Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil
Abstract
1. Introduction
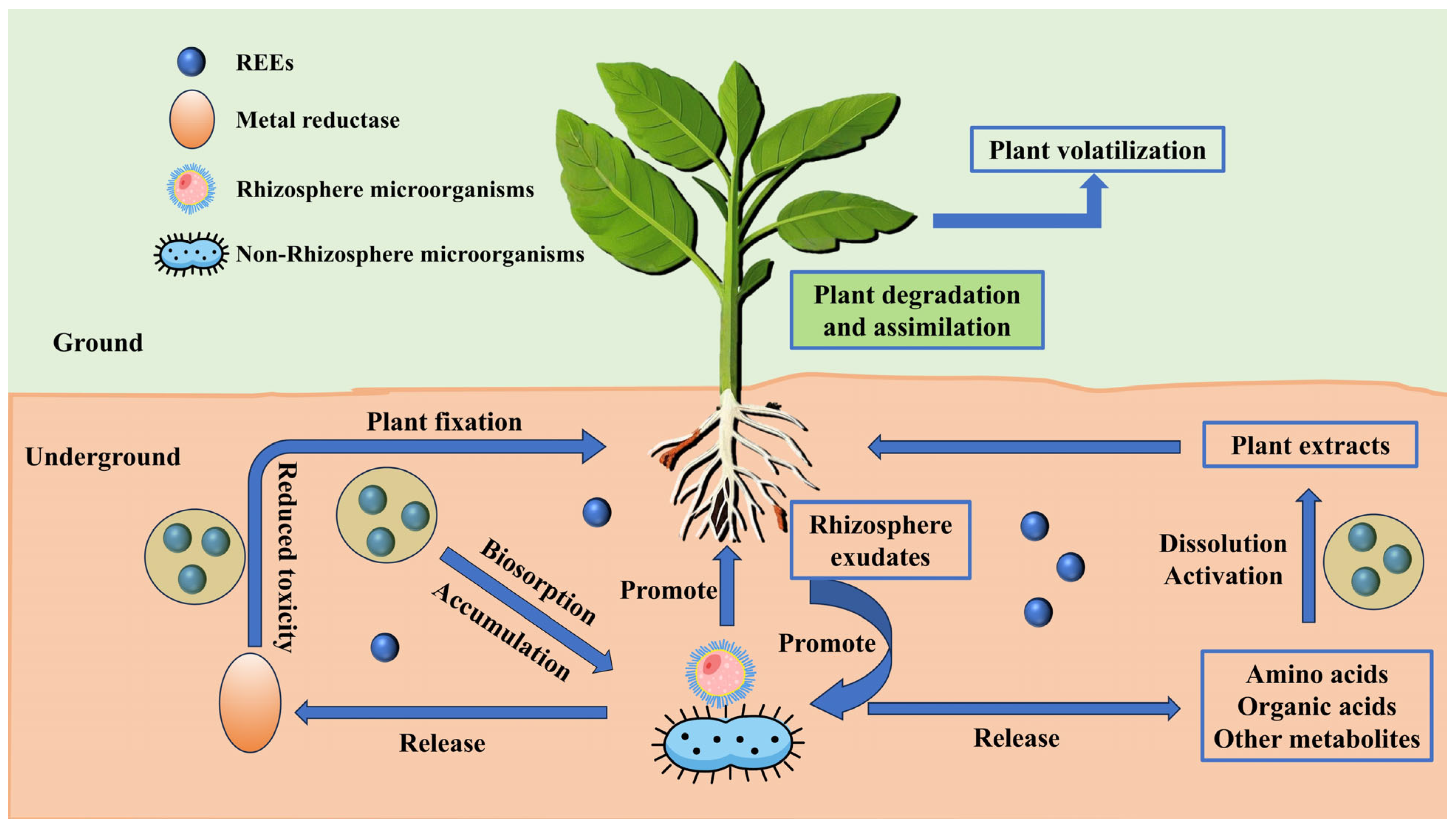
- (i)
- REE Transformation: Metal reductases collaborate with rhizosphere/non-rhizosphere microorganisms to catalyze the transformation of REEs’ chemical forms, regulating their bioavailability.
- (ii)
- Soil Layer Functions: Plants influence REE migration through degradation and fixation, while soil REEs either undergo toxicity reduction before release or diffuse via underground pathways.
- (iii)
- Plant Fixation Mechanisms: Plants accumulate REEs via biosorption, secrete rhizosphere metabolites (e.g., organic acids) to dissolve and activate REEs, and ultimately balance their distribution and toxicity through extraction or release.
- (iv)
- Plant Volatilization: As a core phytoremediation mechanism, plant volatilization not only directly drives the migration and transformation of pollutants within plants, but also enhances the rhizosphere environment and regulates microbial activity through systematic coordination.
2. Environmental Impact of REEs on Soil
3. Interaction Between REEs and Plants
4. Interaction Between REEs and Microorganisms
5. Bioremediation of REE-Contaminated Soil
5.1. Remediation of REE-Contaminated Soil by Plant
5.2. Remediation of REE-Polluted Soil by Microorganisms
5.3. Remediation of REE-Polluted Soil Using the Plant–Microorganism Combined Method
6. Conclusions and Future Prospects
- (i)
- To establish screening criteria for REE hyperaccumulators and decipher key mechanisms, including heavy metal ATPase transporter functions and mycorrhizal interaction dynamics (i.e., Yb/Lu migration pattern in Fagus sylvatica [171]);
- (ii)
- To develop genetically engineered bacteria and a nanomaterial-mediated targeted remediation system to enhance field applicability;
- (iii)
- To quantify the environmental risks of secondary metabolites and establish REE recovery–biomass valorization chains (pyrolysis energy density > 20 MJ/kg).
