler, J.J.; Herzmann, G.; Speegle, M.; Van Gulick, A.E. Experience moderates overlap between object and face recognition, suggesting a common ability. J. Vis. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Litvinenko, L.V.; Golysheva, A.A.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L. Bioaccumulation of molybdate ions by alkanotrophic Rhodococcus leads to significant alterations in cellular ultrastructure and physiology. Ecotoxicol. Environ. Saf. 2024, 274. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.L.; Polley, D.; Gould, C.J. Phenyl Mercuric or Ethyl Mercuric Compounds. Anal. Chem. 1951, 23, 1286–1299. [Google Scholar] [CrossRef]
- Tonomura, K.; Maeda, K.; Futai, F.; Nakagami, T.; Yamada, M. Stimulative vaporization of phenylmercuric acetate by mercury-resistant bacteria. Nature 1968, 217, 644–646. [Google Scholar] [CrossRef]
- Gómez-Jacinto, V.; García-Barrera, T.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vílchez-Lobato, C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg–phytochelatins. Chem.-Biol. Interact. 2015, 238, 82–90. [Google Scholar] [CrossRef]
- Benítez Alvis, S.; Pérez Cordero, A.; Vitola Romero, D. Removal and recovery of mercury in vitro using immobilized live biomass of Chlorella sp. Indian J. Sci. Technol. 2018, 11, 45. [Google Scholar] [CrossRef]
- Peng, Y.; Deng, A.; Gong, X.; Li, X.; Zhang, Y. Coupling process study of lipid production and mercury bioremediation by biomimetic mineralized microalgae. Bioresour. Technol. 2017, 243, 628–630. [Google Scholar] [CrossRef]
- Samadani, M.; El-Khoury, J.; Dewez, D. Tolerance capacity of Chlamydomonas VHLR mutants for the toxicity of mercury. Water Air Soil Pollut. 2020, 231, 167. [Google Scholar] [CrossRef]
- Lown, L.; Vernaz, J.E.; Dunham-Cheatham, S.M.; Gustin, M.S.; Hiibel, S.R. Phase partitioning of mercury, arsenic, selenium, and cadmium in Chlamydomonas reinhardtii and Arthrospira maxima microcosms. Environ. Pollut. 2023, 329, 121679. [Google Scholar] [CrossRef]
- Kelly, D.; Budd, K.; Lefebvre, D.D. Mercury analysis of acid- and alkaline-reduced biological samples: Identification of meta-cinnabar as the major biotransformed compound in algae. Appl. Environ. Microbiol. 2006, 72, 363–365. [Google Scholar] [CrossRef]
- Thabet, J.; Elleuch, J.; Martínez, F.; Abdelkafi, S.; Hernández, L.E.; Fendri, I. Characterization of cellular toxicity induced by sub-lethal inorganic mercury in the marine microalgae Chlorococcum dorsiventrale isolated from a metal-polluted coastal site. Chemosphere 2023, 338, 139391. [Google Scholar] [CrossRef]
- Vela-García, N.; Guamán-Burneo, M.C.; González-Romero, N.P. Efficient bioremediation from metallurgical effluents through the use of microalgae isolated from the amazonic and highlands of Ecuador. Rev. Int. Contam. Ambient. 2019, 35, 917–929. [Google Scholar] [CrossRef]
- Huang, R.; Huo, G.; Song, S.; Li, Y.; Xia, L.; Gaillard, J.-F. Immobilization of mercury using high-phosphate culture-modified microalgae. Environ. Pollut. 2019, 254 Pt A, 112966. [Google Scholar] [CrossRef]
- Yannai, S.; Berdicevsky, I.; Duek, L. Transformations of inorganic mercury by Candida albicans and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1991, 57, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Hoque, E.; Fritscher, J. A new mercury-accumulating Mucor hiemalis strain EH8 from cold sulfidic spring water biofilms. Microbiologyopen 2016, 5, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Shi, Y.; Si, G.; Yang, Q.; Dong, J.; Chen, J. The bioremediation potentials and mercury(II)-resistant mechanisms of a novel fungus Penicillium spp. DC-F11 isolated from contaminated soil. J. Hazard. Mater. 2020, 396, 122638. [Google Scholar] [CrossRef]
- Cardona, G.I.; Escobar, M.C.; Acosta-González, A.; Marín, P.; Marqués, S. Highly mercury-resistant strains from different Colombian Amazon ecosystems affected by artisanal gold mining activities. Appl. Microbiol. Biotechnol. 2022, 106, 2775–2793. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Khosravi-Darani, K.; Rahimifard, N.; Younesi, H. Assessment of Mercury biosorption by Saccharomyces cerevisiae: Response surface methodology for optimization of low Hg (II) concentrations. J. Environ. Chem. Eng. 2018, 6, 4980–4987. [Google Scholar] [CrossRef]
