Seasonal Dynamics of Sediment Microbial Communities at Different Distances from Artificial Reef Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Physicochemical Analyses
2.3. DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.4. Statistical Analyses
3. Results
3.1. Microbial Community Composition
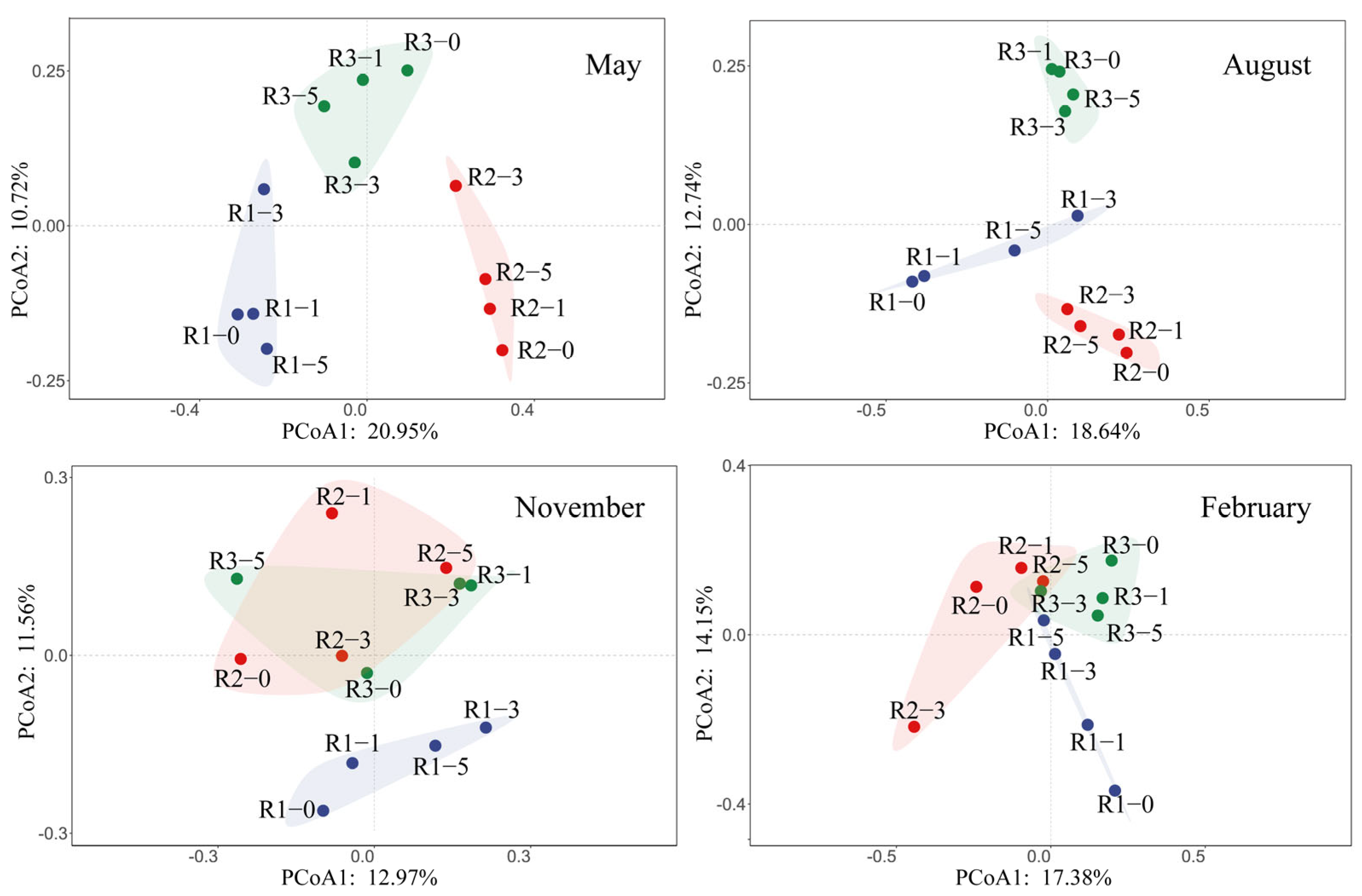
3.2. Spatio-Temporal Dynamics of Microbial Community Structure
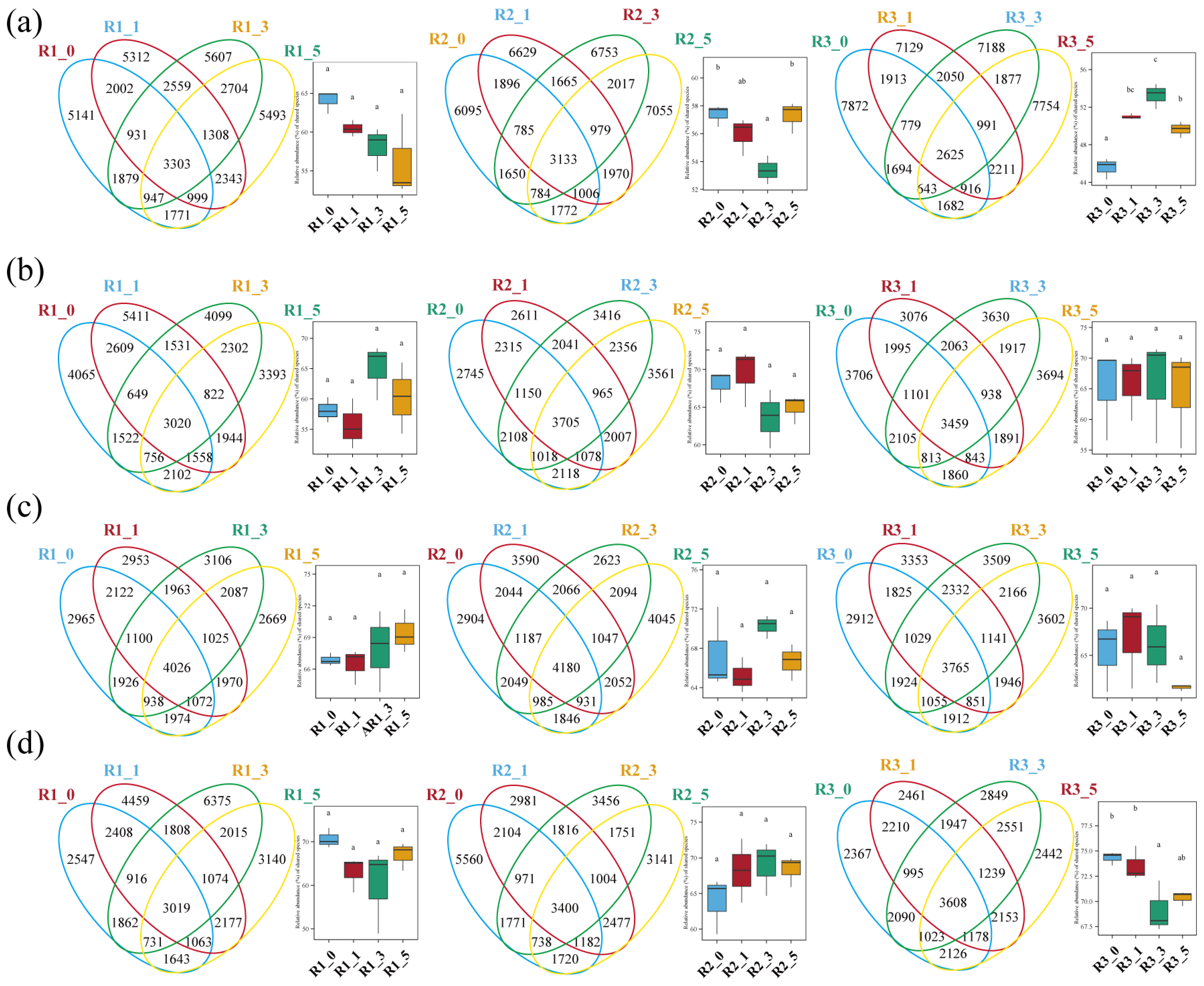
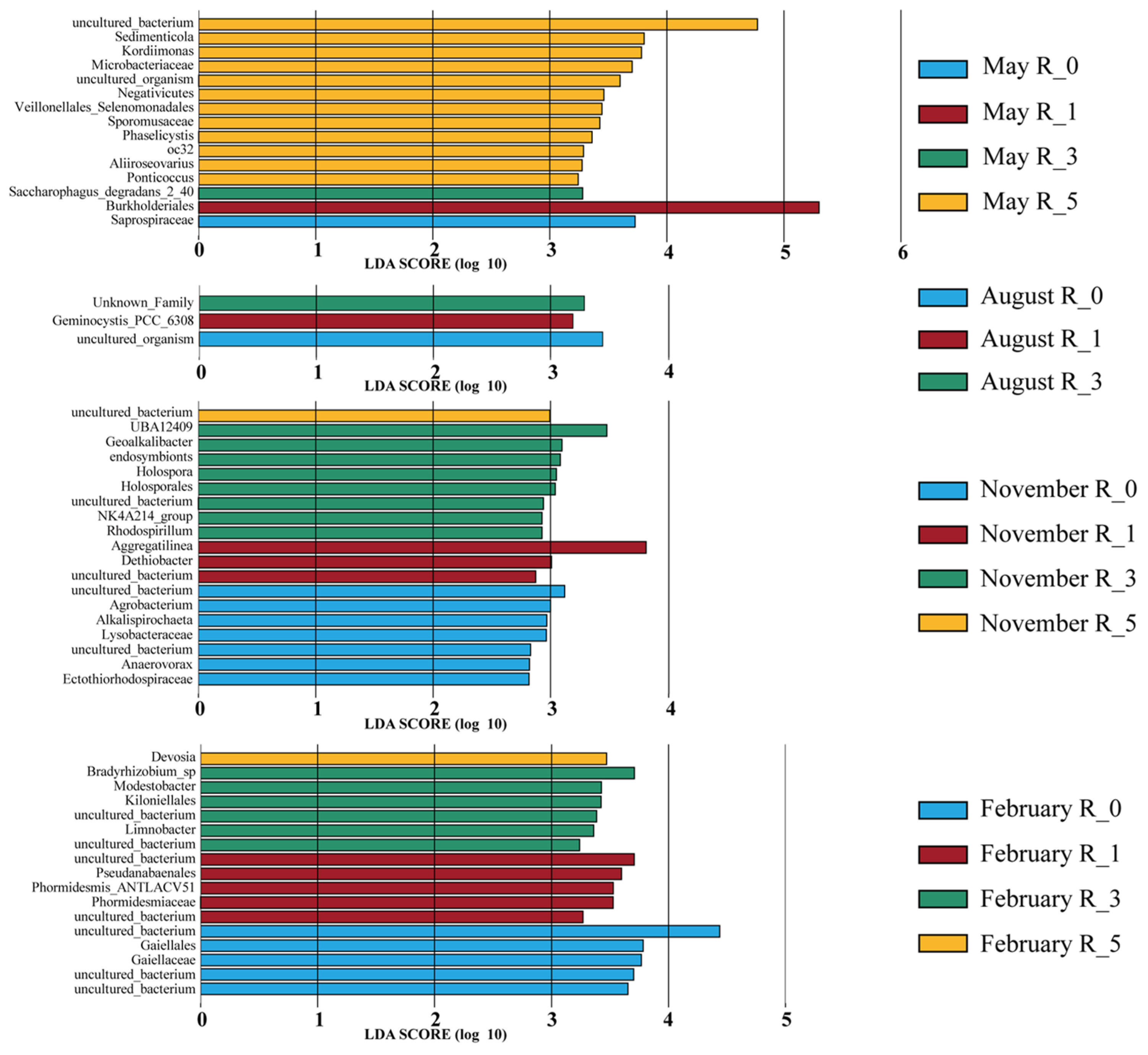
3.3. Differences in Microbial Structure at Various Reef Distances
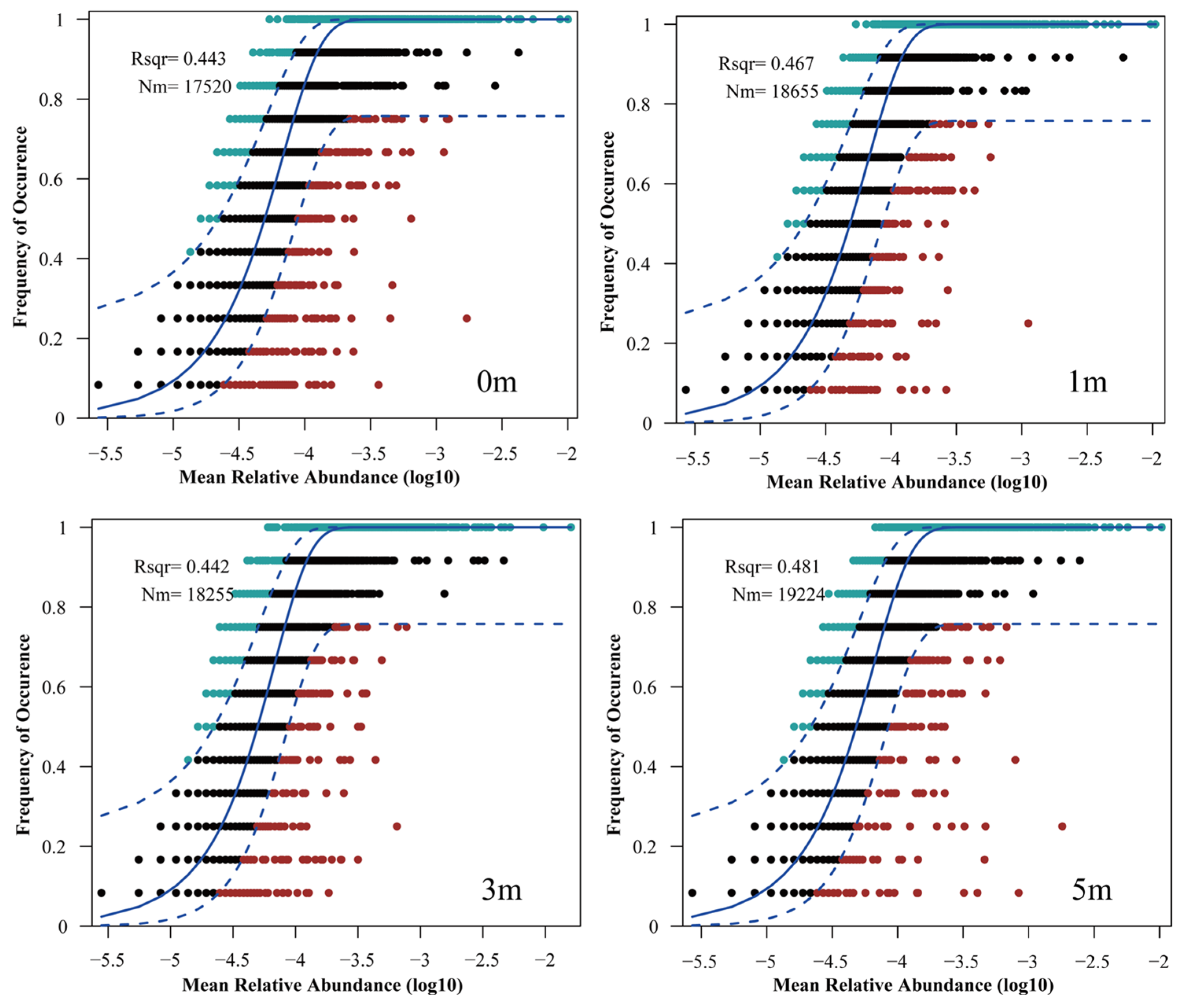
3.4. Community Assembly Mechanisms of Microbes at Varying Reef Distances
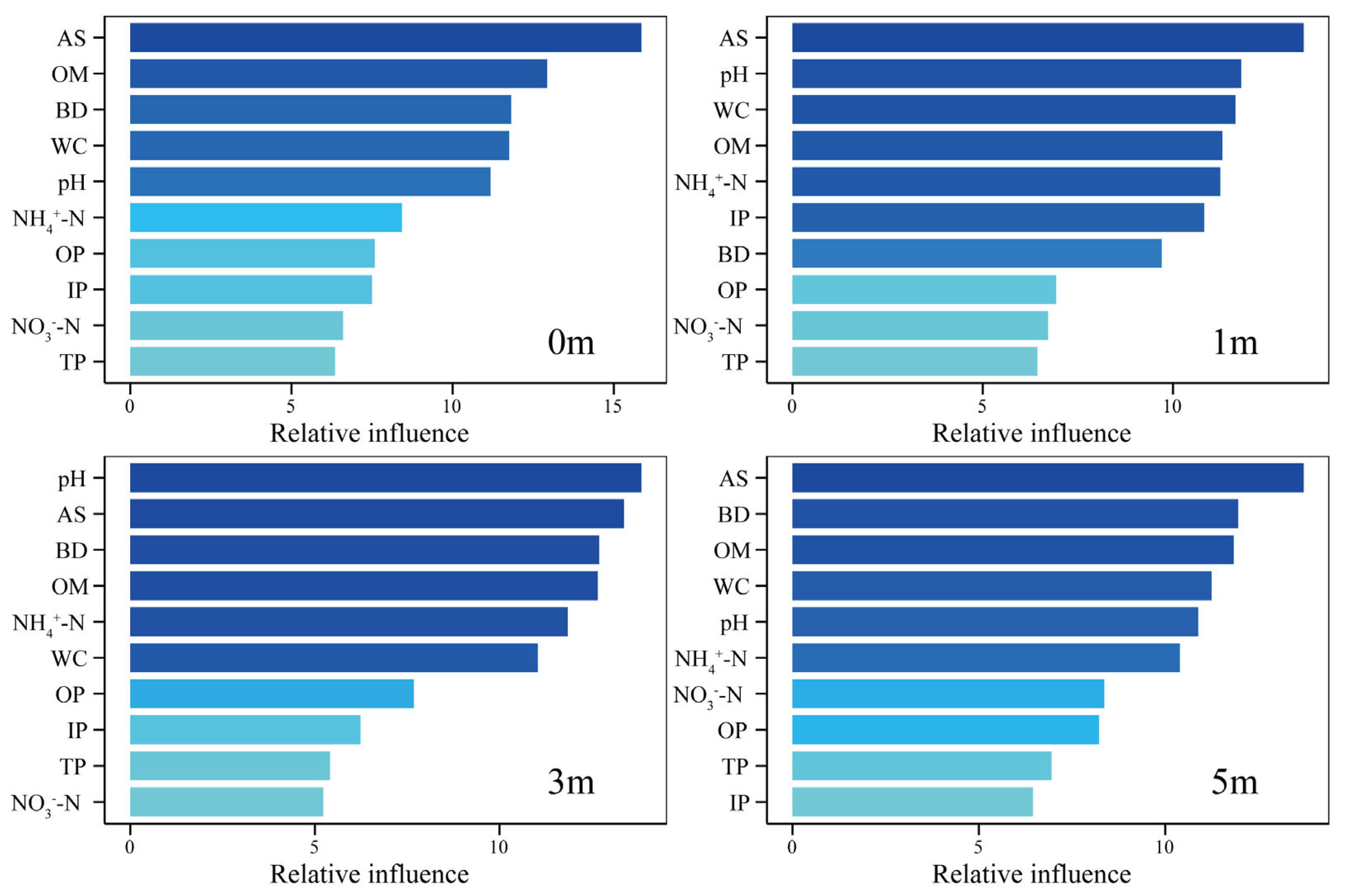
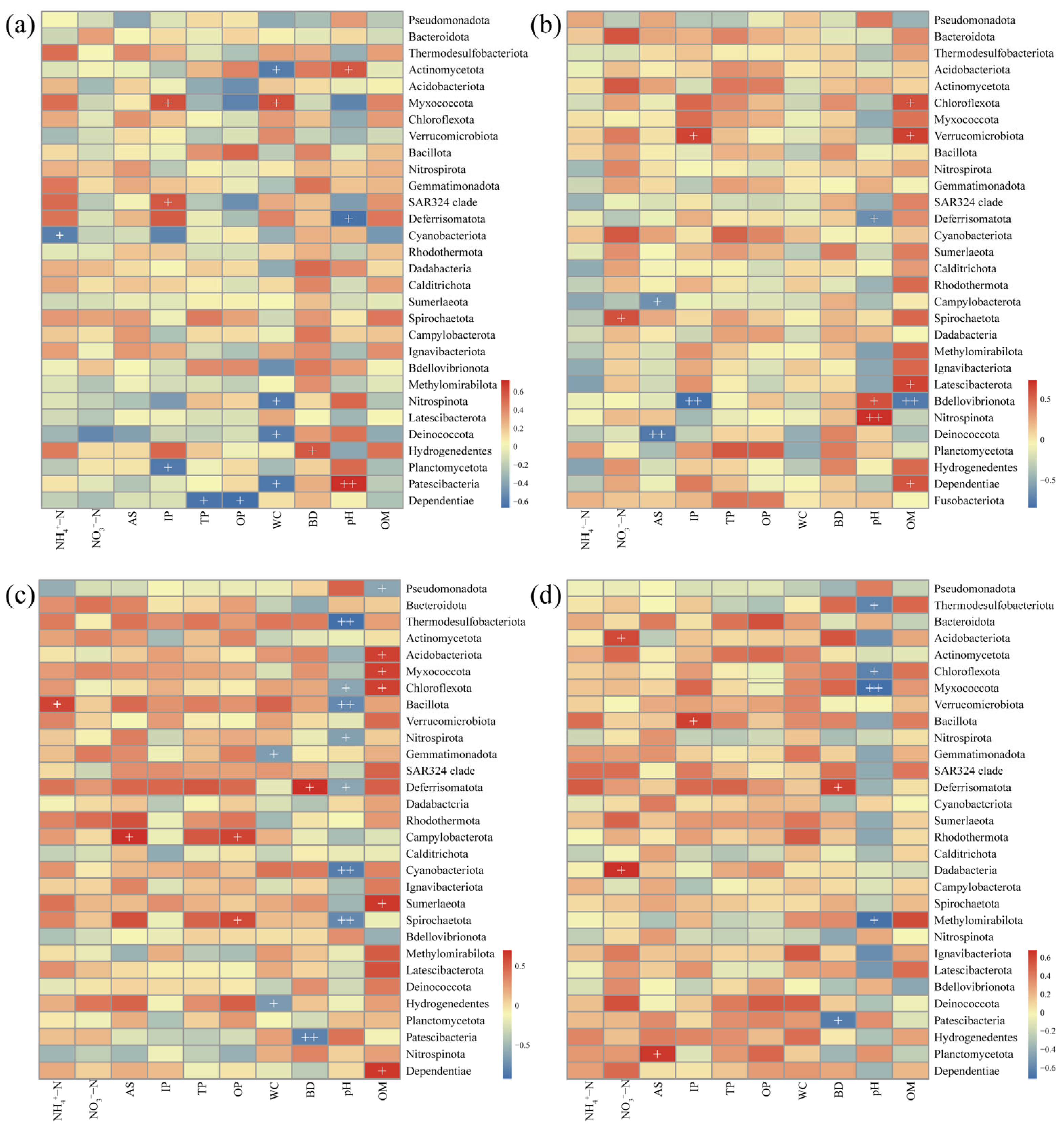
3.5. Relationship Between Dominant Phyla and Environmental Factors
4. Discussion
4.1. Composition and Seasonal Variation in Sediment Microbial Communities at Different Reef Distances
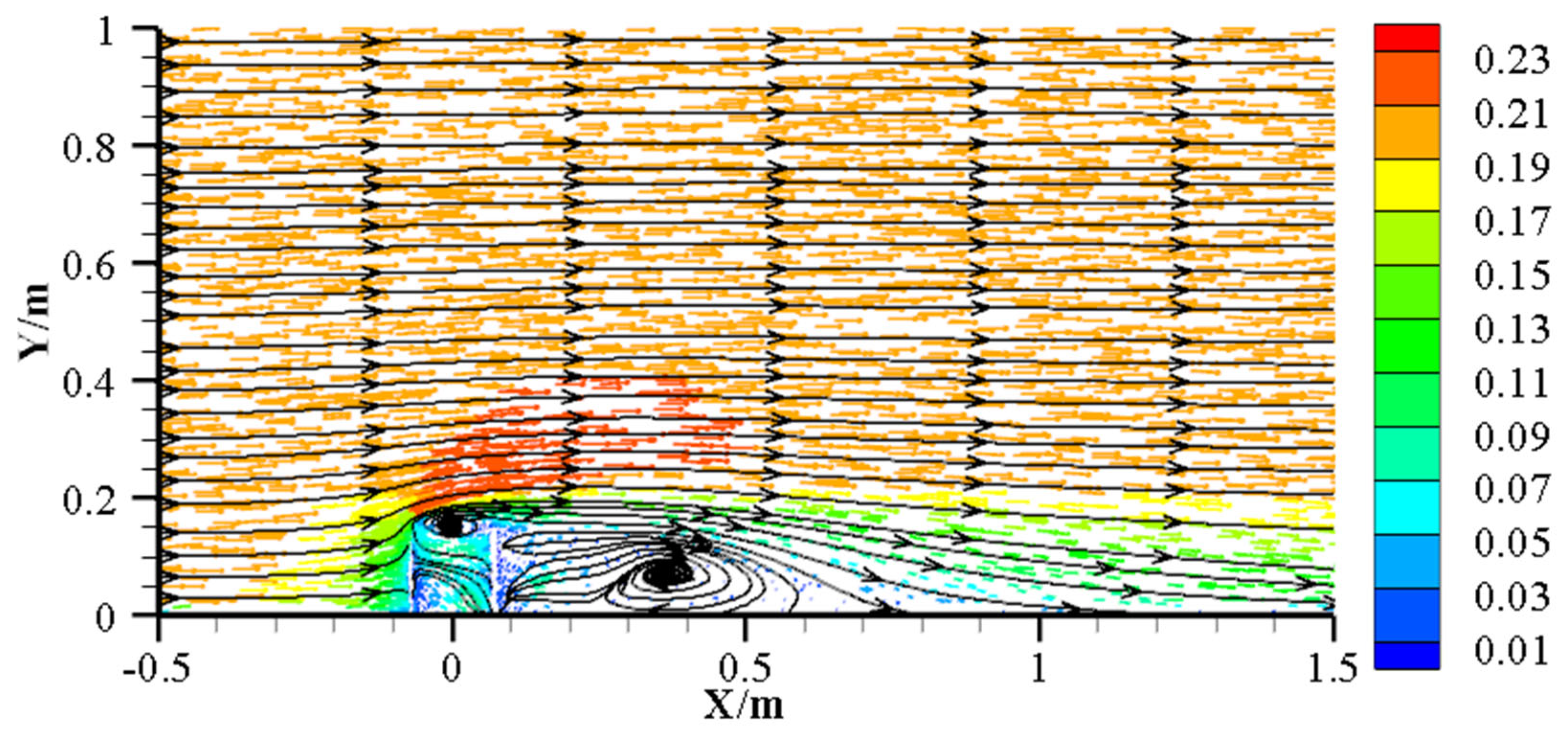
4.2. Impact of AR Units on Microbial Community Structure and Species Distribution Patterns
4.3. Microbial Community Assembly Mechanisms at Different Reef Distances
4.4. Environmental Drivers and Ecological Regulation of Microbial Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Soil Physicochemical Analysis


| Season | Reef–Reef Distance | Acidobacteriota | Thermodesulfobacteriota | Actinomycetota | Bacteroidota | Chloroflexota | Myxococcota | Nitrospirota | Bacillota | Verrucomicrobiota |
|---|---|---|---|---|---|---|---|---|---|---|
| May | R 1-0 | 11.33 b | 13.59 a | 5.15 a | 5.05 b | 1.88 b | ||||
| R 1-1 | 7.63 a | 15.36 ab | 7.55 ab | 3.76 ab | 0.97 ab | |||||
| R 1-3 | 7.41 a | 16.41 b | 8.99 ab | 3.23 ab | 1.2 ab | |||||
| R 1-5 | 6.77 a | 15.99 ab | 10.04 b | 2.77 a | 0.88 a | |||||
| R 2-0 | 8.03 c | 1.12 b | ||||||||
| R 2-1 | 5.54 a | 0.91 ab | ||||||||
| R 2-3 | 6.43 b | 0.77 ab | ||||||||
| R 2-5 | 4.95 a | 0.56 a | ||||||||
| R 3-0 | 5.91 a | 7.2 a | 9.82 b | 2.5 a | 2.91 b | |||||
| R 3-1 | 6.87 b | 7.85 a | 7.34 ab | 2.88 a | 1.17 a | |||||
| R 3-3 | 6.54 ab | 7.53 a | 5.35 a | 3.42 b | 0.51 a | |||||
| R 3-5 | 9.67 c | 11.06 b | 6.53 ab | 2.96 ab | 1.39 a | |||||
| August | R 1-0 | 8.46 a | 7 a | 1.89 a | 2.51 a | 2.78 b | ||||
| R 1-1 | 7.99 a | 7.32 a | 2.27 ab | 2.5 a | 1.87 ab | |||||
| R 1-3 | 11.02 b | 17.64 b | 3.44 b | 4.89 b | 1.68 a | |||||
| R 1-5 | 9.17 a | 9.1 a | 2.68 ab | 3.5 a | 1.74 ab | |||||
| R 2-0 | 11.63 b | 14.41 a | 2.71 b | |||||||
| R 2-1 | 10.75 ab | 15.77 ab | 2.56 b | |||||||
| R 2-3 | 9.67 a | 11.99 a | 1.96 a | |||||||
| R 2-5 | 9.72 a | 19.11 b | 2.57 b | |||||||
| February | R 1-0 | 10.1 c | 14.3 b | 6.32 a | 10.56 a | 5.6 b | 1.63 b | 1.66 a | ||
| R 1-1 | 8.75 bc | 12 b | 7.24 ab | 9.89 a | 3.66 ab | 1.04 a | 2.85 ab | |||
| R 1-3 | 7.57 ab | 7.84 a | 9.26 b | 15.65 ab | 3.11 a | 1.02 a | 6.35 ab | |||
| R 1-5 | 6.09 a | 7.74 a | 6.01 a | 21.04 b | 2.46 a | 0.82 a | 7.39 b | |||
| R 2-0 | 3.92 a | 10.12 b | 0.64 a | |||||||
| R 2-1 | 5.02 ab | 7.41 a | 0.99 ab | |||||||
| R 2-3 | 5.39 b | 9.98 ab | 1.25 b | |||||||
| R 2-5 | 6.24 b | 8.39 ab | 1.16 b | |||||||
| R 3-0 | 5.28 a | |||||||||
| R 3-1 | 6.95 b | |||||||||
| R 3-3 | 7.08 bc | |||||||||
| R 3-5 | 8.26 c |