- (iv)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Q.; Liu, C.; Zhang, X.; Lei, L.; Xiao, C. Selective dissolution and separation of rare earths using guanidine-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2021, 9, 8507–8514. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Wang, D.; Huang, L. Toxic effects of rare earth elements on human health: A Review. Toxics 2024, 12, 317. [Google Scholar] [CrossRef]
- Dang, D.H.; Thompson, K.A.; Ma, L.; Nguyen, H.Q.; Luu, S.T.; Duong, M.T.N.; Kernaghan, A. Toward the circular economy of Rare Earth Elements: A review of abundance, extraction, applications, and environmental impacts. Arch. Environ. Contam. Toxicol. 2021, 81, 521–530. [Google Scholar] [CrossRef]
- Hannington, M.; Jamieson, J.; Monecke, T.; Petersen, S. The abundance of seafloor massive sulfide deposits. Geology 2011, 39, 1155–1158. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L. Recovering rare earth elements from contaminated soils: Critical overview of current remediation technologies. Chemosphere 2021, 265, 129163. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of rare earth elements–a review. Chemosphere 2022, 297, 134259. [Google Scholar] [CrossRef]
- Greenwood, N.N. Chemistry of the Elements; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Teng, D.; Mao, K.; Ali, W.; Xu, G.; Huang, G.; Niazi, N.K.; Feng, X.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC advances 2020, 10, 23221–23232. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.; Khoo, K.S.; Hoang, T.K.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Xu, D.; Fu, R.-; Wang, J.; Shi, Y.; Guo, X. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods-A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, X.; Cao, T.; Tao, L. Advances in research on in situ immobilization of heavy metal in contaminated soil and its effect evaluation. Environ. Sci. Manag. 2016, 41, 98–102. [Google Scholar]
- Kobayashi, K.; Igarashi, Y.; Saito, N.; Higuchi, T.; Sakka, Y.; Suzuki, T.S. Stabilization of the high-temperature phase and total conductivity of yttrium-doped lanthanum germanate oxyapatite. J. Ceram. Soc. Jpn. 2018, 126, 91–98. [Google Scholar] [CrossRef]
- Coey, J. Perspective and prospects for rare earth permanent magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Zhang, S.; Saji, S.E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C.H. Rare-earth incorporated alloy catalysts: Synthesis, properties, and applications. Adv. Mater. 2021, 33, 2005988. [Google Scholar] [CrossRef]
- Zou, D.; Li, H.; Deng, Y.; Chen, J.; Bai, Y. Recovery of lanthanum and cerium from rare earth polishing powder wastes utilizing acid baking-water leaching-precipitation process. Sep. Purif. Technol. 2021, 261, 118244. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, S.; Li, Y.; Wang, T.; Xie, L.; Lu, X. RE (La, Nd and Yb) doped CeO2 abrasive particles for chemical mechanical polishing of dielectric materials: Experimental and computational analysis. Appl. Surf. Sci. 2020, 506, 144668. [Google Scholar] [CrossRef]
- Shi, X.; Cao, B.; Liu, J.; Zhang, J.; Du, Y. Rare-earth-based metal–organic frameworks as multifunctional platforms for catalytic conversion. Small 2021, 17, 2005371. [Google Scholar] [CrossRef] [PubMed]
- Golroudbary, S.R.; Makarava, I.; Kraslawski, A.; Repo, E. Global environmental cost of using rare earth elements in green energy technologies. Sci. Total Environ. 2022, 832, 155022. [Google Scholar] [CrossRef] [PubMed]
- Duchna, M.; Cieślik, I. Rare earth elements in new advanced engineering applications. In Rare Earth Elements-Emerging Advances, Technology Utilization, and Resource Procurement; IntechOpen: London, UK, 2022. [Google Scholar]
- Stratiotou Efstratiadis, V.; Michailidis, N. Sustainable recovery, recycle of critical metals and rare earth elements from waste electric and electronic equipment (circuits, solar, wind) and their reusability in additive manufacturing applications: A review. Metals 2022, 12, 794. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 2002, 9, 143–148. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds-a review. Compos. Part B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Xinde, C.; Xiaorong, W.; Guiwen, Z. Assessment of the bioavailability of rare earth elements in soils by chemical fractionation and multiple regression analysis. Chemosphere 2000, 40, 23–28. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, X.Q. Speciation of rare earth elements in soil and accumulation by wheat with rare earth fertilizer application. Environ. Pollut. 2001, 112, 395–405. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Xu, R.; Ma, X. Study on Plant Diversity of Different Management Period in Rare Earth Elements Mining Area of Fujian Province, South China. J. Anhui Agric. Sci. 2015, 43, 273–275. [Google Scholar]
- Oliveira, J.B.; Biondo, V.; Saab, M.F.; Schwan-Estrada, K.R.F. The use of rare earth elements in the agriculture. Sci. Agrar. Parana. 2014, 13, 171–185. [Google Scholar]
- Li, H.; Jiang, Q.; Li, R.; Zhang, B.; Zhang, J.; Zhang, Y. Passivation of lead and cerium in soil facilitated by biochar-supported phosphate-doped ferrihydrite: Mechanisms and microbial community evolution. J. Hazard. Mater. 2022, 436, 129090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, D.; Zhang, S.; Li, T.; Wang, G.; Xu, X.; Pu, Y.; Nengzi, L. Interaction effects of different chemical fractions of lanthanum, cerium, and fluorine on the taxonomic composition of soil microbial community. BMC Microbiol. 2024, 24, 539. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Yamada, K.; Hanamuro, T.; Tagami, T.; Shimada, K.; Umeda, K. (U-Th)/He thermochronologic analysis of the median tectonic line and associated pseudotachylyte. Geochim. Cosmochim. Acta 2009, 73, A1468. [Google Scholar]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 12483. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Gu, X.; Wang, Y.; Li, K.; Bao, Z. Concentration and fractionation of rare earth elements in soils surrounding rareearth ore area. Rock Miner. Anal. 2019, 38, 137–146. [Google Scholar]