- Oyetibo, G.O.; Miyauchi, K.; Suzuki, H.; Endo, G. Mercury removal during growth of mercury tolerant and self-aggregating Yarrowia spp. AMB Express 2016, 6, 99. AMB Express 2016, 6, 99. [Google Scholar] [CrossRef]
- Sinha, A.; Pant, K.K.; Khare, S.K. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int. Biodeterior. Biodegrad. 2012, 71, 1–8. [Google Scholar] [CrossRef]
- Joshia, G.; Meena, B.; Verma, P.; Nayak, J.; Vinithkumar, N.V.; Dharani, G. Deep-sea mercury resistant bacteria from the Central Indian Ocean: A potential candidate for mercury bioremediation. Mar. Pollut. Bull. 2021, 169, 112549. [Google Scholar] [CrossRef]
- Ekstrom, E.; Morel, F.; Benoit, J. Mercury methylation independent of the acetyl-coenzyme a pathway in sulfate-reducing bacteria. Appl. Environ. Microbiol. 2003, 69, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Aiken, G.R.; Gilmour, C.C. Effect of dissolved organic matter source and character on microbial Hg methylation in Hg-S-DOM solutions. Environ. Sci. Technol. 2013, 47, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Nonnoi, F.; Chinnaswamy, A.; García de la Torre, V.S.; Coba de la Peña, T.; Lucas, M.M.; Pueyo, J.J. Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. and Trifolium spp.) growing in mercury-contaminated soils. Appl. Soil Ecol. 2012, 61, 49–59. [Google Scholar] [CrossRef]
- Touahir, N.; Alouache, S.; Dehane, D. Assessment and characterization of heavy metals resistance bacteria isolated in Southwestern Mediterranean coastal waters (Bou-Ismail Bay): Impacts of anthropogenic activities. Mar. Pollut. Bull. 2023, 192, 115085. [Google Scholar] [CrossRef]
- Mello, I.S.; Targanski, S.; Pietro-Souza, W.; Stachack, F.F.F.; Terezo, A.J.; Soares, M.A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020, 202, 110818. [Google Scholar] [CrossRef]
- Lefebvre, D.D.; Kelly, D.; Budd, K. Biotransformation of Hg (II) by Cyanobacteria. Appl. Environ. Microbiol. 2007, 73, 244–248. [Google Scholar] [CrossRef]
- Mathew, D.C.; Ho, Y.-N.; Gicana, R.G.; Mathew, G.M.; Chien, M.-C.; Huang, C.-C. A rhizosphere-associated symbiont, Photobacterium spp. strain MELD1, and its targeted synergistic activity for phytoprotection against mercury. PLoS ONE 2015, 10, e0121178. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Megharaj, M. Mercury remediation potential of a mercury resistant strain Sphingopyxis sp. SE2 isolated from contaminated soil. J. Environ. Sci. 2017, 51, 128–137. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A. Characterization of mercury-reducing potential bacteria isolated from Keputih non-active sanitary landfill leachate, Surabaya, Indonesia under different saline conditions. J. Environ. Manag. 2019, 241, 114–118. [Google Scholar] [CrossRef]
- Cabral, L.; Giovanella, P.; Gianello, C.; Bento, F.M.; Andreazza, R.; Camargo, F.A.O. Isolation and characterization of bacteria from mercury contaminated sites in Rio Grande do Sul, Brazil, and assessment of methylmercury removal capability of a Pseudomonas putida V1 strain. Biodegradation 2013, 24, 319–331. [Google Scholar] [CrossRef]
- Dabrock, B.; Kesseler, M.; Averhoff, B.; Gottschalk, G. Identification and characterization of a transmissible linear plasmid from Rhodococcus erythropolis BD2 that encodes isopropylbenzene and trichloroethene catabolism. Appl. Environ. Microbiol. 1994, 60, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, T.; Chowdhury, S.P.; Uhl, J.; Rothballer, M. Genome-based characterization of plant-associated Rhodococcus qingshengii RL1 reveals stress tolerance and plant-microbe interaction traits. Front. Microbiol. 2021, 12, 708605. [Google Scholar] [CrossRef]
- Petrus, A.K.; Rutner, C.; Liu, S.; Wang, Y.; Wiatrowski, H.A. Mercury reduction and methyl mercury degradation by the soil bacterium Xanthobacter autotrophicus Py2. Appl. Environ. Microbiol. 2015, 81, 7834–7836. [Google Scholar] [CrossRef]
- Vanella, R.; Verma, S.K. Co2+, Cu2+ and Zn2+ accumulation by cyanobacterium Spirullina platensis. Biotechnol. Prog. 2006, 22, 1282–1293. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging. Mech. Disease 2017, 3, 13. [Google Scholar] [CrossRef]
- Samal, D.P.K.; Sukla, L.B.; Pattanaik, A.; Pradhan, D. Role of microalgae in treatment of acid mine drainage and recovery of valuable metals. Mater. Today Proc. 2020, 30, 346–350. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Mandotra, S.K.; Kumar, N.; Singh, N.K.; Singh, L.; Rai, U.N. Augmentation of arsenic enhances lipid yield and defense responses in alga Nannochloropsis sp. Bioresour. Technol. 2016, 221, 430–437. [Google Scholar] [CrossRef]
