Abstract
The Hepatitis E virus (HEV) is responsible for causing Hepatitis E, a zoonotic disease that has emerged as a significant global health concern, accounting for about 20 million infections and 70,000 deaths annually. Although it is often recognized as a disease that is acute in low-income countries, HEV has also been recognized as a zoonotic disease in high-income countries. The zoonotic transmission requires flexible approaches to effectively monitor the virus, vectors, and reservoirs. However, the environmental monitoring of HEV presents additional challenges due to limitations in current detection methods, making it difficult to accurately assess the global prevalence of the virus. These challenges hinder efforts to fully understand the scope of the disease and to implement effective control measures. This review will explore these and other critical concerns, addressing gaps in HEV research and highlighting the need for improved strategies in the monitoring, prevention, and management of Hepatitis E using a One Health approach.
1. Introduction
Hepatitis E is a liver inflammation caused by the infection of the Hepatitis E virus (HEV). HEV is one of the most common causes of acute hepatitis in low-income countries. However, recently, it has been recognized as a zoonotic disease prevalent in high-income regions (e.g., Europe), where it is acquired mainly from pigs.
The HEV virion comprises 180 copies of capsid protein arranged in an icosahedral capsid. It has three structural domains: S (shell), M (middle), and P (protruding) [1,2,3]. The most conserved across genotypes is the S domain, which forms the icosahedral shell with a jelly-roll fold, serving as a base upon which the other two domains sit [3,4]. The P domain, involved in receptor binding and immune recognition, is subdivided into P1 (threefold protrusions) and P2 (twofold spikes), which contain β-barrel folds and potential polysaccharide-binding sites [2]. The M domain stabilizes the virion by binding to S and P [5]. The N-terminal region is essential for the native T = 3 structure assembly; its absence results in smaller T = 1 virus-like particles. The C-terminal region, while not affecting morphology, is necessary for RNA encapsidation and virion stability [6].
HEV has a single-stranded RNA with positive polarity, measuring about 27–34 nm in diameter and 7.2 kb in length, which includes short non-coding regions and three open reading frames (ORFs) [7]. It belongs to the Hepeviridae family, divided into the Orthohepevirinae subfamily, which infects mammals and birds, and the Parahepevirinae subfamily, which infects fish [8]. Within the genus Paslahepevirus, Paslahepevirus balayani includes eight different genotypes, with HEV genotypes 1 to 4 being the most frequently detected.
HEV transmission mainly occurs enterically through fomites, foodborne, zoonotic, and waterborne transmission routes. HEV genotypes 1 (GT1) and 2 (GT2) cause acute hepatitis outbreaks in humans, are predominant in low-income countries and are primarily transmitted through contaminated water sources. Conversely, genotypes 3 (GT3) and genotypes 4 (GT4) are zoonotic and more commonly found in high-income countries, transmitted mainly through raw or undercooked pork or game meat consumption [9,10]. However, in these countries, waterborne transmission can also occur, since GT3 has been detected in untreated and surface waters in European, North and South American, and Asian countries through human and animal waste, including wastewater and agricultural effluent [11,12,13,14].
According to the World Health Organization (WHO), HEV results in about 20 million new infections (3.3 million symptomatic cases) and over 70.000 deaths annually [15].
A systematic review and meta-analysis examining the global prevalence and risk factors of HEV infection found the highest HEV seroprevalence in Africa (21.76%), followed by Asia (15.80%), Europe (9.31%), North America (8.05%), South America (7.28%), and Oceania (5.99%). A global seroprevalence among the general population was estimated at 12.47% [16].
GT1 and GT2 have higher incidence rates in Asia, Africa, the Middle East, and Central America; when detected in Western countries, they are more often associated with travelers who have traveled to HEV-endemic areas. GT3 is present worldwide, while GT4 has become as significant as GT3 in countries such as China, Japan, Taiwan, Hong Kong, and South Korea [17].
The overall mortality rate for Hepatitis E caused by GT1 and GT2 ranges from 0.2 to 4% [9]. However, the most striking feature of GT1 and GT2 is the high mortality rate among pregnant women, which can reach up to 25% [18]. Regarding GT3 and GT4 genotypes, the infection generally presents as asymptomatic. Acute liver failure is rare in patients with GT3 and GT4 infections, regardless of preexisting liver disease, although there are documented reports from European countries [19,20,21,22]. Although preexisting chronic liver disease may predispose individuals to acute-on-chronic liver failure, a study conducted in France and the United Kingdom found a low prevalence of HEV RNA in patients with decompensated chronic liver disease and in patients with severe forms of acute alcoholic hepatitis [23,24]. Therefore, HEV infection may not predispose individuals to more severe liver disease; however, there are case reports documenting instances where liver transplantation was required due to HEV infection [22].