- Kurzawova, V.; Uhlik, O.; Macek, T.; Mackova, M. Interactions of microbes and plants and their importance in fyto/rhizoremediation in pcb contaminated soil. Listy Cukrov. A Řepařské 2010, 126, 396–397. [Google Scholar]
- Bakhshalizadeh, S.; Liyafoyi, A.R.; Mora-Medina, R.; Ayala-Soldado, N. Bioaccumulation of rare earth elements and trace elements in different tissues of the golden grey mullet (Chelon auratus) in the southern Caspian Sea. Environ. Geochem. Health 2023, 45, 6533–6542. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Mahajan, M.; Gambhir, H.; Khan, A.; Khan, M.I.R. Rare earth metallic elements in plants: Assessing benefits, risks and mitigating strategies. Plant Cell Rep. 2024, 43, 216. [Google Scholar] [CrossRef]
- Shi, K.; Liu, C.; Liu, D.; Lyu, K.; Chen, J.; Wang, X. The accumulation and effect of rare earth element neodymium on the root of rice seedlings. Environ. Sci. Pollut. Res. 2021, 28, 48656–48665. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, W.; Huot, H.; Yang, Y.; Guo, M.; Morel, J.L.; Tang, Y.; Qiu, R. Responses of ramie (Boehmeria nivea L.) to increasing rare earth element (REE) concentrations in a hydroponic system. J. Rare Earths 2022, 40, 840–846. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Pan, H.; Bai, Y.; Hu, Y.; Jin, S. Effects of rare earth elements on bacteria in rhizosphere, root, phyllosphere and leaf of soil–rice ecosystem. Sci. Rep. 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad, M.N.V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D. Role of rare earth elements in plants. Plant Mol. Biol. Report. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.-S.; van Der Ent, A.; Morel, J.L.; Zheng, H.-X.; Wang, G.-B.; Tang, Y.-T.; Qiu, R.-L. Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolacca americana in response to soil properties. Chemosphere 2021, 282, 131096. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Addressing lanthanum toxicity in plants: Sources, uptake, accumulation, and mitigation strategies. Sci. Total Environ. 2024, 929, 172560. [Google Scholar] [CrossRef]
- Zheng, H.-X.; Liu, W.-S.; Sun, D.; Zhu, S.-C.; Li, Y.; Yang, Y.-L.; Liu, R.-R.; Feng, H.-Y.; Cai, X.; Cao, Y. Plasma-membrane-localized transporter NREET1 is responsible for rare earth element uptake in hyperaccumulator Dicranopteris linearis. Environ. Sci. Technol. 2023, 57, 6922–6933. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Feng, L.; Chen, Z.; Owens, G.; Chen, Z. Uptake and transport mechanisms of rare earth hyperaccumulators: A review. J. Environ. Manag. 2024, 351, 119998. [Google Scholar] [CrossRef]
- Egler, S.G.; Niemeyer, J.C.; Correia, F.V.; Saggioro, E.M. Effects of rare earth elements (REE) on terrestrial organisms: Current status and future directions. Ecotoxicology 2022, 31, 689–699. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of rare earth elements as fertilizers and feed additives: A knowledge gap analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef]
- Zocher, A.-L.; Klimpel, F.; Kraemer, D.; Bau, M. Assessing the bioavailability of dissolved rare earths and other trace elements: Digestion experiments with aquatic plant species Lemna minor (“duckweed” reference standard BCR-670). Appl. Geochem. 2021, 134, 105025. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of rare earth elements (REEs) and their roles in plant growth: A review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Zhang, X.; Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 2013, 59, 196–200. [Google Scholar] [CrossRef]
- Sun, L.; Xue, C.; Guo, C.; Jia, C.; Li, X.; Tai, P. Regulatory actions of rare earth elements (La and Gd) on the cell cycle of root tips in rice seedlings (Oryza sativa L.). Chemosphere 2022, 307, 135795. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mukherjee, S.; Al-Munqedhi, B.M.; Kumar, R.; Kalaji, H.M. Salicylic acid and silicon impart resilience to lanthanum toxicity in Brassica juncea L. seedlings. Plant Growth Regul. 2023, 100, 453–466. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Zeng, Y.; Shen, X.; He, Y.; Ling, L.; Cao, L.; Fu, X.; Peng, L.; Chun, C. Effect of the rare earth element lanthanum (La) on the growth and development of citrus rootstock seedlings. Plants 2021, 10, 1388. [Google Scholar] [CrossRef]
- Elbasan, F.; Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Rare-earth element scandium improves stomatal regulation and enhances salt and drought stress tolerance by up-regulating antioxidant responses of Oryza sativa. Plant Physiol. Biochem. 2020, 152, 157–169. [Google Scholar] [CrossRef]
- Qi, J. Uptake, distribution and accumulation of rare earth elements in maize and paddy rice plants. J. Southwest Agric. Univ. 2000, 22, 545–548. [Google Scholar]
- He, C.; Feng, Y.; Deng, Y.; Lin, L.; Cheng, S. A systematic review and meta-analysis on the root effects and toxic mechanisms of rare earth elements. Chemosphere 2024, 363, 142951. [Google Scholar] [CrossRef]
- Dong, C.; Jiao, C.; Xie, C.; Liu, Y.; Luo, W.; Fan, S.; Ma, Y.; He, X.; Lin, A.; Zhang, Z. Effects of ceria nanoparticles and CeCl3 on growth, physiological and biochemical parameters of corn (Zea mays) plants grown in soil. NanoImpact 2021, 22, 100311. [Google Scholar] [CrossRef]
- Romero-Freire, A.; González, V.; Groenenberg, J.; Qiu, H.; Auffan, M.; Cotelle, S.; Giamberini, L. Cytotoxicity and genotoxicity of lanthanides for Vicia faba L. are mediated by their chemical speciation in different exposure media. Sci. Total Environ. 2021, 790, 148223. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.S.; Montanha, G.S.; Marques, J.P.R.; Almeida, E.D.; Yabuki, L.N.M.; Menegario, A.A.; Carvalho, H.W.P.D. Foliar application of rare earth elements on soybean (Glycine max (L)): Effects on biometrics and characterization of phytotoxicity. J. Rare Earths 2020, 38, 10. [Google Scholar] [CrossRef]
- d’Aquino, L.; Morgana, M.; Carboni, M.A.; Staiano, M.; Antisari, M.V.; Re, M.; Lorito, M.; Vinale, F.; Abadi, K.M.; Woo, S.L. Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol. Biochem. 2009, 41, 2406–2413. [Google Scholar] [CrossRef]