| Season | Reef Distance | OM (%) | pH | BD (g/cm3) | WC (%) | NH4+-N (μg/g) | NO3−-N (μg/g) | AS (mg/kg) | IP (μg/g) | TP (μg/g) | OP (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| May | R_0 | 5.6 ± 2.4 | 9.12 ± 0.35 | 0.08 ± 0.02 | 41.16 ± 11.7 | 4.25 ± 0.46 | 1.29 ± 1.11 | 25.61 ± 26.72 | 157.09 ± 140.15 | 219.93 ± 190.59 | 62.84 ± 67.77 |
| R_1 | 5.65 ± 1.8 | 9.13 ± 0.28 | 0.08 ± 0.02 | 53.02 ± 13.92 | 5.19 ± 0.21 | 0 ± 0 | 38.05 ± 33.06 | 134.65 ± 123.41 | 201.98 ± 181.16 | 67.33 ± 58.69 | |
| R_3 | 6.54 ± 0.83 | 9.22 ± 0.35 | 0.09 ± 0.02 | 51.07 ± 16.51 | 4.28 ± 0.33 | 1.93 ± 3.34 | 40.37 ± 38.74 | 161.58 ± 139.93 | 273.79 ± 237.97 | 112.21 ± 99.25 | |
| R_5 | 6.7 ± 1.57 | 9.19 ± 0.31 | 0.08 ± 0.02 | 53.44 ± 18.99 | 4.31 ± 0.95 | 5.15 ± 7.31 | 33.4 ± 31.59 | 152.61 ± 134.88 | 255.84 ± 222.48 | 103.23 ± 101.06 | |
| August | R_0 | 6.94 ± 1.4 | 9.2 ± 0.05 | 0.09 ± 0.02 | 42.91 ± 10.28 | 2.82 ± 2.45 | 5.15 ± 4.86 | 64.63 ± 11.39 | 121.19 ± 105.17 | 350.1 ± 303.49 | 228.91 ± 199.27 |
| R_1 | 6.47 ± 0.32 | 9.2 ± 0.42 | 0.08 ± 0.03 | 67.03 ± 31.33 | 2.8 ± 2.44 | 9.65 ± 12.06 | 54.99 ± 26.9 | 116.7 ± 104.59 | 341.12 ± 295.42 | 224.42 ± 196.21 | |
| R_3 | 5.92 ± 1.85 | 8.9 ± 0.13 | 0.09 ± 0 | 53.2 ± 4.05 | 3.68 ± 3.25 | 12.23 ± 15 | 85.64 ± 56.02 | 143.63 ± 125.11 | 345.61 ± 299.38 | 201.98 ± 175.05 | |
| R_5 | 6.12 ± 1.35 | 9.24 ± 0.28 | 0.07 ± 0.02 | 54.01 ± 1.89 | 2.84 ± 2.46 | 4.5 ± 4.02 | 67.13 ± 18.61 | 134.65 ± 117.39 | 336.63 ± 293.47 | 201.98 ± 181.16 | |
| November | R_0 | 6.35 ± 1.72 | 8.98 ± 0.41 | 0.07 ± 0.02 | 78.41 ± 43.64 | 3.78 ± 0.69 | 5.15 ± 2.95 | 51.83 ± 17.51 | 210.96 ± 77.74 | 287.26 ± 7.77 | 76.3 ± 74.16 |
| R_1 | 7.61 ± 0.87 | 9.05 ± 0.23 | 0.08 ± 0.02 | 68.21 ± 29.85 | 4.91 ± 1.53 | 7.08 ± 8.92 | 58.26 ± 7.87 | 242.37 ± 23.32 | 287.26 ± 7.77 | 44.88 ± 28.03 | |
| R_3 | 6.22 ± 2.35 | 9.24 ± 0.12 | 0.07 ± 0.01 | 45.22 ± 14.82 | 4.03 ± 1 | 3.22 ± 1.11 | 44.81 ± 9.24 | 193 ± 50.98 | 318.68 ± 15.55 | 125.68 ± 66.42 | |
| R_5 | 6.59 ± 1.71 | 9.22 ± 0.3 | 0.07 ± 0.02 | 62.72 ± 11.15 | 3.56 ± 0.24 | 3.22 ± 1.11 | 56 ± 34.26 | 201.98 ± 35.63 | 377.03 ± 61.71 | 175.05 ± 81.91 | |
| February | R_0 | 10.01 ± 6.46 | 9.12 ± 0.21 | 0.09 ± 0.01 | 44.88 ± 5.5 | 4.03 ± 0.03 | 2.57 ± 2.23 | 34.81 ± 2.75 | 228.91 ± 48.55 | 327.65 ± 56.06 | 98.74 ± 89.66 |
| R_1 | 12.09 ± 8.48 | 9.07 ± 0.09 | 0.09 ± 0 | 51.28 ± 6.03 | 3.89 ± 0.26 | 1.29 ± 1.11 | 53.5 ± 21.01 | 233.4 ± 47.29 | 323.17 ± 110.22 | 89.77 ± 89.66 | |
| R_3 | 11.55 ± 3.8 | 9.14 ± 0.05 | 0.09 ± 0.01 | 49.6 ± 1.18 | 3.99 ± 0.29 | 1.93 ± 0 | 61.71 ± 35.59 | 237.89 ± 41.14 | 336.63 ± 40.4 | 98.74 ± 81.16 | |
| R_5 | 7.45 ± 0.73 | 8.89 ± 0.04 | 0.08 ± 0.01 | 67.16 ± 11.89 | 3.68 ± 0.64 | 7.72 ± 3.34 | 31.48 ± 11.58 | 206.47 ± 28.03 | 363.56 ± 58.69 | 157.09 ± 60.72 |
References
- Reimer, J. A longer-term view on benthic communities on artificial reefs: It’s all about location. Peer Community Ecol. 2022, 1, 100093. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Rountos, K.J.; Essington, T.E.; Santora, C.; Pauly, D.; Watson, R.; Sumaila, U.R.; Boersma, P.D.; Boyd, I.L.; Conover, D.O.; et al. The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 2014, 15, 43–64. [Google Scholar] [CrossRef]