- Danouche, M.; El-Ghachtouli, N.; El-Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Nateras-Ramírez, O.; Martínez-Macias, M.R.; Sánchez-Machado, D.I.; López-Cervantes, J.; Aguilar-Ruiz, R.J. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresour. Technol. Rep. 2022, 17, 100932. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Shi, K.; Gao, Z.; Shi, T.-Q.; Song, P.; Ren, L.-J.; Huang, H.; Ji, X.-J. Reactive oxygen species-mediated cellular stress response and lipid accumulation in oleaginous microorganisms: The state of the art and future perspectives. Front. Microbiol. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; dell’Anno, F.; Sansone, C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Jebapriya, G.R.; Gnanadoss, J.J. Bioremediation of textile dye using white-rot fungi: A review. Int. J. Curr. Res. Rev. 2013, 5, 1–13. [Google Scholar]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microbial. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Goutam, J.; Sharma, J.; Singh, R.; Sharma, D. Fungal-mediated bioremediation of heavy metal–polluted environment. In Microbial Rejuvenation of Polluted Environment; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; pp. 51–76. [Google Scholar] [CrossRef]
- Bellion, M.; Courbot, M.; Jacob, C. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef]
- Siddiquee, S.; Rovina, K.; Azad, S.A. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem Technol. 2015, 7, 384–395. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2315–2318. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of Rhizobia. Materials 2016, 9, 419–423. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, W.-J.; Wu, T.-H.; Hon, C.-L. Effects of extracellular polymeric substances on the bioaccumulation of mercury and its toxicity toward the cyanobacterium Microcystis aeruginosa. J. Environ. Sci. Health Tox. Hazard. Subst. Environ. Eng. 2014, 49, 1371–1375. [Google Scholar] [CrossRef]
- Chen, H.W.; Wu, Y.-Y.; Li, Y.-X.; Huang, W.-J. Methylmercury accumulation and toxicity to cyanobacteria: Implications of extracellural polymeric substances and growth properties. Water Environ. Res. 2014, 86, 627–632. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zheng, Y.; Ge, Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotoxicol. Environ. Saf. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- Barra, L.; Greco, S. The Potential of microalgae in phycoremediation submitted. In Microalgae—Current and Potential Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Shivaji, S.; Dronamaraju, S.V.L. Scenedesnus rotundus isolated from the petroleum effluent employs alternate mechanisms of tolerance to elevated levels of Cadmium and Zinc. Sci. Rep. 2019, 9, 8485. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El-Ghachtouli, N.; El-Baouchi, A.; El-Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Gielen, H.; Remans, T.; Vangronsveld, J.; Cuypers, A. MicroRNAs in metal stress: Specific roles or secondary responses? In. J. Mol. Sci. 2012, 13, 15826–15847. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 8034. [Google Scholar] [CrossRef]
- Chakravorty, M.; Nanda, M.; Bisht, B.; Sharma, R.; Kumar, S.; Mishra, A.; Vlaskin, M.S.; Chauhan, P.K.; Kumar, V. Heavy metal tolerance in microalgae: Detoxification mechanisms and applications. Aquat. Toxicol. 2023, 260. [Google Scholar] [CrossRef]
- Prigione, V.; Zerlottin, M.; Refosco, D.; Tigini, V.; Anastasi, A.; Varese, G.C. Chromium removal from a real tanning effluent by autochthonous and allochthonous fungi. Bioresour. Technol. 2009, 100, 2770–2776. [Google Scholar] [CrossRef]
- Gadd, G.M.; Ramsay, L.; Crawford, J.W.; Ritz, K. Nutritional influence on fungal colony growth and biomass distribution in response to toxic metals. FEMS Microbiol. Lett. 2001, 204, 311–316. [Google Scholar] [CrossRef]
- Cherrad, S.; Girard, V.; Dieryckx, C.; Gonçalves, I.R.; Dupuy, J.-W.; Bonneu, M.; Rascle, C.; Job, C.; Job, D.; Vacher, S.; et al. Proteomic analysis of proteins secreted by Botrytis cinerea in response to heavy metal toxicity. Metallomics 2012, 4, 835–846. [Google Scholar] [CrossRef]