- d’Aquino, L.; Tommasi, F. Rare earth elements and microorganisms. In Rare Earth Elements in Human and Environmental Health: At Crossroads Between Toxicity and Safety; CRC Press: Boca Raton, FL, USA, 2016; pp. 117–131. [Google Scholar]
- Técher, D.; Grosjean, N.; Sohm, B.; Blaudez, D.; Le Jean, M. Not merely noxious? Time-dependent hormesis and differential toxic effects systematically induced by rare earth elements in Escherichia coli. Environ. Sci. Pollut. Res. 2020, 27, 5640–5649. [Google Scholar] [CrossRef]
- Bayer, M.; Bayer, M. Lanthanide accumulation in the periplasmic space of Escherichia coli B. J. Bacteriol. 1991, 173, 141–149. [Google Scholar] [CrossRef]
- Merroun, M.; Chekroun, K.B.; Arias, J.; Gonzalez-Munoz, M. Lanthanum fixation by Myxococcus xanthus: Cellular location and extracellular polysaccharide observation. Chemosphere 2003, 52, 113–120. [Google Scholar] [CrossRef]
- Li, W.; Zhao, R.; Xie, Z.; Chen, X.; Shen, P. Effects of La3+ on growth, transformation, and gene expression of Escherichia coli. Biol. Trace Elem. Res. 2003, 94, 167–177. [Google Scholar]
- Liu, P.; Liu, Y.; Lu, Z.; Zhu, J.; Dong, J.; Pang, D.; Shen, P.; Qu, S. Study on biological effect of La3+ on Escherichia coli by atomic force microscopy. J. Inorg. Biochem. 2004, 98, 68–72. [Google Scholar]
- Dong, W.; Li, S.; Camilleri, E.; Korza, G.; Yankova, M.; King, S.M.; Setlow, P. Accumulation and release of rare earth ions by spores of Bacillus species and the location of these ions in spores. Appl. Environ. Microbiol. 2019, 85, e00956-19. [Google Scholar] [CrossRef]
- Zhang, X.; Al-Dossary, A.; Hussain, M.; Setlow, P.; Li, J. Applications of Bacillus subtilis spores in biotechnology and advanced materials. Appl. Environ. Microbiol. 2020, 86, e01096-20. [Google Scholar] [CrossRef]
- d’Aquino, L.; Carboni, M.; Woo, S.L.; Morgana, M.; Nardi, L.; Formisano, E.; Lorito, M. Effect of rare earth application on the growth of Trichoderma spp.; several plant pathogenic fungi. J. Plant Pathol. 2004, 86, 316. [Google Scholar]
- Aruguete, D.M.; Aldstadt, J.H., III; Mueller, G.M. Accumulation of several heavy metals and lanthanides in mushrooms (Agaricales) from the Chicago region. Sci. Total Environ. 1998, 224, 43–56. [Google Scholar] [CrossRef]
- Horiike, T.; Yamashita, M. A new fungal isolate, Penidiella sp. strain T9, accumulates the rare earth element dysprosium. Appl. Environ. Microbiol. 2015, 81, 3062–3068. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.; Guo, Y.; Zhang, S.; Chang, L.; Qi, Y.; Li, X.; Liu, J.; Guo, W.; Zhao, J. Comparative insights into influences of co-contamination by rare-earth elements and heavy metals on soil bacterial and fungal communities. J. Soils Sediments 2022, 22, 2499–2515. [Google Scholar] [CrossRef]
- Dou, C.; Lian, B. Role of rock-inhabiting fungi in dissolution, migration and concentration of rare earth elements in dolomite. Bull. Mineral. Petrol. Geochem. 2010, 29, 57–62. [Google Scholar]
- Gao, Y.; Zeng, F.; Yi, A.; Ping, S.; Jing, L. Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol. Trace Elem. Res. 2003, 91, 253–265. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Saxena, G.; Mulla, S. Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2020; Volume II. [Google Scholar]
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Su, R.; Wang, Y.; Huang, S.; Chen, R.; Wang, J. Application for ecological restoration of contaminated soil: Phytoremediation. Int. J. Environ. Res. Public Health 2022, 19, 13124. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, A.; Gan, Y.; Chen, Z.; Wang, X. Advances in bioremediation technologies of contaminated soils by heavy metal in metallic mines. Environ. Sci. Technol. 2010, 33, 106–112. [Google Scholar]
- Diels, L.; De Smet, M.; Hooyberghs, L.; Corbisier, P. Heavy metals bioremediation of soil. Mol. Biotechnol. 1999, 12, 149–158. [Google Scholar] [CrossRef]
- Merten, D.; Grawunder, A.; Lonschinski, M.; Lorenz, C.; Büchel, G. Rare earth element patterns related to bioremediation processes in a site influenced by acid mine drainage. In Proceedings of the IMWA Symposium 2007: Water in Mining environments, Sardinia, Italy, 27–31 May 2007; pp. 233–237. [Google Scholar]
- Feng, A.; Xiao, X.; Ye, C.; Xu, X.; Zhu, Q.; Yuan, J.; Hong, Y.; Wang, J. Isolation and characterization of Burkholderia fungorum Gan-35 with the outstanding ammonia nitrogen-degrading ability from the tailings of rare-earth-element mines in southern Jiangxi, China. AMB Express 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, C.; Gao, L.; Long, J.; Xu, H.; Yang, R. Anomalous concentrations and environmental implications of rare earth elements in the rock-soil-moss system in the black shale area. Chemosphere 2022, 307, 135770. [Google Scholar] [CrossRef]
- Schmoger, M.E.; Oven, M.; Grill, E. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 2000, 122, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Kumar, N.; Singh, N.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I. Basic concepts on heavy metal soil bioremediation. ejmp ep Eur. J. Miner. Process. Environ. Prot. 2003, 3, 58–66. [Google Scholar]
- Adeoye, A.O.; Adebayo, I.A.; Afodun, A.M.; Ajijolakewu, K.A. Benefits and limitations of phytoremediation: Heavy metal remediation review. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 227–238. [Google Scholar]
- Zhuang, G. Current phytoremediation technologies and applications. J. Phys. Conf. Ser. 2023, 2608, 012054. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Rabbani, M.; Rabbani, M.T.; Muthoni, F.; Sun, Y.; Vahidi, E. Advancing phytomining: Harnessing plant potential for sustainable rare earth element extraction. Bioresour. Technol. 2024, 401, 130751. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, M.; Liu, W.; Guo, M.; Zheng, H.; Huot, H.; Jally, B.; Tang, Y.; Laubie, B.; Simonnot, M. Element case studies: Rare earth elements. In Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 471–483. [Google Scholar]