- Smith, J.A.; Lowry, M.B.; Champion, C.; Suthers, I.M. A designed artificial reef is among the most productive marine fish habitats: New metrics to address ‘production versus attraction’. Mar. Biol. 2016, 163, 188. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, W.; Chen, H.; Chen, P.; Jia, X. The mechanism and research progress on fish attraction technique for artificial reefs. Mar. Fish. 2010, 32, 225–230. [Google Scholar] [CrossRef]
- Reeds, K.A.; Smith, J.A.; Suthers, I.M.; Johnston, E.L. An ecological halo surrounding a large offshore artificial reef: Sediments, infauna, and fish foraging. Mar. Environ. Res. 2018, 141, 30–38. [Google Scholar] [CrossRef]
- Davis, N.; VanBlaricom, G.R.; Dayton, P.K. Man-made structures on marine sediments: Effects on adjacent benthic communities. Mar. Biol. 1982, 70, 295–303. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Song, X.; Xu, M.; Yang, H. Effects of artificial reefs on the meiofaunal community and benthic environment—A case study in Bohai Sea, China. Mar. Pollut. Bull. 2019, 140, 179–187. [Google Scholar] [CrossRef]
- Galván, D.E.; Parma, A.M.; Iribarne, O.O. Influence of predatory reef fishes on the spatial distribution of Munida gregaria (=M. subrugosa) (Crustacea; Galatheidae) in shallow Patagonian soft bottoms. J. Exp. Mar. Biol. Ecol. 2008, 354, 93–100. [Google Scholar] [CrossRef]
- Wilding, T.A. The benthic impacts of the Loch Linnhe Artificial Reef. Hydrobiologia 2006, 555, 345–353. [Google Scholar] [CrossRef]
- Becker, A.; Lowry, M.B.; Fowler, A.M.; Taylor, M.D. Hydroacoustic surveys reveal the distribution of mid-water fish around two artificial reef designs in temperate Australia. Fish. Res. 2023, 257, 106509. [Google Scholar] [CrossRef]
- Pennifold, M.; Davis, J. Macrofauna and nutrient cycling in the Swan River Estuary, Western Australia: Experimental results. Hydrol. Process. 2001, 15, 2537–2553. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Tang, Y.; Zhao, F.; Luo, Y. Numerical Analysis of the Flow Effect of the Menger-Type Artificial Reefs with Different Void Space Complexity Indices. Symmetry 2021, 13, 1040. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, L.; Song, M.; Jiang, Z.; Liang, Z. The effects of flow field on the succession of the microbial community on artificial reefs. Mar. Pollut. Bull. 2023, 191, 114920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liang, Z.; Zhu, L.; Liu, Y. Numerical simulation of effect of guide plate on flow field of artificial reef. Ocean Eng. 2016, 116, 236–241. [Google Scholar] [CrossRef]
- Kim, D.; Woo, J.; Na, W.-B. Intensively stacked placement models of artificial reef sets characterized by wake and upwelling regions. Mar. Technol. Soc. J. 2017, 51, 60–70. [Google Scholar] [CrossRef]
- Xia, X.; Li, Y.; Li, J.; Gong, P.; Huang, J.; Lu, J. Effect of oyster shell filling in artificial reefs on flow field environment and assessing the potential of carbon fixation. J. Sea Res. 2024, 202, 102537. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.-x.; Gong, P.-h.; Guan, C.-t. Numerical simulation and PIV experimental study of the effect of flow fields around tube artificial reefs. Ocean Eng. 2017, 134, 96–104. [Google Scholar] [CrossRef]