- Lorenzo-Gutiérrez, D.; Gómez-Gil, L.; Guarro, J.; Roncero, M.I.G.; Fernández-Bravo, A.; Capilla, J.; López-Fernández, L. Role of the Fusarium oxysporum metallothionein Mt1 in resistance to metal toxicity and virulence. Metallomics 2019, 11, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Fazli, M.; Soleimani, N.; Mehrasbi, M.; Darabian, S.; Mohammadi, J.; Ramazani, A. Highly cadmium tolerant fungi: Their tolerance and removal potential. J. Environ. Health Sci. Eng. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Azzahra, N.A.; Bingöl, Ö.A.; Izbiańska-Jankowska, K.; Jelonek, T.; Deckert, J.; Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M. Cadmium stress reprograms ROS/RNS homeostasis in Phytophthora infestans (Mont.) de Bary. Int. J. Mol. Sci. 2020, 21, 8375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, G.; Chen, G.; Yan, M.; Chen, A.; Du, J.; Huang, J.; Yi, B.; Zhou, Y.; He, X.; et al. The effect of heavy metal-induced oxidative stress on the enzymes in white rot fungus Phanerochaete chrysosporium. Biotechnol. Appl. Biochem. 2015, 175, 1281–1293. [Google Scholar] [CrossRef]
- Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L.; Vlasov, D. Organic acids induce tolerance to zinc- and copper-exposed fungi under various growth conditions. Curr. Microbiol. 2015, 70, 520–527. [Google Scholar] [CrossRef]
- Fomina, M.A.; Alexander, I.J.; Colpaert, J.V.; Gadd, G.M. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil. Biol. Biochem. 2005, 37, 851–866. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Hong, J.W.; Park, J.Y.; Gadd, G.M. Pyrene degradation and copper and zinc uptake by Fusarium solani and Hypocrea lixii isolated from petrol station soil. J. Appl. Microbiol. 2010, 108, 2030–2040. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Sheng, F.; Qing, H. Adsorption characteristics of Copper (II), Zinc (II) and Mercury (II) by four kinds of immobilized fungi residues. Ecotoxicol. Environ. Saf. 2018, 147, 357–366. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Driessen, A.J.M. Phylogenetic analysis of fungal ABC transporters. BMC Genom. 2010, 11, 177. [Google Scholar] [CrossRef]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S. Differential expression of metallothioneins in response to heavy metals and their involvement in metal tolerance in the symbiotic basidiomycete Laccaria bicolor. Microbiology 2014, 160, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Cho, M.; Hu, G.; Caza, M.; Horianopoulos, L.C.; Kronstad, J.W.; Jung, W.H. Vacuolar zinc transporter Zrc1 is required for detoxification of excess intracellular zinc in the human fungal pathogen Cryptococcus neoformans. J. Microbiol. 2018, 56, 65–71. [Google Scholar] [CrossRef]
- Urai, M.; Aizawa, T.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Neilan, B.; Couperwhite, I.; Nakajima, M.; Sunairi, M. Structural analysis of an extracellular polysaccharide produced by a benzene tolerant bacterium Rhodococcus sp. 33. Carbohydr. Res. 2006, 341, 616–623. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.; Ivshina, I.B. Hydrocarbon and metal-polluted soil bioremediation: Progress and challenges. Microbiol. Aust. 2018, 39, 133–136. [Google Scholar] [CrossRef]
- Faisal, M.; Hasnain, S. Comparative study of Cr (VI) uptake and reduction in industrial effluent by Ochrobacterium intermedium and Brevibacterium sp. Biotechnol. Lett. 2004, 26, 1623–1628. [Google Scholar] [CrossRef]
- Henson, W.R.; Hsu, F.-F.; Dantas, G.; Moon, T.S.; Foston, M. Lipid metabolism of phenol-tolerant Rhodococcus opacus strains for lignin bioconversion. Biotechnol. Biofuels. 2018, 11, 1556–1565. [Google Scholar] [CrossRef]
- Sokolovska, I.; Rozenberg, R.; Riez, C.; Rouxhet, P.G.; Agathos, S.N.; Wattiau, P. Carbon source-induced modifications in the mycolic acid content and cell wall permeability of Rhodococcus erythropolis E1. Appl. Environ. Microbiol. 2003, 69, 7019–7027. [Google Scholar] [CrossRef]
- Ledin, M. Accumulation of metals by microorganisms-processes and importance for soil systems. Earth-Sci. Rev. 2000, 51, 1–31. [Google Scholar] [CrossRef]