- Liu, W.-S.; van Der Ent, A.; Erskine, P.D.; Morel, J.L.; Echevarria, G.; Spiers, K.M.; Montargès-Pelletier, E.; Qiu, R.-L.; Tang, Y.-T. Spatially resolved localization of lanthanum and cerium in the rare earth element hyperaccumulator fern Dicranopteris linearis from China. Environ. Sci. Technol. 2020, 54, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Morabito, D.; Bourgerie, S. Contrasted tolerance of Agrostis capillaris metallicolous and non-metallicolous ecotypes in the context of a mining technosol amended by biochar, compost and iron sulfate. Environ. Geochem. Health 2021, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Okoroafor, P.U.; Ogunkunle, C.O.; Heilmeier, H.; Wiche, O. Phytoaccumulation potential of nine plant species for selected nutrients, rare earth elements (REEs), germanium (Ge), and potentially toxic elements (PTEs) in soil. Int. J. Phytoremediation 2022, 24, 1310–1320. [Google Scholar] [CrossRef]
- Sergeeva, A.; Zinicovscaia, I.; Grozdov, D.; Yushin, N. Assessment of selected rare earth elements, HF, Th, and U in the Donetsk region using moss bags technique. Atmos. Pollut. Res. 2021, 12, 101165. [Google Scholar] [CrossRef]
- Bouslimi, H.; Dridi, N.; Ferreira, R.; Brito, P.; Caçador, I.; Hidouri, S.; Sleimi, N. Appraisal of the physiological response of Cakile maritima and Brassica juncea for tolerating Lanthanum stress. J. Mar. Sci. Eng. 2023, 12, 65. [Google Scholar] [CrossRef]
- Wood, B.W.; Grauke, L.J. The rare-earth metallome of pecan and other Carya. J. Am. Soc. Hortic. Sci. 2011, 136, 389–398. [Google Scholar] [CrossRef]
- Anawar, H.; Freitas, M.d.; Canha, N.; Dionísio, I.; Dung, H.; Galinha, C.; Pacheco, A. Assessment of bioaccumulation of REEs by plant species in a mining area by INAA. J. Radioanal. Nucl. Chem. 2012, 294, 377–381. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Chen, Z. A review of in situ phytoextraction of rare earth elements from contaminated soils. Int. J. Phytoremediation 2022, 24, 557–566. [Google Scholar] [CrossRef]
- Liu, W.-S.; Zheng, H.-X.; Liu, C.; Guo, M.-N.; Zhu, S.-C.; Cao, Y.; Qiu, R.-L.; Morel, J.L.; van Der Ent, A.; Tang, Y.-T. Variation in rare earth element (REE), aluminium (Al) and silicon (Si) accumulation among populations of the hyperaccumulator Dicranopteris linearis in southern China. Plant Soil 2021, 461, 565–578. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Li, W.; Chen, Z. Effects of clipping intensity on the physiology of dicranopteris pedata and its interroot soil in the rare-earth-mining area in Southern China. Sustainability 2024, 16, 664. [Google Scholar] [CrossRef]
- van der Ent, A.; Nkrumah, P.N.; Purwadi, I.; Erskine, P.D. Rare earth element (hyper) accumulation in some Proteaceae from Queensland, Australia. Plant Soil 2023, 485, 247–257. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, C.; Liu, W.-S.; Guo, M.-N.; Morel, J.L.; Huot, H.; Yu, H.-J.; Tang, Y.-T.; Qiu, R.-L. Accumulation and fractionation of rare earth elements (REEs) in the naturally grown Phytolacca americana L. in southern China. Int. J. Phytoremediation 2018, 20, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ding, T.-X.; Liu, W.-S.; Tang, Y.-T.; Qiu, R.-L. Phosphorus mediated rhizosphere mobilization and apoplast precipitation regulate rare earth element accumulation in Phytolacca americana. Plant Soil 2023, 483, 697–709. [Google Scholar] [CrossRef]
- Jalali, J.; Lebeau, T. The role of microorganisms in mobilization and phytoextraction of rare earth elements: A review. Front. Environ. Sci. 2021, 9, 688430. [Google Scholar] [CrossRef]
- Brito, P.; Mil-Homens, M.; Caçador, I.; Caetano, M. Changes in REE fractionation induced by the halophyte plant Halimione portulacoides, from SW European salt marshes. Mar. Chem. 2020, 223, 103805. [Google Scholar] [CrossRef]
- Brito, P.; Caetano, M.; Martins, M.D.; Caçador, I. Effects of salt marsh plants on mobility and bioavailability of REE in estuarine sediments. Sci. Total Environ. 2021, 759, 144314. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. 13 Innovative techniques utilized for bioremediation of rare earth elements to attain a sustainable world. In Rare Earth Elements: Processing, Catalytic Applications and Environmental Impact; De Gruyter: Berlin, Germany; Boston, MA, USA, 2023; pp. 241–258. [Google Scholar]
- Miclean, M.; Levei, E.A.; Tanaselia, C.; Cadar, O. Rare earth elements transfer from soil to vegetables and health risks associated with vegetable consumption in a former mining area. Agronomy 2023, 13, 1399. [Google Scholar] [CrossRef]
- Khan, A.M.; Yusoff, I.; Abu Bakar, N.K.; Abu Bakar, A.F.; Alias, Y.; Mispan, M.S. Accumulation, uptake and bioavailability of rare earth elements (Rees) in soil grown plants from ex-mining area in Perak, Malaysia. Appl. Ecol. Environ. Res. 2017, 15, 117–133. [Google Scholar] [CrossRef]
- Bibak, A.; Stümp, S.; Knudsen, L.; Gundersen, V. Concentrations of 63 elements in cabbage and sprouts in Denmark. Commun. Soil. Sci. Plant Anal. 1999, 30, 2409–2418. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.; Asher, C. Rare earth elements and plant growth: II. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continuously flowing nutrient solutions. J. Plant Nutr. 1995, 18, 1977–1989. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, N.; Dai, Y.; Ahmad, Z.; Li, Y.; Han, S.; Zhang, H.; Chen, J.; Yang, J. Accumulation and distribution characteristics of rare earth elements (REEs) in the naturally grown marigold (Tagetes erecta L.) from the soil. Environ. Sci. Pollut. Res. 2023, 30, 46355–46367. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, H.; Wang, Y. Study on the contents of trace rare earth elements and their distribution in wheat and rice samples by RNAA. J. Radioanal. Nucl. Chem. 1994, 179, 377–383. [Google Scholar] [CrossRef]
- Xiong, X.; Xiao, J.; Hu, R.; Wang, J.; Wang, Y.; Wang, X.; Deng, Q. Formation and evolution of a paleosol across the lower silurian-lower permian boundary in Zunyi District, Northern Guizhou, China and its paleoenvironment and paleoclimate implications. Acta Geol. Sin.-Engl. Ed. 2015, 89, 2012–2029. [Google Scholar]