- Kim, D.; Woo, J.; Yoon, H.-S.; Na, W.-B. Wake lengths and structural responses of Korean general artificial reefs. Ocean Eng. 2014, 92, 83–91. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Li, P.; Liu, L.; Li, Z.-H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environ. Res. 2022, 214, 114202. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Pommier, T.; Canback, B.; Riemann, L.; Bostrom, K.H.; Simu, K.; Lundberg, P.; Tunlid, A.; Hagstrom, A. Global patterns of diversity and community structure in marine bacterioplankton. Mol. Ecol. 2007, 16, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yu, H.; Sheng, H.; Chen, C.; Tang, Y.; Liang, Z. Seasonal variations and co-occurrence networks of bacterial communities in the water and sediment of artificial habitat in Laoshan Bay, China. PeerJ 2022, 9, e12705. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Orsi, W.D.; Edgcomb, V.P.; Christman, G.D.; Biddle, J.F. Gene expression in the deep biosphere. Nature 2013, 499, 205–208. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, Y. Response of sediment microbial communities to the flow effect of the triangular artificial reef: A simulation-based experimental study. Mar. Environ. Res. 2024, 198, 106546. [Google Scholar] [CrossRef]
- Geng, M.; Zhang, W.; Hu, T.; Wang, R.; Cheng, X.; Wang, J. Eutrophication causes microbial community homogenization via modulating generalist species. Water Res. 2022, 210, 118003. [Google Scholar] [CrossRef]
- Lin, G.; Lin, X. Bait input altered microbial community structure and increased greenhouse gases production in coastal wetland sediment. Water Res. 2022, 218, 118520. [Google Scholar] [CrossRef]
- Inagaki, F.; Hinrichs, K.U.; Kubo, Y.; Bowles, M.W.; Heuer, V.B.; Hong, W.L.; Hoshino, T.; Ijiri, A.; Imachi, H.; Ito, M.; et al. Exploring deep microbial life in coal-bearing sediment down to ~2.5 km below the ocean floor. Science 2015, 349, 420–424. [Google Scholar] [CrossRef]
- Babcock, K.K.; Cesbron, F.; Patterson, W.F.; Garner, S.B.; Waidner, L.A.; Caffrey, J.M. Changing Biogeochemistry and Invertebrate Community Composition at Newly Deployed Artificial Reefs in the Northeast Gulf of Mexico. Estuaries Coasts 2020, 43, 680–692. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Liang, Z.; Sun, L.; Nie, Z.; Wang, J.; Xie, W.; Jiang, Z. Numerical Study of Efficiency Indices to Evaluate the Effect of Layout Mode of Artificial Reef Unit on Flow Field. J. Mar. Sci. Eng. 2021, 9, 770. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Z.; Chai, Z.; Cong, W.; Zhu, L.; Guo, Z.; Song, M.; Ma, J.; Guo, T.; Zhang, W.; et al. Construction and evolution of artificial reef ecosystems: Response and regulation of marine microorganisms. Environ. Pollut. 2025, 367, 125610. [Google Scholar] [CrossRef] [PubMed]
- Bunse, C.; Pinhassi, J. Marine Bacterioplankton Seasonal Succession Dynamics. Trends Microbiol. 2017, 25, 494–505. [Google Scholar] [CrossRef]
- Jiao, N.; Zhu, C.; Liu, J.; Luo, T.; Bai, M.; Yu, Z.; Chen, Q.; Rinkevich, B.; Weinbauer, M.; Thomas, H.; et al. A roadmap for Ocean Negative Carbon Emission eco-engineering in sea-farming fields. Innov. Geosci. 2023, 1, 100029. [Google Scholar] [CrossRef]
- Jeanthon, C.; L’Haridon, S.; Cueff, V.; Banta, A.; Reysenbach, A.-L.; Prieur, D. Thermodesulfobacterium hydrogeniphilum sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent at Guaymas Basin, and emendation of the genus Thermodesulfobacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 765–772. [Google Scholar] [CrossRef]