Virus Replication
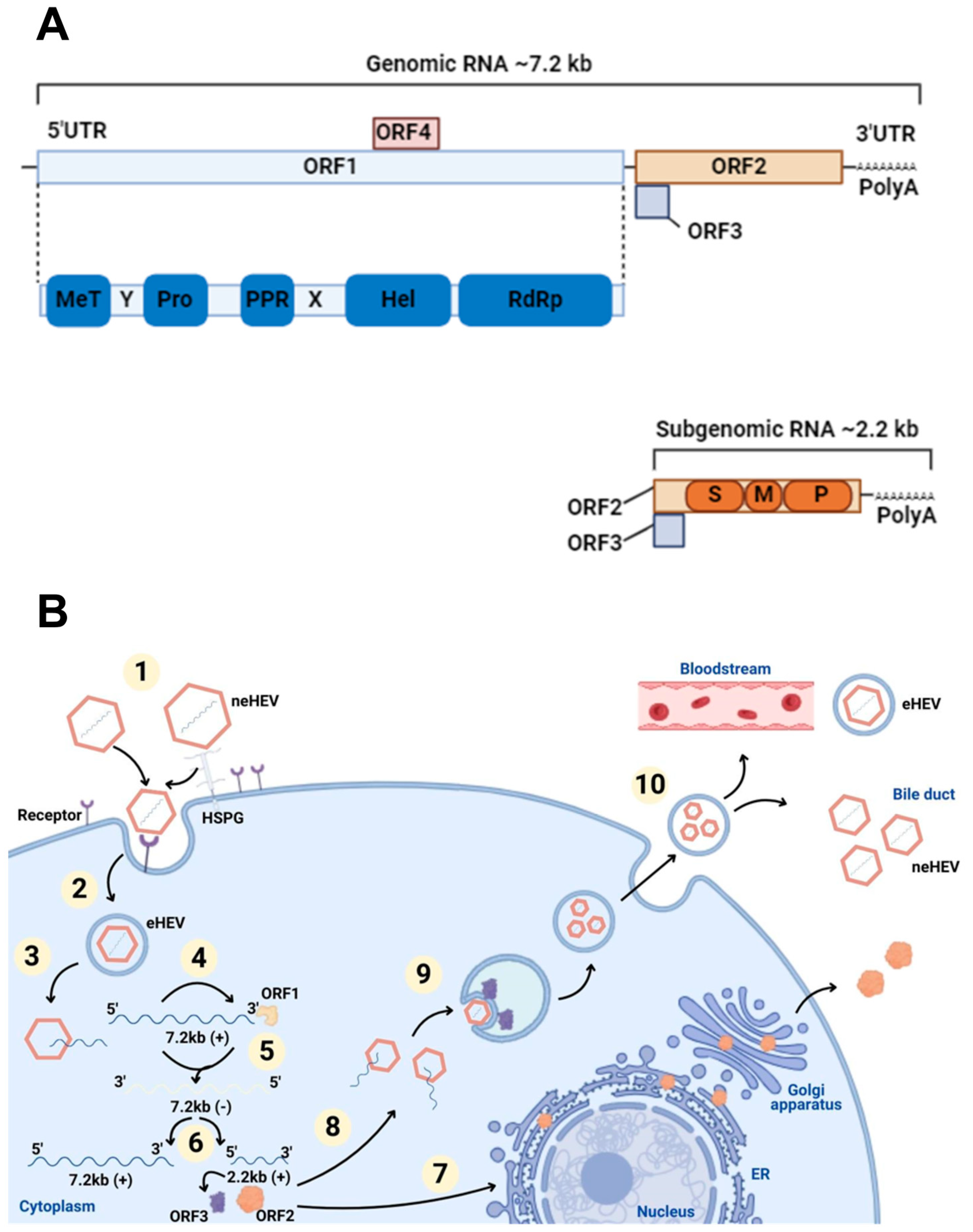
HEV viral RNA is generated as genomic RNA (7.2 kb) and a subgenomic RNA (2.2 kb). The subgenomic RNA initiates near the ORF1 stop codon, enabling the expression of ORF2 and ORF3. ORF1 facilitates viral RNA replication by encoding a polyprotein known as the HEV replicase; ORF2 has a longer form with a signal peptide for secretion (ORF2s) and a shorter form in the capsid (ORF2i). It encodes the capsid protein, which, as previously described, contains the S, M, and P structural domains; ORF3 encodes a phosphoprotein involved in morphogenesis and release of virions [7,10]. In the case of HEV1, it exhibits an additional reading frame, ORF4, which synthesizes a stress-induced protein in the endoplasmic reticulum necessary for the proper functioning of the viral RNA polymerase (Figure 1) [25].
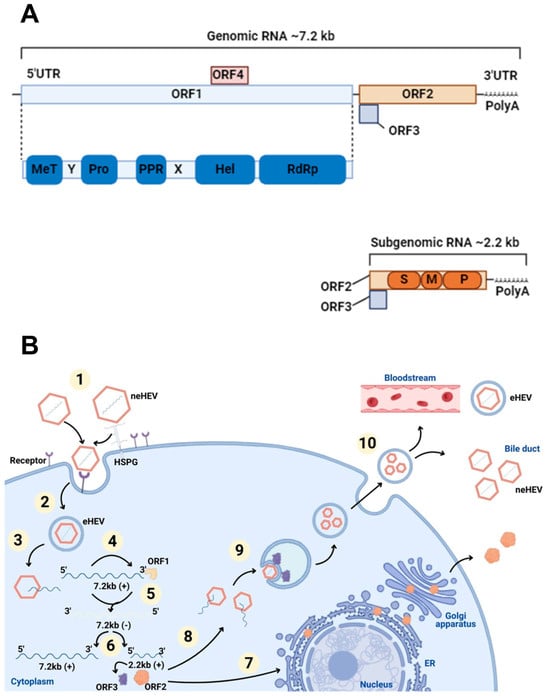
Figure 1.