- Ozaki, T.; Enomoto, S.; Minai, Y.; Ambe, S.; Makide, Y. A survey of trace elements in pteridophytes. Biol. Trace Elem. Res. 2000, 74, 259–273. [Google Scholar] [CrossRef]
- Jally, B.; Laubie, B.; Chour, Z.; Muhr, L.; Qiu, R.; Morel, J.L.; Tang, Y.; Simonnot, M.-O. A new method for recovering rare earth elements from the hyperaccumulating fern Dicranopteris linearis from China. Miner. Eng. 2021, 166, 106879. [Google Scholar] [CrossRef]
- Mohsin, M.; Salam, M.M.A.; Nawrot, N.; Kaipiainen, E.; Lane, D.J.; Wojciechowska, E.; Kinnunen, N.; Heimonen, M.; Tervahauta, A.; Peräniemi, S. Phytoextraction and recovery of rare earth elements using willow (Salix spp.). Sci. Total Environ. 2022, 809, 152209. [Google Scholar] [CrossRef]
- Wutscher, H.K.; Perkins, R.E. Acid extractable rare earth elements in Florida citrus soils and trees. Commun. Soil Sci. Plant Anal. 1993, 24, 2059–2068. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, W.; Dong, J.; Yang, T.; Shangguan, Z.; Qu, J.; Li, X.; Tan, X. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 2022, 438, 129557. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zeng, G.-M.; Niu, Q.-Y.; Liu, Y.; Zhou, L.; Jiang, L.-H.; Tan, X.-f.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef]
- Li, S.; Yan, X.; Zhang, M.; Sun, Q.; Zhu, X. Microbial remediation technology for heavy metal contamination of mine soil. Chemoecology 2024, 34, 47–59. [Google Scholar] [CrossRef]
- Kalsi, A.; Celin, S.M.; Bhanot, P.; Sahai, S.; Sharma, J.G. Microbial remediation approaches for explosive contaminated soil: Critical assessment of available technologies, Recent innovations and Future prospects. Environ. Technol. Innov. 2020, 18, 100721. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A. Bioleaching of phosphate minerals using Aspergillus niger: Recovery of copper and rare earth elements. Metals 2020, 10, 978. [Google Scholar] [CrossRef]
- Andres, Y.; Maccordick, H.J.; Hubert, J.C. Complexes of mycobactin from mycobacterium smegmatis with scandium yttrium and lanthanum. Biol. Met. 1991, 4, 207–210. [Google Scholar] [CrossRef]
- Andrès, Y.; Thouand, G.; Boualam, M.; Mergeay, M. Factors influencing the biosorption of gadolinium by micro-organisms and its mobilisation from sand. Appl. Microbiol. Biotechnol. 2000, 54, 262–267. [Google Scholar] [CrossRef]
- Texier, A.C.; Andres, Y.; Cloirec, P.L. Selective biosorption of lanthanide (La, Eu, Yb) ions by an immobilized bacterial biomass. Water Sci. Technol. 2000, 42, 91–94. [Google Scholar] [CrossRef]
- Challaraj Emmanuel, E.; Vignesh, V.; Anandkumar, B.; Maruthamuthu, S. Bioaccumulation of cerium and neodymium by Bacillus cereus isolated from rare earth environments of Chavara and Manavalakurichi, India. Indian J. Microbiol. 2011, 51, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Dong, W.; Ning, Z.; Song, Y.; Hu, K. Recovery of terbium by Lysinibacillus sp. DW018 isolated from ionic rare earth tailings based on microbial induced calcium carbonate precipitation. Front. Microbiol. 2024, 15, 1416731. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.C.; Ananthi, T.; Anandkumar, B.; Maruthamuthu, S. Accumulation of rare earth elements by siderophore-forming Arthrobacter luteolus isolated from rare earth environment of Chavara, India. J. Biosci. 2012, 37, 25–31. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Xu, C.-L.; Chen, Y.-Y. Bacillus sp. Z2 immobilized rare earth yttrium and reduced the absorption of Y in rice. China Environ. Sci. 2023, 43, 927–934. [Google Scholar]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.-i.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, X.; He, H.; Li, J.; Ma, L.; Tan, W.; Zhong, Y.; Zhu, J.; Zhou, M.-F.; Dong, H. Microorganisms accelerate REE mineralization in supergene environments. Appl. Environ. Microbiol. 2022, 88, e00632-22. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.H.; Zhang, Z.C.; Bao, Z.H.; Hao, L.J.; Diao, F.W.; Li, F.Y.; Guo, W. Claroideoglomus etunicatum affects the structural and functional genes of the rhizosphere microbial community to help maize resist Cd and La stresses. Environ. Pollut. 2022, 307, 119559. [Google Scholar] [CrossRef]
- Moriwaki, H.; Yamamoto, H. Interactions of microorganisms with rare earth ions and their utilization for separation and environmental technology. Appl. Microbiol. Biotechnol. 2013, 97, 1–8. [Google Scholar] [CrossRef]
- Giese, E.C. Biosorption as green technology for the recovery and separation of rare earth elements. World J. Microbiol. Biotechnol. 2020, 36, 52. [Google Scholar] [CrossRef]
- Ngwenya, B.T.; Mosselmans, J.F.W.; Magennis, M.; Atkinson, K.D.; Tourney, J.; Olive, V.; Ellam, R.M. Macroscopic and spectroscopic analysis of lanthanide adsorption to bacterial cells. Geochim. Cosmochim. Acta 2009, 73, 3134–3147. [Google Scholar] [CrossRef]
- Kang, X.; Csetenyi, L.; Gadd, G.M. Colonization and bioweathering of monazite by Aspergillus niger: Solubilization and precipitation of rare earth elements. Environ. Microbiol. 2021, 23, 3970–3986. [Google Scholar] [CrossRef]
- Schijf, J.; Byrne, R. Stability constants for mono-and dioxalato-complexes of Y and the REE, potentially important species in groundwaters and surface freshwaters. Geochim. Cosmochim. Acta 2001, 65, 1037–1046. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Liu, Y.; Bian, L.; Wang, X.; Zhou, Z.; Huang, Y. Bioleaching of rare earth elements from bastnaesite-bearing rock by actinobacteria. Chem. Geol. 2018, 483, 544–557. [Google Scholar] [CrossRef]
- Brantley, S.; Liermann, L.; Bau, M.; Wu, S. Uptake of trace metals and rare earth elements from hornblende by a soil bacterium. Geomicrobiol. J. 2001, 18, 37–61. [Google Scholar]
- Kastori, R.; Putnik-Delić, M.; Maksimović, I. Rare earth elements, microorganisms, and control of plant diseases. Contemp. Agric. 2024, 73, 228–237. [Google Scholar] [CrossRef]
- Vítová, M.; Mezricky, D. Microbial recovery of rare earth elements from various waste sources: A mini review with emphasis on microalgae. World J. Microbiol. Biotechnol. 2024, 40, 189. [Google Scholar] [CrossRef]