- Larsbrink, J.; McKee, L.S. Chapter Two-Bacteroidetes bacteria in the soil: Glycan acquisition, enzyme secretion, and gliding motility. Adv. Appl. Microbiol. 2020, 110, 63–98. [Google Scholar] [CrossRef]
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agric. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 00744. [Google Scholar] [CrossRef]
- Li, L.; Huang, D.; Hu, Y.; Rudling, N.M.; Canniffe, D.P.; Wang, F.; Wang, Y. Globally distributed Myxococcota with photosynthesis gene clusters illuminate the origin and evolution of a potentially chimeric lifestyle. Nat. Commun. 2023, 14, 6450. [Google Scholar] [CrossRef]
- Nan, B.; Bandaria, J.N.; Moghtaderi, A.; Sun, I.-H.; Yildiz, A.; Zusman, D.R. Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories. Proc. Natl. Acad. Sci. USA 2013, 110, E1508–E1513. [Google Scholar] [CrossRef]
- Treuner-Lange, A.; Chang, Y.-W.; Glatter, T.; Herfurth, M.; Lindow, S.; Chreifi, G.; Jensen, G.J.; Søgaard-Andersen, L. PilY1 and minor pilins form a complex priming the type IVa pilus in Myxococcus xanthus. Nat. Commun. 2020, 11, 5054. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Francis, T.B.; Ferraro, M.; Hehemann, J.-H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota are specialist consumers of sulfated methyl pentoses during diatom blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef]
- Solyanikova, I.; Golovleva, L. Physiological and biochemical properties of actinobacteria as the basis of their high biodegradative activity. Appl. Biochem. Microbiol. 2015, 51, 143–149. [Google Scholar] [CrossRef]
- Vick, S.H.; Greenfield, P.; Tetu, S.G.; Midgley, D.J.; Paulsen, I.T. Genomic and phenotypic insights point to diverse ecological strategies by facultative anaerobes obtained from subsurface coal seams. Sci. Rep. 2019, 9, 16186. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, X.; Cheng, L.; Teng, B. Experimental study of local scour around subsea caissons in steady currents. Coast. Eng. 2012, 60, 30–40. [Google Scholar] [CrossRef]
- Xie, C.-J.; Yao, L.; Tang, R.; Han, S.; Yang, S.; Alwathnani, H.; Rensing, C.; Liu, G.-H.; Zhou, S.-G. Azotosporobacter soli gen. nov., sp. nov., a novel nitrogen-fixing bacterium isolated from paddy soil. Antonie van Leeuwenhoek 2024, 117, 79. [Google Scholar] [CrossRef]
- Vesth, T.; Ozen, A.; Andersen, S.C.; Kaas, R.S.; Lukjancenko, O.; Bohlin, J.; Nookaew, I.; Wassenaar, T.M.; Ussery, D.W. Veillonella, firmicutes: Microbes disguised as gram negatives. Stand. Genom. Sci. 2013, 9, 431–448. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.-W.; Whitman, W.B. Kordiimonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1–12. [Google Scholar]
- Deng, Y.; Li, C.-J.; Zhang, J.; Liu, W.-H.; Yu, L.-Y.; Zhang, Y.-Q. Extensive genomic study characterizing three Paracoccaceae populations and revealing Pseudogemmobacter lacusdianii sp. nov. and Paracoccus broussonetiae sp. nov. Microbiol. Spectr. 2024, 12, e0108824. [Google Scholar] [CrossRef]