The genome and life cycle of HEV. (A) HEV genome. Genomic RNA has a short 5′ untranslated region (UTR), three open reading frames (ORF), a 3′ UTR, and a 3′ polyadenylated (PolyA) tail. ORF1 (blue box) boards the domains: methyltransferase (MeT); Y domain (Y); cysteine protease (PRO); polyproline region (PPR); domain X (X); helicase (Hel); RNA polymerase (RdRp). ORF2 (orange box) and ORF3 (purple box) are translated from subgenomic RNA developed in viral replication. ORF2 contains three domains: S (shell), M (middle), and P (protruding). ORF4 (pink box) is found only in HEV1. (B) The life cycle of HEV. (1) Viral attachment: non-enveloped particles of the virus (neHEV) attach to receptors using HSPGs (heparan sulfate proteoglycans) present on the surface of liver cells as an anchoring point; (2) endocytosis: quasi-enveloped HEV (eHEV) particles internalize by clathrin-mediated endocytosis; (3) the release of genomic RNA: positive sense strand is released into the cytoplasm after dissociating from the viral capsid; (4) translation: genomic RNA is used as mRNA for the ORF1 protein production process; (5) RNA replication: a viral replicase contained by ORF1 synthesizes negative-sense intermediate strand RNA that acts as a template for transcription of genomic and subgenomic RNA; (6) translation of ORF2 and ORF3 from subgenomic RNA; (7) non-productive ORF2 pathway: part of the protein produced passes through the endoplasmic reticulum (ER) and is secreted out of the cell; (8) ORF2 productive pathway: the ORF2 capsid protein assembles with the genomic RNA and virions are formed; (9) packaging and assembly: virions are surrounded by a lipid bilayer vesicle and are incorporated into ORF3; (10) virus release: virions are released from cells, by the viroporin activity of ORF3 through the endosomal sorting complexes required for the transport (ESCRT), and can go into the bloodstream as eHEV or into the bile as neHEV. Created in Biorender.
HEV virions exist as non-enveloped (neHEV), secreted in feces, and as qua-si-enveloped (eHEV) virions, found in circulating blood and in the supernatant of in-fected cell cultures. The knowledge regarding the eHEV entry mechanism is poorly understood, but it is known that, upon contact with target cells, neHEV interacts with heparan sulfate proteoglycans. eHEV enters the cell in clathrin-dependent endocytosis via the dynamin-2 and membrane cholesterol pathways [26]. The PI3K pathway and actin were also identified as crucial for virus internalization and intracellular trafficking [27,28].
Once inside the cells, viral RNA is uncoated (a process that remains poorly understood) translated using the host’s translation machinery, while the viral replicase mediates genome replication and release. In short, the viral genomic RNA serves as mRNA for ORF1 polyprotein translation and produces functional enzymes and do-mains. The viral replicase RNA-dependent RNA polymerase (RdRp) synthesizes a complementary negative-sense RNA strand that serves as a template for HEV genome replication and the transcription of subgenomic RNA (sgRNA), which is responsible for the translation of ORF2 and ORF3 proteins [29,30]. The secreted form of the ORF2 protein undergoes post-translational modifications and functions as an immune decoy. In contrast, the capsid-associated form of ORF2 self-assembles into virus-like particles and packages genomic RNA into progeny virions [31]. The ORF3 protein interacts with microtubules and various host cellular proteins to modulate the intracellular environment in favor of HEV replication [32,33]. Moreover, ORF3 binds to the tumor susceptibility gene 101 (TSG101) protein, a component of the ESCRT pathway, facilitating the budding of nascent virions into multivesicular bodies (MVBs) [34]. These MVBs eventually fuse with the plasma membrane, releasing virions into the bloodstream as lipid-enveloped particles (eHEV) or into the bile duct, where bile salts degrade the quasi-envelope [35].
2. Sources of Hepatitis E in the Environment and Pathways of Environmental Transmission
2.1. Contaminated Water Sources
In low-income countries, the main mode of transmission of HEV is via the fecal-oral route, through contaminated water, which can be attributed to limited access to water, sanitation, hygiene, and health services, as well as inadequate disposal and wastewater treatment [36]. When viral particles are released from feces into the environment, they can directly contaminate drinking, recreational, and surface waters [37]. The last one poses a significant risk to the food production chain since it can indirectly reach shellfish culture and irrigation water, and impact vegetables, fruits, and seafood, to name a few [38]. Furthermore, the vicinity of pig production also represents a contamination risk to surface water, due to runoff waters, percolation events, or agronomic use of pig slurry [39,40]. In addition to inadequate sanitation treatment, the infectious particles in the water are also due to the extremely resistant feature of non-enveloped virions, such as HEV. They are persistent and resistant to several factors, such as drying, UV, and chemical treatments. These factors enhance the virus’s ability to transmit to naïve hosts through the environment and confer distinct advantages of survival and transmission within sensitive populations [41].