- Wegner, C.-E.; Westermann, M.; Steiniger, F.; Gorniak, L.; Budhraja, R.; Adrian, L.; Küsel, K. Extracellular and intracellular lanthanide accumulation in the methylotrophic Beijerinckiaceae bacterium RH AL1. Appl. Environ. Microbiol. 2021, 87, e03144-20. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Volesky, B.; Garcia, O., Jr. Biosorption of lanthanum using Sargassum fluitans in batch system. Hydrometallurgy 2002, 67, 31–36. [Google Scholar] [CrossRef]
- Kazy, S.K.; Das, S.K.; Sar, P. Lanthanum biosorption by a Pseudomonas sp.: Equilibrium studies and chemical characterization. J. Ind. Microbiol. Biotechnol. 2006, 33, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Zak, M.T.; Papangelakis, V.G.; Allen, D.G. Biosorption of lanthanum by acidophile Euglena mutabilis biofilms and the role of extracellular polymeric substances. Algal Res. 2023, 72, 103111. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Guo, H.; Wang, X.; Yang, C. Adsorption isotherms of lanthanum to soil constituents and effects of pH, EDTA and fulvic acid on adsorption of lanthanum onto goethite and humic acid. Chem. Speciat. Bioavailab. 2001, 13, 75–81. [Google Scholar]
- Dong, W.; Wang, X.; Bian, X.; Wang, A.; Du, J.; Tao, Z. Comparative study on sorption/desorption of radioeuropium on alumina, bentonite and red earth: Effects of pH, ionic strength, fulvic acid, and iron oxides in red earth. Appl. Radiat. Isot. 2001, 54, 603–610. [Google Scholar]
- Takahashi, Y.; Châtellier, X.; Hattori, K.H.; Kato, K.; Fortin, D. Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats. Chem. Geol. 2005, 219, 53–67. [Google Scholar] [CrossRef]
- Sponza, D.T. Extracellular polymer substances and physicochemical properties of flocs in steady and unsteady-state activated sludge systems. Process Biochem. 2002, 37, 983–998. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, K.; Meng, X.; Shen, L.; Qiu, G.; Wang, Y.; Zhao, H. Study on bioleaching methods and microbial-mineral interaction of ion-adsorption type rare earth ore. J. Environ. Manag. 2025, 382, 125422. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Chibuike, G. Use of mycorrhiza in soil remediation: A review. Sci. Res. Essays 2013, 8, 1679–1687. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Siciliano, A.; Staropoli, A.; Vinale, F.; Nicoletti, R.; DellaGreca, M.; Guida, M.; Salvatore, F.; Iuliano, M. Interaction of the fungal metabolite harzianic acid with rare-earth cations (La3+, Nd3+, Sm3+, Gd3+). Molecules 2022, 27, 1959. [Google Scholar] [CrossRef]
- Yu, D.M. The microorganism-plant system for remediation of soil exposed to coal mining. Foods Raw Mater. 2021, 9, 406–418. [Google Scholar]
- Weissenhorn, I.; Leyval, C. Spore germination of arbuscular mycorrhizal fungi in soils differing in heavy metal content and other parameters. Eur. J. Soil Biol. 1996, 32, 165–172. [Google Scholar]
- Sharma, N.; Tapwal, A. Mycorrhizal symbiosis in Taxus: A review. Mycorrhiza 2024, 34, 173–180. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2010. [Google Scholar]
- Lupwayi, N.; Hamel, C.; Tollefson, T. Soil Biology of the Canadian Prairies. Agric. Soils Prairies 2010, 3, 16–24. [Google Scholar]
- Guo, W.; Zhao, R.; Zhao, W.; Fu, R.; Guo, J.; Bi, N.; Zhang, J. Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. Appl. Soil Ecol. 2013, 72, 85–92. [Google Scholar] [CrossRef]
- A Alsherif, E.A.; Sonbol, H.; AbdElgawad, H.; Ramadan, A.; Korany, S.M.; Crecchio, C.; Ulhassan, Z.; Skalicky, M.; Yang, X.; Brestic, M. Arbuscular mycorrhizal fungi improve tolerance of wheat plants under soil Europium contamination. Plant Soil 2024, 505, 881–895. [Google Scholar] [CrossRef]
- Aloufi, F.A.; Halawani, R.F. Differential AMF-mediated biochemical responses in sorghum and oat plants under environmental impacts of neodymium nanoparticles. Plant Physiol. Biochem. 2025, 219, 109348. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Ionic charge, radius, and potential control root/soil concentration ratios of fifty cationic elements in the organic horizon of a beech (Fagus sylvatica) forest podzol. Sci. Total Environ. 2004, 329, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.F.; Cleland, W. Lanthanide-adenosine 5′-triphosphate complexes: Determination of their dissociation constants and mechanism of action as inhibitors of yeast hexokinase. Biochemistry 1983, 22, 5507–5513. [Google Scholar] [CrossRef]
- Moriwaki, H.; Masuda, R.; Yamazaki, Y.; Horiuchi, K.; Miyashita, M.; Kasahara, J.; Tanaka, T.; Yamamoto, H. Application of freeze-dried powders of genetically engineered microbial strains as adsorbents for rare earth metal ions. ACS Appl. Mater. Interfaces 2016, 8, 26524–26531. [Google Scholar] [CrossRef]
- Yan, S.; Xu, S.; Lei, S.; Gao, Y.; Chen, K.; Shi, X.; Guo, Y.; Bilyera, N.; Yuan, M.; Yao, H. Hyperaccumulator extracts promoting the phytoremediation of rare earth elements (REEs) by Phytolacca americana: Role of active microbial community in rhizosphere hotspots. Environ. Res. 2024, 252, 118939. [Google Scholar] [CrossRef]
- Zaharescu, D.G.; Burghelea, C.I.; Dontsova, K.; Presler, J.K.; Maier, R.M.; Huxman, T.; Domanik, K.J.; Hunt, E.A.; Amistadi, M.K.; Gaddis, E.E. Ecosystem composition controls the fate of rare earth elements during incipient soil genesis. Sci. Rep. 2017, 7, 43208. [Google Scholar] [CrossRef] [PubMed]
- del Rocío Bustillos-Cristales, M.; Corona-Gutierrez, I.; Castañeda-Lucio, M.; Águila-Zempoaltécatl, C.; Seynos-García, E.; Hernández-Lucas, I.; Muñoz-Rojas, J.; Medina-Aparicio, L.; Fuentes-Ramírez, L.E. Culturable facultative methylotrophic bacteria from the cactus Neobuxbaumia macrocephala possess the locus xoxF and consume methanol in the presence of Ce3+ and Ca2+. Microbes Environ. 2017, 32, 244–251. [Google Scholar] [CrossRef]
- Khan, M.A.; Cheng, Z.; Xiao, X.; Khan, A.R.; Ahmed, S.S. Ultrastructural studies of the inhibition effect against Phytophthora capsici of root exudates collected from two garlic cultivars along with their qualitative analysis. Crop Prot. 2011, 30, 1149–1155. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018. (In Chinese)
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.