- Gomes, A.F.R.; Almeida, M.C.; Sousa, E.; Resende, D.I.S.P. Siderophores and metallophores: Metal complexation weapons to fight environmental pollution. Sci. Total Environ. 2024, 932, 173044. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhiqi, Q.; Tan, H.; Cao, L. Siderophore production by actinobacteria. BioMetals 2014, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Barkay, T.; Miller, S.M.; Summers, A.O. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 2003, 27, 355–384. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Robison, T.; Wiatrowski, H. The impact of ionic mercury on antioxidant defenses in two mercury-sensitive anaerobic bacteria. BioMetals 2013, 26, 1023–1026. [Google Scholar] [CrossRef]
- Podar, M.; Gilmour, C.C.; Brandt, C.C.; Soren, A.; Brown, S.D.; Crable, B.R.; Palumbo, A.V.; Somenahally, A.C.; Elias, D.A. Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Sci. Adv. 2015, 1, e1500675. [Google Scholar] [CrossRef]
- Desrosiers, M.; Planas, D.; Mucci, A. Total mercury and methylmercury accumulation in periphyton of Boreal Shield lakes: Influence of watershed physiographic characteristics. Sci. Total Environ. 2006, 355, 248–250. [Google Scholar] [CrossRef]
- Ranchou-Peyruse, M.; Monperrus, M.; Bridou, R.; Duran, R. Overview of mercury methylation capacities among anaerobic bacteria including representatives of the sulphate-reducers: Implications for environmental studies. Geomicrobiol. J. 2009, 26, 1–8. [Google Scholar] [CrossRef]
- Podar, P.T.; Peyton, K.; Soren, A.; Wilpiszeski, R.L.; Gilmour, C.C.; Elias, D.A.; Podar, M. Complete genome sequence of Desulfobulbus oligotrophicus Prop6, an anaerobic Deltabacterota strain that lacks mercury methylation capability. Microbiol. Resour. Announc. 2021, 10, e00002-21. [Google Scholar] [CrossRef]
- Kerin, E.J.; Gilmour, C.C.; Roden, E.; Suzuki, M.T.; Coates, J.D.; Mason, R.P. Mercury methylation by dissimilatory iron-reducing bacteria. Appl. Environ. Microbiol. 2006, 72, 7919–7921. [Google Scholar] [CrossRef]
- Bravo, A.G.; Peura, S.; Buck, M.; Ahmed, O.; Mateos-Rivera, A.; Ortega, S.H.; Schaefer, J.K.; Bouchet, S.; Tolu, J.; Björn, E.; et al. Methanogens and iron-reducing bacteria: The overlooked members of mercury-methylating microbial communities in Boreal lakes. Appl. Environ. Microbiol. 2018, 84, e01774-18. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yee, N.; Barkay, T. Microbial Transformations in the Mercury Cycle. In Environmental Chemistry and Toxicology of Mercury; Liu, G., Cai, Y., O’Driscoll, N., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 155–191. [Google Scholar]
- Ignatenko, A.V. Analysis of waste waters toxicity and detoxication during their biological treatment. Technol. Elimin. Chem. Hazards 2022, 6, 21–46. [Google Scholar] [CrossRef]
- François, F.; Lombard, C.; Guigner, J.-M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.-L.; Pignol, D.; et al. Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl. Environ. Microbiol. 2012, 78, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.K.; Rocks, S.S.; Zheng, W.; Liang, L.; Gu, B.; Morel, F.M.M. Active transport, substrate specificity, and methylation of Hg (II) in anaerobic bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 8715–8717. [Google Scholar] [CrossRef]
- Boyd, E.S.; Barkay, T. The mercury resistance operon: From an origin in a geothermal environment to an efficient detoxification machine. Front. Microbiol. 2012, 3, 349. [Google Scholar] [CrossRef]
- Christakis, C.A.; Barkay, T.; Boyd, E.S. Expanded diversity and phylogeny of mer genes broadens mercury resistance paradigms and reveals an origin for MerA among thermophilic archaea. Front. Microbiol. 2021, 12, 682605. [Google Scholar] [CrossRef]
- Maynaud, G.; Brunel, B.; Yashiro, E.; Mergeay, M.; Cleyet-Marel, J.-C.; Le Quéré, A. CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a PIB-2-type ATPase involved in cadmium and zinc resistance. Res. Microbiol. 2014, 165, 175–189. [Google Scholar] [CrossRef]
- Dash, H.R.; Sahu, M.; Mallick, B.; Das, S. Functional efficiency of MerA protein among diverse mercury resistant bacteria for efficient use in bioremediation of inorganic mercury. Biochimie 2017, 142, 207–215. [Google Scholar] [CrossRef]
- Fagorzi, C.; Checcucci, A.; diCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Harnessing Rhizobia to improve heavy-metal phytoremediation by legumes. Genes 2018, 9, 542. [Google Scholar] [CrossRef]