- Garcia, R.O.; Reichenbach, H.; Ring, M.W.; Müller, R. Phaselicystis flava gen. nov., sp. nov., an arachidonic acid-containing soil myxobacterium, and the description of Phaselicystidaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1524–1530. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Nielsen, P.H. The Family Saprospiraceae. In The Prokaryotes: Other Major Lineages of Bacteria and The Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 863–889. [Google Scholar] [CrossRef]
- Weiner, R.M.; Taylor, L.E., II; Henrissat, B.; Hauser, L.; Land, M.; Coutinho, P.M.; Rancurel, C.; Saunders, E.H.; Longmire, A.G.; Zhang, H.; et al. Complete Genome Sequence of the Complex Carbohydrate-Degrading Marine Bacterium, Saccharophagus degradans Strain 2-40T. PLoS Genet. 2008, 4, e1000087. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Bej, A.K.; Marsic, D.; Whitman, W.B.; Krader, P. Spirochaeta dissipatitropha sp. nov., an alkaliphilic, obligately anaerobic bacterium, and emended description of the genus Spirochaeta Ehrenberg 1835. Int. J. Syst. Evol. Microbiol. 2009, 59, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Dirren, S.; Posch, T. Promiscuous and specific bacterial symbiont acquisition in the amoeboid genus Nuclearia (Opisthokonta). FEMS Microbiol. Ecol. 2016, 92, fiw105. [Google Scholar] [CrossRef]
- Sravanthi, T.; Tushar, L.; Sasikala, C.; Ramana, C.V. Alkalispirochaeta cellulosivorans gen. nov., sp. nov., a cellulose-hydrolysing, alkaliphilic, halotolerant bacterium isolated from the gut of a wood-eating cockroach (Cryptocercus punctulatus), and reclassification of four species of Spirochaeta as new combinations within Alkalispirochaeta gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 1612–1619. [Google Scholar] [CrossRef]
- Matthies, C.; Evers, S.; Ludwig, W.; Schink, B. Anaerovorax odorimutans gen. nov., sp. nov., a putrescine-fermenting, strictly anaerobic bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 1591–1594. [Google Scholar] [CrossRef]
- Greene, A.C.; Patel, B.K.C.; Yacob, S. Geoalkalibacter subterraneus sp. nov., an anaerobic Fe(III)- and Mn(IV)-reducing bacterium from a petroleum reservoir, and emended descriptions of the family Desulfuromonadaceae and the genus Geoalkalibacter. Int. J. Syst. Evol. Microbiol. 2009, 59, 781–785. [Google Scholar] [CrossRef]
- Gu, Y.; Zhu, X.; Lin, F.; Shen, C.; Li, Y.; Ao, L.; Fan, W.; Ren, C.; Xu, Y. Caproicibacterium amylolyticum gen. nov., sp. nov., a novel member of the family Oscillospiraceae isolated from pit clay used for making Chinese strong aroma-type liquor. Int. J. Syst. Evol. Microbiol. 2021, 71, 004789. [Google Scholar] [CrossRef]
- Ferguson, S.J.; Jackson, J.B.; McEwan, A.G. Anaerobic respiration in the Rhodospirillaceae: Characterisation of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol. Rev. 1987, 3, 117–143. [Google Scholar] [CrossRef]
- Nakahara, N.; Nobu, M.K.; Takaki, Y.; Miyazaki, M.; Tasumi, E.; Sakai, S.; Ogawara, M.; Yoshida, N.; Tamaki, H.; Yamanaka, Y.; et al. Aggregatilinea lenta gen. nov., sp. nov., a slow-growing, facultatively anaerobic bacterium isolated from subseafloor sediment, and proposal of the new order Aggregatilineales ord. nov. within the class Anaerolineae of the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2019, 69, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Merkel, A.Y. Dethiobacteraceae fam. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley and Sons: Hoboken, NJ, USA, 2022; pp. 1–2. [Google Scholar]