In high-income countries, HEV-related outbreaks and cases linked to contaminated water are rarely reported. However, some authors highlight a gap in understanding the potential for waterborne transmission in these settings, as studies conducted in France suggest that contaminated water may play a role in the country’s HEV epidemiology [42,43].
Table 1 highlights the global burden of HEV outbreaks, demonstrating significant geographical and numerical disparities in its impact. The largest documented outbreak was in China in 1986-1988, affecting approximately 120,000 individuals due to water supply contamination. In comparison, India experienced a large epidemic in 1990–1991 with 79,091 cases, or a reduction of approximately 34% compared to the Chinese outbreak. Similarly, 16,175 cases of HEV occurred in Turkmenistan along with a collapse of the water distribution system in 1985—more than 23 times more cases than in Nepal (692 cases in 1995), which were the result of drinking water contaminated with fecal matter contaminated drinking water. Bangladesh also experienced a major outbreak in 2008–2009, comprising 4751 cases from wastewater contamination of the municipal water supply, while Pakistan had only 133 reported cases in 1987, a difference of nearly 35-fold.

Table 1.
HEV waterborne outbreaks worldwide.
In Africa, Uganda and Somalia had serious outbreaks. Uganda had more than 10,196 cases in 2007, while Somalia had 11,413 cases in 1988–1989, approximately 12% more cases than in Uganda. Both outbreaks resulted from the consumption of untreated river water or public water system breakdowns. These trends show the substantial public health challenge posed by HEV in these regions, likely driven by factors such as inadequate sanitation, and contaminated water supplies. Outbreaks in Mexico, Cuba, or Iraq represented fewer than 300 cases, which may reflect more effective containment measures, improved surveillance, or possible underreporting. Cumulatively, the evidence indicates a strong qualitative correlation between the magnitude of HEV outbreaks and the concurrence of poor water sanitation, dense population centers, and poorly established public health infrastructure—circumstances that, if present, appear to facilitate extensive transmission of the virus through contaminated water supplies. These patterns emphasize the vulnerability of low-income countries to HEV, highlighting the critical need for targeted interventions, including improved water quality, sanitation measures, and access to healthcare.
Apart from the outbreaks, recently, rat HEV, belonging to the Orthohepevirus C species, has been reported infecting humans in Hong Kong, Canada, and Spain, including both immunocompromised patients and an immunocompetent individual [89,90,91,92,93]. Additionally, rat HEV has been detected in wastewater, raising concerns about potential environmental exposure [94,95]. While zoonotic transmission from rats to humans has been suggested, the exact sources and routes remain unknown.
2.2. Animal Reservoirs and Foodborne Transmission
In 1990, using an experimental model, Balayan reported HEV infection in domestic pigs for the first time [96]. In 1997, the first case of HEV infection in domestic pigs was identified in the USA. This isolate was genetically related to the human HEV strains from the USA and showed cross-reactivity with antibodies against the human HEV capsid protein [97]. Pigs generally do not show clinical symptoms associated with hepatitis, although the presence of hepatitis has already been demonstrated by microscopy [98].
HEV is endemic on domestic swine farms worldwide, and pigs older than three to four months have been found to have antibodies against HEV [99,100]. Since the virus was first identified in domestic swine, many countries have reported finding the virus or antibodies in their pig herds, such as Argentina [101], Brazil [102,103,104], Colombia [105], Czech Republic [106], Denmark [107], France [108], Italy [106], Korea [109], Serbia [110], and Spain [106,111].
The zoonotic potential of genotypes GT3 and GT4 has been well documented, as they have been described in humans as well as in Sus scrofa domesticus (domestic pigs) and Sus scrofa (wild boars) [98,112] Recently, the range of wild and domestic hosts infected with GT3 and GT4 has increased, and the genotypes circulating in wild ungulates have similarities with human genotypes [98,113].
Among animals in Bulgaria, HEV seroprevalence varies from 32.2% in sheep, 24.4% in goats, 21.1% in dogs, 17.7% in cats, 8.3% in horses to 7.7% in cattle [114]. A study conducted in Irish breeding pigs in 2015 highlighted that 81% of pigs were seropositive, indicating a high HEV infection rate [115]. In the UK, a study reported that 92.8% of pigs were IgG-positive, with 20.5% HEV RNA-positive at the point of slaughter [116].
Over the years, studies conducted in several countries have assessed the presence of HEV genetic material or specific antibodies in wild boars. HEV presence has been confirmed in France [117], Germany [118,119], Italy [120,121,122,123], Netherlands [14], Portugal [112,124], Spain [125,126,127], and Sweden [128].
In 2003, the first identification of HEV genetic material in deer occurred. Along with this identification, the first evidence of foodborne transmission of HEV emerged, associated with an outbreak of acute hepatitis that affected four members of the same family, who consumed raw meat from Japanese deer (Cervus nippon) meat [113,129]. After this first report, studies in other countries detected HEV in deer, such as Japan [130,131,132], Spain [125], Netherlands [14], and Uruguay [133].