- Gionfriddo, C.M.; Stott, M.B.; Power, J.F.; Ogorek, J.M.; Krabbenhoft, D.P.; Wick, R.; Holt, K.; Chen, L.-X.; Thomas, B.C.; Banfield, J.F. Genome-resolved metagenomics and detailed geochemical speciation analyses yield new insights into microbial mercury cycling in geothermal springs. Appl. Environ. Microbiol. 2020, 86, e00176-20. [Google Scholar] [CrossRef]
- Hui, C.Y.; Ma, B.-C.; Hu, S.-Y.; Wu, C. Tailored bacteria tackling with environmental mercury: Inspired by natural mercuric detoxification operons. Environ. Pollut. 2024, 341, 123016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, G.; Kang, X.; Zhang, X.; Guo, J.; Wang, S.; Chen, Y.; Xue, L. Aquatic bacteria rheinheimera tangshanensis new ability for mercury pollution removal. Int. J. Mol. Sci. 2023, 24, 5009. [Google Scholar] [CrossRef] [PubMed]
- Barkay, T.; Kritee, K.; Boyd, E.; Geesey, G. A thermophilic bacterial origin and subsequent constraints by redox, light and salinity on the evolution of the microbial mercuric reductase. Environ. Microbiol. 2010, 12, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, D.S.; Poulain, A.J. A physiological role for Hg (II) during phototrophic growth. Nat. Geosci. 2016, 9, 121–122. [Google Scholar] [CrossRef]
- Wood, J.M.; Kennedy, F.S.; Rosen, C.G. Synthesis of methyl-mercury compounds by extracts of a methanogenic bacterium. Nature 1968, 220, 173–174. [Google Scholar] [CrossRef]
- Hamdy, M.; Noyes, O. Formation of methyl mercury by bacteria. Appl. Microbiol. 1975, 30, 424–432. [Google Scholar] [CrossRef]
- Bridou, R.; Monperrus, M.; Gonzalez, P.R.; Guyoneaud, R.; Amouroux, D. Simultaneous determination of mercury methylation and demethylation capacities of various sulfate-reducing bacteria using species-specific isotopic tracers. Environ. Toxicol. Chem. 2011, 30, 337–344. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, J.; Fan, F.; Wang, S.; Wang, D.; Huang, Y. Effects of redox-modified biochar on mercury reduction and methylation on electron transfer in Geobacter sulfurreducens PCA. Bioresour. Technol. 2025, 427, 132423. [Google Scholar] [CrossRef]
- Sone, Y.; Mochizuki, Y.; Koizawa, K.; Nakamura, R.; Pan-Hou, H.; Itoh, T.; Kiyono, M. Mercurial-resistance determinants in Pseudomonas strain K-62 plasmid pMR68. AMB Express 2013, 3, 41. [Google Scholar] [CrossRef]
- Bhat, A.; Sharma, R.; Desigan, K.; Lucas, M.M.; Mishra, A.; Bowers, R.M.; Woyke, T.; Epstein, B.; Tiffin, P.; Pueyo, J.J.; et al. Horizontal gene transfer of the Mer operon is associated with large effects on the transcriptome and increased tolerance to mercury in nitrogen-fixing bacteria. BMC Microbiol. 2024, 24, 247. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Peshkur, T.A.; Korobov, V.P. Efficient uptake of cesium ions by Rhodococcus cells. Microbiology 2002, 71, 357–361. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Kostina, L.V. Adaptive mechanisms of nonspecific resistance to heavy metal ions in alkanotrophic actinobacteria. Russ. J. Ecol. 2013, 44, 123–130. [Google Scholar] [CrossRef]
- Bell, J.M.L.; Philp, J.C.; Kuyukina, M.S.; Ivshina, I.B.; Dunbar, S.A.; Cunningham, C.J.; Anderson, P. Methods evaluating vanadium tolerance in bacteria isolated from crude oil contaminated land. J. Microbiol. Methods 2004, 58, 87–100. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Turner, R.J.; Zannoni, D.; Cappelletti, M. Processing of metals and metalloids by Actinobacteria: Cell resistance mechanisms and synthesis of metal(loid)-based nanostructures. Microorganisms 2020, 8, 2027. [Google Scholar] [CrossRef]
- Li, Q.; Liu, T.; Deng, P. Recovery of mercury and lead from wastewater by sulfide precipitation-flotation. In Characterization of Minerals, Metals, and Materials, 2nd ed.; Carpenter, J.S., Bai, C., Escobedo, J.P., Hwang, J.-Y., Ikhmayies, S., Li, B., Li, J., Monteiro, S.N., Peng, Z., Zhang, M., Eds.; Springer Cham: Orlando, FL, USA, 2015; pp. 667–674. [Google Scholar] [CrossRef]
- Onaizi, S.A. Simultaneous mercury removal from wastewater and hydrogen sulfide scavenging from sour natural gas using a single unit operation. J. Clean. Prod. 2022, 380, 134900. [Google Scholar] [CrossRef]
- Piao, H.; Bishop, P. Stabilization of mercury-containing wastes using sulfide. Environ. Pollut. 2006, 139, 498–506. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cai, X.-L.; Zhang, Z.-B.; Zhang, L.-B.; Wang, S.-X.; Peng, J.-H. Separation and enrichment of elemental sulfur and mercury from hydrometallurgical zinc residue using sodium sulfide. Trans. Nonferrous Met. Soc. China 2015, 25, 640–646. [Google Scholar] [CrossRef]