- Balk, M.; Altinbaş, M.; Rijpstra, W.I.; Sinninghe Damsté, J.S.; Stams, A.J. Desulfatirhabdium butyrativorans gen. nov., sp. nov., a butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. [Google Scholar] [CrossRef]
- Liesack, W.; Finster, K. Phylogenetic Analysis of Five Strains of Gram-Negative, Obligately Anaerobic, Sulfur-Reducing Bacteria and Description of Desulfuromusa gen. nov., Including Desulfuromusa kysingii sp. nov., Desulfuromusa bakii sp. nov., and Desulfuromusa succinoxidans sp. nov. Int. J. Syst. Evol. Microbiol. 1994, 44, 753–758. [Google Scholar] [CrossRef]
- Albuquerque, L.; França, L.; Rainey, F.A.; Schumann, P.; Nobre, M.F.; da Costa, M.S. Gaiella occulta gen. nov., sp. nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. nov. and Gaiellales ord. nov. Syst. Appl. Microbiol. 2011, 34, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Yee, B.; Oertli, G.E.; Fuerst, J.A.; Staley, J.T. Reclassification of the polyphyletic genus Prosthecomicrobium to form two novel genera, Vasilyevaea gen. nov. and Bauldia gen. nov. with four new combinations: Vasilyevaea enhydra comb. nov., Vasilyevaea mishustinii comb. nov., Bauldia consociata comb. nov. and Bauldia litoralis comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Kämpfer, P.; Schleifer, K.H. Limnobacter thiooxidans gen. nov., sp. nov., a novel thiosulfate-oxidizing bacterium isolated from freshwater lake sediment. Int. J. Syst. Evol. Microbiol. 2001, 51, 1463–1470. [Google Scholar] [CrossRef]
- Tamulonis, C.; Postma, M.; Kaandorp, J. Modeling filamentous cyanobacteria reveals the advantages of long and fast trichomes for optimizing light exposure. PLoS ONE 2011, 6, e22084. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhao, F.; Tang, Y. Experimental Study of Deflector on Scour Reduction Around Artificial Reef. Int. Conf. Offshore Mech. Arct. Eng. 2024, 87813, V004T005A008. [Google Scholar] [CrossRef]
- Tang, Y.; Long, X.; Wang, X.; Zhao, F.; Huang, L. Effect of Reefs Spacing on Flow Field Around Artificial Reef Based on the Hydrogen Bubble Experiment. Int. Conf. Ocean Offshore Arct. Eng. 2017, 57748, V07BT06A017. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef]
- Freches, A.; Fradinho Joana, C. The biotechnological potential of the Chloroflexota phylum. Appl. Environ. Microbiol. 2024, 90, e0175623. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Pinto, O.H.B.; Quirino, B.F.; de Freitas, M.A.M.; Thompson, F.L.; Thompson, C.; Kruger, R.H. Genome-resolved metagenomic analysis of Great Amazon Reef System sponge-associated Latescibacterota bacteria and their potential contributions to the host sponge and reef. Front. Microbiomes 2023, 2, 1206961. [Google Scholar] [CrossRef]
- Flieder, M.; Buongiorno, J.; Herbold, C.W.; Hausmann, B.; Rattei, T.; Lloyd, K.G.; Loy, A.; Wasmund, K. Novel taxa of Acidobacteriota implicated in seafloor sulfur cycling. ISME J. 2021, 15, 3159–3180. [Google Scholar] [CrossRef]