Several studies have reported evidence of HEV exposure or infection in various animal species, either through the detection of anti-HEV antibodies or HEV RNA, suggesting that these animals may play a potential role in the epidemiology of the virus, such as cattle [134,135,136,137,138], sheep [135,137,139,140,141], goat [137,140,142,143,144,145], buffalos [137] and mooses [146,147]. In addition to these species, HEV has also been detected in other mammals such as, rabbits [148,149,150,151,152,153,154,155], mongooses [156,157,158], ferrets [159], camels [160,161,162], musks [163], rats [164,165,166,167,168,169,170,171,172,173,174,175], mice [176,177], voles [176,178], minks [179,180], shrews [171], foxes [181], bats [182,183,184], monkeys [185,186,187,188], bisons [189], bears [190], macaques [1,188,191,192,193,194,195,196,197,198,199], cats [200,201], chimpanzees [186,198,202,203,204,205], leopards [190], dogs [136,200,206,207], pigs [97], donkeys [208], brown hares [153], langurs [191], horses [209,210], gerbils [211], tamarins [192], raccons [212], dolphins [213], deers [131,132,210,214,215,216], muntjacs [210], wild boars [117,119,127,131,189,215,217,218,219,220,221,222], and yaks [223]. There are also reports of HEV circulating in clams [224], and mussels [225,226,227,228]. In addition to chickens [229,230], cranes [190], pheasants [190], griffons [231], buzzards [232], ducks [230], pigeons [232], geeses [230], little egrets [233], little owls [232], thrushes [232], sparrows [234], and turkeys [235].
The consumption of raw or undercooked pork products has been identified as a primary risk factor for HEV infection in high-income countries [236]. Products with the highest rate of HEV infection include those made from meat, liver, or viscera when consumed raw or undercooked [98,237]. The virus present in the liver of commercial pigs remains fully infectious and incubating the contaminated meat at a temperature of 56 °C, equivalent to a medium-rare cooking condition, did not completely inactivate the virus [238,239].
Studies from various countries have reported HEV contamination in a variety of meat products. In the food production chain, the slaughter process poses a significant risk of cross-contamination through the use of virus-contaminated tools or handling practices. Di Bartolo [106] detected HEV on the floor, working surfaces, workers hands, and aprons during pig dissection, indicating that the initial production areas (from bleeding to evisceration) are at higher risk for fecal contamination and highlighting potential hazards for workers [106].
Since the first report of foodborne HEV [129], the number of sporadic cases and small outbreaks has increased over the years (Table 2).

Table 2.
Foodborne cases and outbreaks related to HEV worldwide. * Raw pork liver sausages.
The data in Table 2 allow us to recognize patterns in the foodborne spread of HEV, primarily due to the ingestion of raw or uncooked meat products from either domestic or wild animals. The data show foodborne HEV outbreaks that were reported in France and Japan, with at least six separate incidents taking place in Japan alone from 2003 to 2006. The fact that the incidents took place over several years reveals the persistent nature of foodborne HEV threats in the region, typically GT3 or GT4.
In France, figatelli and other pork products were the sources of outbreaks. These were also caused by GT3, once more indicating its zoonotic and foodborne significance in Europe. Notably, an individual French outbreak took place over the years (2007–2009) with at least three cases, showing that certain traditional foods can be ongoing sources of HEV exposure if not properly cooked or processed.
Spain also reported two foodborne cases, one each in 2012 (linked to pork meat) and 2015 (linked to wild boar meat), the latter involving eight individuals, one of the largest foodborne clusters in the table. This outbreak alone accounts for almost 20% of all reported foodborne HEV cases here, indicating the very high risk from consumption of wild game meat.
In South Korea (2009), a single HEV case was associated with the consumption of wild boar bile juice, demonstrating alternative exposures other than through meat, possibly through organ-derived products or traditional medicine. In China (2018), a foodborne outbreak caused by pork meat affected 41 people, which is both the largest outbreak in the table and the only outbreak with more than ten cases. This outbreak is particularly noteworthy because it shows that foodborne HEV outbreaks are not limited to small, localized events, and given the right circumstances—i.e., the wide distribution of contaminated meat—they can take on large proportions. There is one report from the United Arab Emirates (2012), where HEV genotype 7 was identified in a case resulting from the consumption of camel milk. The report is important because it extends the known portfolio of HEV genotypes implicated in foodborne transmission and also because it resurrects questions concerning the role of non-porcine animals in HEV ecology and human infection, especially where raw milk or other animal products are culturally significant.
Trends and conclusions given here are drawn solely from reported events and outbreaks as presented in Table 2. They hence provide the characteristics and outcomes of study samples with documented foodborne HEV transmission events worldwide.
3. Current Concentration Methods and Their Limitations and Challenges
The effectiveness of HEV detection in food and environmental matrices is greatly dependent on the viral concentration approach employed, since recovery efficiency directly influences the sensitivity and reliability of downstream analyses. The concentration of HEV in environmental samples faces several overarching challenges, including the wide range of matrices such as water, wastewater, meat, and vegetables, to name a few; beyond the different approaches that must be taken to ensure viral recovery in each one [254,255,256]. The concentration step is essential for virus detection, as viral contamination in food and environmental samples typically occurs at low levels and in highly diluted forms. Furthermore, concentrating viruses from larger sample volumes enhances the sensitivity of detection methods [257,258,259].