- Wagner-Döbler, I. Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl. Microbiol. Biotechnol. 2003, 62, 124–133. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Okoronkwo, N.E.; Igwe, J.C.; Okoronkwo, I.J. Environmental impacts of mercury and its detoxification from aqueous solutions. Afr. J. Biotechnol. 2007, 6, 335–340. [Google Scholar]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.-F.; Lebrun, L. Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J. Membr. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Wang, L.; Liu, F.; Lin, C.-J.; Zhang, L.; Wang, F. Spatial distribution and accumulation of Hg in soil surrounding a Zn/Pb smelter. Sci. Total Environ. 2014, 496, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Mohn, F.H. Use of chlorophyll fluorescence in metal-stress research: A case study with the green microalga Scenedesmus. Ecotoxicol. Environ. Saf. 2003, 55, 64–69. [Google Scholar] [CrossRef]
- Shanmugaprakash, M.; Sivakumar, V. Development of experimental design approach and ANN-based models for determination of Cr(VI) ions uptake rate from aqueous solution onto the solid biodiesel waste residue. Bioresour. Technol. 2013, 148, 550–559. [Google Scholar] [CrossRef]
- Yadav, M.; Rani, K.; Chauhan, M.K.; Panwar, A. Evaluation of mercury adsorption and removal efficacy of pulverized Chlorella (C. vulgaris). J. Appl. Phycol. 2020, 32, 1253–1262. [Google Scholar] [CrossRef]
- do Nascimento, F.H. Dynamic interactions of Hg(II) with the surface of green microalgae Chlamydomonas reinhardtii studied by stripping chronopotentiometry. Algal Res. 2017, 24, 347–353. [Google Scholar] [CrossRef]
- Torres, M.J.; Bellido-Pedraza, C.M.; Llamas, A. Applications of the microalgae chlamydomonas and its bacterial consortia in detoxification and bioproduction. Life 2024, 14, 940. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Simansky, S.; Sanabria, A.; Márová, I.; Garbayo, I.; Vílchez, C. Interaction of naturally occurring phytoplankton with the biogeochemical cycling of mercury in aquatic environments and its effects on global Hg pollution and public health. Microorganisms 2023, 11, 2034. [Google Scholar] [CrossRef]
- Aksu, Z.; Isoglu, I.A. Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Fu, Y.-Q.; Li, S.; Zhu, H.Y.; Jiang, R.; Yin, L.F. Biosorption of copper (II) from aqueous solution by mycelial pellets of Rhizopus oryzae. Afr. J. Biotechnol. 2012, 11, 1403–1411. [Google Scholar] [CrossRef]
- Kumar, R.; Pundir, S.P.; Dhir, B.; Sharma, A. Potential of some fungal and bacterial species in bioremediation of heavy metals. J. Nucl. Phys. Mater. Sci. Radiat. 2014, 1, 213–223. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tang, D.; Dai, J.; Tang, X.; Bao, Y.; Ning, J.; Zhen, Q.; Song, H.; Leger, R.J.S.; Fang, W. Bioremediation of mercury-polluted soil and water by the plant symbiotic fungus Metarhizium robertsii. Proc. Natl. Acad. Sci. USA 2022, 119, e2214513119. [Google Scholar] [CrossRef]
- Kavčič, A.; Mikuš, K.; Debeljak, M.; van Elteren, J.T.; Arčon, I.; Kodre, A.; Kump, P.; Karydas, A.G.; Migliori, A.; Czyzycki, M.; et al. Localization, ligand environment, bioavailability and toxicity of mercury in Boletus spp. and Scutiger pes-caprae mushrooms. Ecotoxicol. Environ. Saf. 2019, 184, 109623. [Google Scholar] [CrossRef]
- Elahi, A.; Rehman, A. Oxidative stress, chromium-resistance and uptake by fungi: Isolated from industrial wastewater. Braz. Arch. Biol. Technol. 2017, 60, 1–14. [Google Scholar] [CrossRef]
- Cruz, K.; Guezennec, J.; Barkay, T. Binding of Hg by bacterial extracellular polysaccharide: A possible role in Hg tolerance. Appl. Microbiol. Biotechnol. 2017, 101, 5–7. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Andrews, S.; Megharaj, M. Mercury toxicity to terrestrial biota. Ecol. Indic. 2017, 74, 451–462. [Google Scholar] [CrossRef]
- Nascimento, A.M.; Chartone-Souza, E. Operon mer: Bacterial resistance to mercury and potential for bioremediation of contaminated environments. Genet. Mol. Res. 2003, 2, 92–101. [Google Scholar]
- Zeng, X.; Tang, J.-X.; Jiang, P.; Liu, H.-W.; Dai, Z.-M.; Liu, X.-D. Isolation, characterizationand extraction of mer gene of Hg2+ resisting strain D2. Trans. Nonferrous Met. Soc. China 2010, 20, 507–512. [Google Scholar] [CrossRef]