- Fang, Y.; Yuan, Y.; Liu, J.; Wu, G.; Yang, J.; Hua, Z.; Han, J.; Zhang, X.; Li, W.; Jiang, H. Casting Light on the Adaptation Mechanisms and Evolutionary History of the Widespread Sumerlaeota. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, I.; Rawls, M.; Coykendall, D.K.; Foustoukos, D.I. Deferrisoma palaeochoriense sp. nov., a thermophilic, iron(III)-reducing bacterium from a shallow-water hydrothermal vent in the Mediterranean Sea. Int. J. Syst. Evol. Microbiol. 2016, 66, 830–836. [Google Scholar] [CrossRef]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef]
- Zhu, B.; Karwautz, C.; Andrei, S.; Klingl, A.; Pernthaler, J.; Lueders, T. A novel Methylomirabilota methanotroph potentially couples methane oxidation to iodate reduction. mLife 2022, 1, 323–328. [Google Scholar] [CrossRef]
- Fang, G.; Yu, H.; Sheng, H.; Tang, Y.; Liang, Z. Comparative analysis of microbial communities between water and sediment in Laoshan Bay marine ranching with varied aquaculture activities. Mar. Pollut. Bull. 2021, 173, 112990. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Mohamad, O.A.A.; Gao, L.; Xie, Y.-G.; Abdugheni, R.; Huang, Y.; Li, L.; Fang, B.-Z.; Li, W.-J. Sediment prokaryotic microbial community and potential biogeochemical cycle from saline lakes shaped by habitat. Microbiol. Res. 2023, 270, 127342. [Google Scholar] [CrossRef]
- Daims, H.; Nielsen Jeppe, L.; Nielsen Per, H.; Schleifer, K.-H.; Wagner, M. In Situ Characterization ofNitrospira-Like Nitrite-Oxidizing Bacteria Active in Wastewater Treatment Plants. Appl. Environ. Microbiol. 2001, 67, 5273–5284. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.-Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef]
- Yan, J.-F.; Xiang, L.; Zhang, B.-Y.; Tang, C.; Xie, Y.-Q.; Li, Y.-W.; Feng, N.-X.; Liu, B.-L.; Li, H.; Cai, Q.-Y.; et al. Mechanism and Association between Microbial Nitrogen Transformation in Rhizosphere and Accumulation of Ciprofloxacin in Choysum (Brassica parachinensis). Environ. Sci. Technol. 2023, 57, 16053–16064. [Google Scholar] [CrossRef]
- Vendrell, P.F.; Zupancic, J. Determination of soil nitrate by transnitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1990, 21, 1705–1713. [Google Scholar] [CrossRef]
- Sims, G.K.; Ellsworth, T.R.; Mulvaney, R.L. Microscale determination of inorganic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 1995, 26, 303–316. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Department of Agriculture, US: Washington, DC, USA, 1954. [Google Scholar]
- Kilmer, V.J.; Nearpass, D. The determination of available sulfur in soils. Soil Sci. Soc. Am. J. 1960, 24, 337–340. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Tang, Y. Seasonal Dynamics of Sediment Microbial Communities at Different Distances from Artificial Reef Units. Microorganisms 2025, 13, 1194. https://doi.org/10.3390/microorganisms13061194
Zhu M, Tang Y. Seasonal Dynamics of Sediment Microbial Communities at Different Distances from Artificial Reef Units. Microorganisms. 2025; 13(6):1194. https://doi.org/10.3390/microorganisms13061194
Chicago/Turabian StyleZhu, Meiling, and Yanli Tang. 2025. "Seasonal Dynamics of Sediment Microbial Communities at Different Distances from Artificial Reef Units" Microorganisms 13, no. 6: 1194. https://doi.org/10.3390/microorganisms13061194
APA StyleZhu, M., & Tang, Y. (2025). Seasonal Dynamics of Sediment Microbial Communities at Different Distances from Artificial Reef Units. Microorganisms, 13(6), 1194. https://doi.org/10.3390/microorganisms13061194