The main methods used for HEV concentration include adsorption-elution, ultracentrifugation, filtration, and polyethylene glycol (PEG) precipitation. Membrane-based methods are already consolidated and most used for virus detection in water samples [260,261,262,263]. This method has already been standardized for Norovirus and Hepatitis A, as described in the ISO 15216-1:2017 [264] and it has been tested for HEV detection with adjustments that require a new validation for these specific conditions [37,265]. Briefly, it consists of passing a certain volume of water through a polarized membrane to which the viruses will adsorb via electrostatic interactions. After this, a solution will elute viruses from the membrane. Samples can pass through a secondary concentration process or undergo nucleic acid extraction after elution from the membrane. Depending on the type of wastewater used and how the wastewater is treated, membrane-based methods can be adopted if a clarification step is added to prevent filter clogging [266,267,268,269]. Membrane-based methods often require sample preparation and are highly dependent on the physical-chemical properties of the analyzed sample for optimal viral recovery. Nevertheless, membrane adsorption remains a straightforward and adaptable technique capable of concentrating viruses from substantial volumes of water or wastewater samples, a distinct advantage in detecting HEV.
PEG is a common precipitating agent used in concentration methods for decades in different matrices [270], including multiple HEV concentration protocols. PEG binds to water molecules, forming a protein pellet containing viruses and other proteins [271].
In HEV concentration in meat products, PEG also precipitates proteins such as myoglobin and hemoglobin, which can inhibit the PCR reaction [272]. In the same way, in pork livers, the presence of bile salts is a major challenge as it is known to be a PCR inhibitor [273]. To address this, other steps are implemented to clarify the sample and eliminate lipids and proteins that can interfere with further molecular analysis. Clarifying steps may include centrifugation for phase separation, organic solvents to separate the protein phase from the rest of the sample, and emulsifiers for better lipid removal.
Compared to other options, PEG is inexpensive. However, it is a labor-intensive technique that requires time and meticulous handling to execute, potentially affecting the reproducibility of results across laboratories. Related to HEV, PEG methods are shown to be less effective in HEV recovery from meat products compared with ultrafiltration and ultracentrifugation [272,274]. In wastewater, PEG is also shown to have lower recovery rates than ultracentrifugation [275]. However, ultrafiltration and ultracentrifugation are much more expensive techniques requiring costly equipment and resources to execute.
Ultracentrifugation is used in a variety of matrices for viral recovery [262,276,277]. The technique consists of virus concentration from aqueous solutions and organic homogenates using considerable force to disassociate both the solid and aqueous parts. Ultracentrifugation has already been assessed to recover HEV from environmental samples. This technique effectively clarifies and concentrates viral particles, although it may also co-concentrate impurities, reducing a large volume to a smaller one. Although it can be easily standardized across laboratories, its primary limitation is the high cost of the equipment, making it less accessible in resource-limited settings.
Ultrafiltration is a widely employed method for recovering viruses from environmental samples [278,279,280]. This technique does not require sample preacidification, can simultaneously concentrate multiple pathogens, and achieves high recovery rates [281]. It allows the concentration of large water volumes, up to 1000 liters, but typically necessitates an additional concentration step since the elution process uses 200–500 mL of eluent [282,283,284,285].
The ultrafiltration process is based on size exclusion and includes various approaches, such as dead-end ultrafiltration (DEUF), tangential flow ultrafiltration (TFUF), hollow-fiber ultrafiltration, vortex flow filtration, electronegative filtration, and centrifugal ultrafiltration. DEUF forces water perpendicularly through hollow fiber membranes, trapping particles larger than the pore size, and is particularly suitable for processing large volumes of water [285,286]. TFUF, in contrast, uses a tangential flow to minimize microbial adherence to the membrane, with the retentate concentrating larger particles [287]. Similarly, hollow-fiber ultrafiltration operates in a tangential flow pattern, where viruses exceeding the membrane cutoff are retained and eluted for further analysis [288].
Vortex flow filtration employs Taylor vortices generated by rotating a cylindrical filter, preventing particles from adhering to the membrane surface as water is filtered. Although this automated method shows potential, it is rarely applied for concentrating human pathogenic viruses in environmental water samples [289]. Electronegative filtration, a standard method for concentrating viral particles from wastewater, involves altering the sample’s surface charge to enhance virus binding to electronegative filters [290,291]. Finally, centrifugal ultrafiltration uses centrifugal force to drive liquids through ultrafilters, selectively retaining viral particles while removing smaller contaminants and excess liquid [292].
Each variation of ultrafiltration offers unique advantages depending on the sample type and analytical requirements, making it a versatile tool for viral concentration in environmental studies.