| Plants | REEs | Remediation Mechanisms | References |
|---|---|---|---|
| Brachythecium campestre Ceratodon purpureus | La, Ce, Nd, Sm, Yb | Rhizofiltration | Sergeeva et al., 2021 [102] |
| Cakile maritim Brassica juncea | La | Phytoextraction | Bouslimi et al., 2023 [103] |
| Carya illinoinensis | Ce, La, Nd, Y | Phytoextraction | Wood et al., 2011 [104] |
| Carlina corymbosa Erica australis Lavandula luisierra | La, Ce | Phytostabilization | Anawar et al., 2012 [105] |
| Dicranopteris dichotoma Dicranopteris linearis Dicranopteris pedata | La, Ce, Pr, Nd, Sm, Eu | Phytoextraction | Chen et al., 2022 [106] Liu et al., 2021 [107] Lin et al., 2024 [108] |
| Helicia australasica | Y, Ce, Dy, Er, Tm, Yb, Lu | Phytostabilization | van der Ent et al., 2023 [109] |
| Phytolacca Americana L. | Ce, Y | Phytoextraction | Ming et al., 2018 [110] Liu et al., 2023 [111] |
| Pronephrium triphyllum | La, Ce, Nd, Sm, Eu, Tb, Yb and Lu | Rhizofiltration | Jalali et al., 2021 [112] |
| Rice | Nd, Dy, La | Phytoextraction | Sun et al., 2022 [56] |
| Salt marshes | Y, La | Phytostabilization | Brito et al., 2020 [113] Brito et al., 2021 [114] |
| Microorganisms | REEs | Results | References |
|---|---|---|---|
| Aspergillus niger | Ce, La, Nd | The REE oxalates are precipitated by metabolites produced during fungal growth and adsorbed onto the mycelium. | Castro et al., 2020 [131] |
| Mycobacterium smegmatis | La, Sc, Y | The formation of REE (La, Sc, and Y)–siderophore complexes was observed in Mycobacterium smegmatis. | Andres et al., 1991 [112,132] |
| Bacillus subtilis Saccharomyces cerevisiae Pseudomonas aeruginosa Ralstonia metallidurans Mycobacterium smegmatis | Gd | The study determined the binding affinity and maximum biosorption capacity of Gd3+, ranging from 350 μmol/g in Bacillus subtilis to 5.1 μmol/g in Saccharomyces cerevisiae. | Andres et al., 2000 [133] |
| Pseudomonasaeruginosa | La, Eu, Yb | Under conditions of pH 5.0 and a flow rate of 0.76 m/h, the removal capacities for lanthanide cations (2 mmol/L) were 198 μmol/g for La3+, 167 μmol/g for Eu3+, and 192 μmol/g for Yb3+ (±10%). | Texier et al., 2000 [134] |
| Bacillus cereus | Ce, Nd | Ce and Nd demonstrated significant cellular accumulation in the two experimental groups, with dry weight accumulation levels reaching 3.02 μmol/g (Ce) and 1.40 μmol/g (Nd) in the first group, and further increasing to 7.05 μmol/g (Ce) and 3.17 μmol/g (Nd) in the second group. | Challarj et al., 2011 [135] |
| Lysinbacillus sp. DW018 | Tb | DW018 can recover Tb3+ from wastewater, achieving a recovery rate as high as 98.28% after 10 min of treatment. | Bian et al., 2024 [136] |
| Arthrobacter luteolus | Sc, Sm | The bacterial cells exhibit a high uptake rate for LREEs, such as Sm and Sc. | Emmanuel et al., 2012 [137] |
| Bacillus sp. Z2 | Y | It can effectively reduce the bioavailability of Y3+. | Wang et al., 2023 [138] |
| Galdieria sulphuraria | Nd, Dy, La | The algae achieved over 90% recovery efficiency for Nd (III), Dy (III), and La (III) at a concentration of 0.5 ppm, and maintained stability within a pH range of 1.5–2.5. | Minoda et al., 2015 [139] |
| Plants | Microorganisms | Outcomes of Combined Effects | References |
|---|---|---|---|
| Rice | Anaeromyces Obacter Georgfuchsia | These rhizospheric bacteria can significantly increase the content of REEs in rice, making it notably higher than that in soil from non-rare-earth mining areas. | Zhang et al., 2022 [45] |
| Helianthus annuus | Bacillus sp. | The combined system significantly enhanced the phytoextraction capacity of REEs, with the concentrations of Ce, La, Nd, and Y reaching 4.4, 38.3, 3.4, and 21 times those of the control group, respectively. | Jalali et al., 2021 [112] |
| Sorghum bicolor L. | Glomus versiforme | The concentrations of La, Ce, Pr, and Nd in the roots of sorghum increased by approximately 70%. | Guo et al., 2013 [168] |
| Phytolacca americana | Rhodanobacter | Rhodanobacter promotes the growth of Phytolacca americana, thereby enhancing the phytoremediation efficiency of Phytolacca americana for REEs. | Yan et al., 2024 [174] |
| Buffalo grass | Rhizophagus | The activity of arbuscular mycorrhizae typically increases the mass of REEs in plant tissues by a factor of 1.2 to 1.6. | Zaharescu et al., 2017 [175] |
| Neobuxbaumia macrocephala | Actinobacteria Sphingobacteriia | The bacteria isolated from the surface, rhizosphere, and stems of this plant exhibit metabolic characteristics dependent on REEs (Ce). | del Rocío et al., 2017 [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Song, Y.; Wang, L.; Jian, W.; Zhou, Q. Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil. Microorganisms 2025, 13, 1282. https://doi.org/10.3390/microorganisms13061282
Dong W, Song Y, Wang L, Jian W, Zhou Q. Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil. Microorganisms. 2025; 13(6):1282. https://doi.org/10.3390/microorganisms13061282
Chicago/Turabian StyleDong, Wei, Yuexin Song, Luyao Wang, Wenchao Jian, and Qian Zhou. 2025. "Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil" Microorganisms 13, no. 6: 1282. https://doi.org/10.3390/microorganisms13061282
APA StyleDong, W., Song, Y., Wang, L., Jian, W., & Zhou, Q. (2025). Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil. Microorganisms, 13(6), 1282. https://doi.org/10.3390/microorganisms13061282