- Chen, C.; Yu, H.; Zhao, J.; Li, B.; Qu, L.; Liu, S.; Zhang, P.; Chai, Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Perspect. 2006, 114, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, J.; Hanson, T.E.; Barkay, T.; Boyd, J.M. Superoxide dismutase and pseudocatalase increase tolerance to Hg(II) in Thermus thermophilus HB27 by maintaining the reduced bacillithiol pool. mBio 2019, 10, e00183-19. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Du, P.; Shendi, A.A.I.; Yu, B. Mercury bioremediation in aquatic environment by genetically modified bacteria with self-controlled biosecurity circuit. J. Clean. Prod. 2022, 337, 130524. [Google Scholar] [CrossRef]
- Harithsa, S.; Kerkar, S.; Bharathi, P.A.L. Mercury and lead tolerance in hypersaline sulfate-reducing bacteria. Mar. Pollut. Bull. 2002, 44, 726–732. [Google Scholar] [CrossRef]
- Li, R.; Qi, L.; Ibeanusi, V.; Badisa, V.; Brooks, S.; Chen, C. Reduction and bacterial adsorption of dissolved mercuric ion by indigenous bacteria at the Oak Ridge Reservation site. Chemosphere 2021, 280, 130629. [Google Scholar] [CrossRef]
- Blum, P.W.; Hershey, A.E.; Tsui, M.T.-K.; Hammerschmidt, C.R.; Agather, A.M. Methylmercury and methane production potentials in North Carolina Piedmont stream sediments. Biogeochemistry 2018, 137, 181–195. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef]
- Komijani, M.; Shamabadi, N.S.; Shahin, K.; Eghbalpour, F.; Tahsili, M.R.; Bahram, M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Environ. Pollut. 2021, 274, 116569. [Google Scholar] [CrossRef]
- Putri, W.A.; Rahayu, H.M.; Khasanah, A.U.; Sembiring, L. Identification of mercury-resistant Streptomyces isolated from Cyperus rotundus L. rhizosphere and molecular cloning of mercury (II) reductase gene. J. Biotechnol. 2021, 26, 206–213. [Google Scholar] [CrossRef]
- Undabarrena, A.; Ugalde, J.A.; Seeger, M.; Cámara, B. Genomic data mining of the marine actinobacteria Streptomyces sp. H-KF8 unveils insights into multi-stress related genes and metabolic pathways involved in antimicrobial synthesis. PeerJ 2017, 5, e2912. [Google Scholar] [CrossRef]
- Lin, Y.B.; Wang, X.Y.; Li, H.F.; Wang, N.N.; Wang, H.X.; Tang, M.; Wei, G.-H. Streptomyces zinciresistens sp. nov., a zinc-resistant actinomycete isolated from soil from a copper and zinc mine. Int. J. Syst. Evol. Microbiol. 2011, 61 Pt 3, 616–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahbub, K.R.; King, W.L.; Siboni, N.; Nguyen, V.K.; Rahman, M.M.; Megharaj, M.; Seymour, J.R.; Franks, A.E.; Labbate, M. Long-lasting effect of mercury contamination on the soil microbiota and its co-selection of antibiotic resistance. Environ. Pollut. 2020, 265 Pt B, 115057. [Google Scholar] [CrossRef]
- Hall, J.P.J.; Harrison, E.; Pärnänen, K.; Virta, M.; Brockhurst, M.A. The impact of mercury selection and conjugative genetic elements on community structure and resistance gene transfer. Front. Microbiol. 2020, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.K.; Yagi, J.; Reinfelder, J.R.; Cardona, T.; Ellickson, K.M.; Tel-Or, S.; Barkay, T. Role of the bacterial organomercury lyase (MerB) in controlling methylmercury accumulation in mercury-contaminated natural waters. Environ. Sci. Technol. 2004, 38, 4304–4311. [Google Scholar] [CrossRef]
- David, G.; Blondeau, K.; Renouard, M.; Lewit-Bentley, A. Crystallization and preliminary analysis of Escherichia coli YodA. Acta Crystallogr. D Biol. Crystallogr. 2002, 58 Pt 7, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, C.J.; Brown, N.L.; Hobman, J.L. Zinc dependence of zinT (yodA) mutants and binding of zinc, cadmium and mercury by ZinT. Biochem. Biophys. Res. Commun. 2007, 364, 66–71. [Google Scholar] [CrossRef]
- Murphy, V.; Hughes, H.; McLoughlin, P. Comparative study of chromium biosorption by red, green and brown seaweed biomass. Chemosphere 2008, 70, 1128–1134. [Google Scholar] [CrossRef]
- Plaza, J.; Viera, M.; Donati, E.; Guibal, E. Biosorption of mercury by Macrocystis pyrifera and Undaria pinnatifida: Influence of zinc, cadmium and nickel. J. Environ. Sci. (China) 2011, 23, 1778–1786. [Google Scholar] [CrossRef]
- Pongratz, R.; Heumann, K.G. Production of methylated mercury, lead, and cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere 1999, 39, 89–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).