3.1. Waterborne HEV Studies
The study of Salvador [37] assessed HEV presence in drinking and surface water using three concentration methods. Large water volumes were filtered using NanoCeram® PAC-AG electropositive filters (Nanoceram Filters, Caslte Rock, CO, USA). The filters were eluted with 3% beef extract, and the eluate underwent organic flocculation, followed by sample concentration using Vivaspin® (Sartorius, Göttingen, Germany) concentrators through centrifugation. The results evidenced HEV RNA in 77.8% of surface water and 66.7% of drinking water and infectious HEV in 23.0% of surface water and 27.7% of drinking water [37]. Hennechart-Collette [265] tested a method for detecting Hepatitis A (HAV), HEV, and norovirus in tap and bottled drinking water adapted from the ISO/DIS 16140-4:2018 [293]. In this method, virus particles are concentrated using one filter with a 47 mm positively charged membrane with a pore size of 0.45 μm. The recovery rates for HEV ranged from 27.87% to 53.54% when analyzed from pure RNA extracts based on the water samples. The LOD95 (Limit of Detection) for HEV was determined to be 2.8 genome copies per milliliter of water sample, and the LOQ (Limit of Quantification) was also 2.8 genome copies per milliliter of water sample [265].
Wang [294] described a method to concentrate HEV in raw and still tap water as well as sewage. Firstly, the samples are centrifuged and filtered twice through Nano-Ceram® cartridge filters at an average flow rate of 2.5 L/min, where viruses are electrostatically attached to the filter. Viruses are eluted from the filters and filtered through a Sartobran® Capsule filter (0.65/0.45 μm) (Sartorius, Göttingen, Germany). Finally, the filtrate is ultracentrifuged. In tap water, 10–130 International Units (IUs) of HEV RNA/mL (subtypes GT3a and GT3c/i) were identified [294].
Cuevas-Ferrando [255] evaluated various virus concentration methods for wastewater and drinking water. For influent wastewater, two approaches were used: an ultracentrifugation-based method (UC) and an aluminum hydroxide adsorption–precipitation method (Al). In the UC method, influent water underwent sequential centrifugation and ultracentrifugation. In the Al method, water was treated with aluminum hydroxide, centrifuged, and resuspended in 3% beef extract, with the concentrate recovered by additional centrifugation. For drinking water, the primary concentration utilized a DEUF system, followed by a secondary concentration using PEG precipitation and centrifuge filtration using Amicon® Ultra-15 tubes (Merck KGaA, Darmstadt, Germany). The study evaluated HEV recovery and detection in water samples, finding average recoveries of 15.2%, 19.9%, and 16.9% in influent, effluent, and drinking water, respectively, with detection limits of 103–104 IU/L. A one-year pilot study assessed HEV prevalence in influent and effluent water from four influent and effluent water in waste and two drinking-water treatment plants using three RT-qPCR assays. HEV was detected in 30.65% of influent samples, with an average concentration of 6.3 × 103 IU/L, but not in effluent or drinking-water samples. Despite HEV circulation in the Valencian region, limited assay sensitivity and unknown waterborne infective doses prevented definitive risk assessments of environmental contamination [255].
Wassaf [295] provided HEV detection results by molecular detection on environmental samples, specifically in wastewater samples, using centrifugation and PEG precipitation with HEV (GT3, subtypes a, b, and c) detected in 6.3% of raw sewage samples and 3.2% of riverine samples [295], while Miura’s [296] study used crossflow ultrafiltration and PEG to concentrate effluent samples and recovered concentrations varying from 5.8 to 3.0 RNAc/L, apart from those <LOD (2.2 log RNAc/L) [296].
3.2. Foodborne HEV Studies
The study conducted by Son [274] compared different buffer and concentration methods to detect HEV RNA in swine liver samples, including (i) phosphate-buffered saline (PBS, pH 7.4) using ultrafiltration; (ii) threonine buffer using PEG precipitation and ultrafiltration; and (iii) glycine buffer using ultrafiltration and PEG precipitation. Using phosphate-buffered saline ultrafiltration, real-time RT-PCR detected HEV in 6 of the 26 samples. Using threonine buffer with PEG precipitation and ultrafiltration, real-time RT-PCR detected HEV in 1 and 3 of the 26 samples, respectively. When glycine buffer was used combined with ultrafiltration and PEG precipitation, real-time RT-PCR detected HEV in 1 and 3 samples of the 26 samples, respectively. For nested RT-PCR, all samples tested negative regardless of the type of elution buffer or concentration method used [274].
Chatonnat [297] evaluated the presence of HEV RNA in pork liver pâtés and raw pork livers. Pâté was vortexed with internal control virus suspension, TRI Reagent Solution® (Molecular Research Center, Inc., Cincinnati, OH, USA), and centrifuged. Raw pork liver was homogenized with internal control virus suspension, TRI Reagent Solution®, RNaseOUT™ (Thermo Fisher Scientific, Carlsbad, CA, USA), and glass beads in a Bead Genie device (Scientific Industries, Bohemia, NY, USA), followed by a centrifugation step. HEV RNA was detected in 29% of the pâtés and 4% of the raw pork livers [297].
The study of Martin-Latil [263] evaluated three virus elution–concentration methods for food samples containing raw pig liver, using three elution buffers: distilled water, PBS (pH 7.4), and TGEB (Tris–HCl, glycine, beef extract, pH 9.5). The methods differ in their handling of viral particles before nucleic acid extraction. In short, Method 1 consisted of sample homogenization in an elution buffer, incubation, and centrifugation, followed by treatment with PEG and 0.3 M NaCl, and centrifugation. Method 2 followed the same steps as Method 1 up to the PEG precipitation and centrifugation. However, the pellet was resuspended in buffer, mixed with chloroform–butanol (1:1), and centrifuged. In Method 3, chloroform–butanol (1:1) was added to the filtrate early to purify viral particles before PEG precipitation and centrifugation. After centrifugation, the aqueous phase containing viruses was subjected to the steps of Method 1 for concentration. The mean recovery rate of HEV from pig liver sausages was 3.94%, and the recovery increased to 18.38% in Figatelli sausages [263].
Randazzo [256] study evaluated methods to concentrate HEV from lettuce and wastewater. For lettuces, Method A was based on the ISO 15216-1:2017 [264], including lettuce elution on TGBE buffer, centrifugation, pH adjusting, PEG and NaCl supplementing, and centrifugation again.
Method B was a modified version of ISO 15216-1:2017 [264], including lettuce elution in TGBE buffer, and PEG supplementing. In Method C, viruses were eluted in lettuce using buffered peptone water (BPW) with Pulsifier equipment. The filtrate was treated with PEG and NaCl, centrifuged, and resuspended in PBS. Sewage samples were concentrated by ultracentrifugation. Irrigation water samples were concentrated by filtration.
The ISO 15216 protocol resulted in average HEV recoveries of 2% in vegetables, while no detection was reported in lettuce or irrigation waters. HEV was detected in sewage only (10/14) [256].
One of the major challenges in both foodborne and waterborne HEV studies is the lack of standardized protocols for viral concentration and detection. In foodborne studies, the wide variety of food matrices—ranging from raw liver to pâtés and cured meats—necessitates the use of diverse methodologies. A similar issue is observed in waterborne studies, where although the impact of the matrix type (e.g., wastewater, river water, drinking water, irrigation water) is generally less pronounced, the same lack of protocol standardization persists. Across both contexts, differences in buffer type, sample size, elution conditions, and RNA extraction kits often result in substantially variable detection rates. Therefore, variations in reported HEV prevalence across studies may reflect as much due to methodological variability as to actual differences in contamination levels, making it essential to harmonize methods for reliable comparison and risk assessment.
4. Current Detection Methods and Their Limitations and Challenges
4.1. Molecular Techniques
Since pork products are important sources of HEV infection in humans, it is necessary to implement protocols to detect the presence of infectious viruses in pork and pork-derived products [298]. Predominantly, molecular methods for detecting HEV, specifically RT-PCR (Reverse Transcription Polymerase Chain Reaction), have been employed in pork products [299].
Szabo [300] aimed to investigate the distribution of HEV-positive meat products in Germany and carried out different methodologies for homogenizing and extracting sausage samples, followed by identification through real-time RT-PCR. The extraction method using TRI Reagent® yielded the best results. Among the 120 retail samples tested, 21% were HEV-positive, including 20% of raw sausages and 22% of liver sausages. Positive samples were subsequently tested by a nested RT-PCR to get sequences for comparison with other strains. 18 out of 25 were confirmed as HEV-positive, indicating the presence of different viral strain subtypes of GT3. The study showed a high presence of HEV in pork products and optimized extraction protocols, resulting in an efficient methodology for detecting HEV in different types of sausages [300].
It has been found that the RT-qPCR technique achieves greater sensitivity in detecting HEV compared to variations of nested PCR. In this regard, Marziali [301] applied the methodology TaqMan qPCR assay previously described by Jothikumar [302] with internal control (MS2 phage), aiming to study the prevalence of HEV in pig production farms in Argentina, as well as to perform genotyping analysis of positive samples. For this, 135 porcine fecal samples were collected from pigs from 16 different establishments in the central region of Argentina. The genetic material extraction was performed with the NucleoSpin RNA Virus Kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany). HEV detection used the TaqMan qPCR assay targeting a well-conserved sequence within the overlapping HEV ORF2/3 region, and multiplex was used for MS2 detection. The results showed an HEV positivity rate of 8.1% (11/135 samples), with these samples derived from six different locations. The infection rate of confined pigs was 8.7%, compared to 6.3% for outdoor pigs [301].
Son [274] used the Jothikumar protocol [302] and compared it with a nested PCR to determine which methodology had greater sensitivity for detecting HEV. For this, HEV-positive human and porcine fecal samples were used as positive controls, as well as HEV-positive porcine livers. Liver, feces, and porcine bile samples were collected at slaughterhouses. RNA extraction from the samples was performed with the RNeasy Mini Kit (Qiagen, Hilden, Germany). The results showed that 11.1% of bile samples and 12.7% of porcine fecal samples tested positive for HEV using nested PCR. In comparison, 42.2% of bile samples and 15.2% of porcine fecal samples were positive for HEV in real-time RT-PCR. Overall, positive samples varied according to the buffer and molecular method used. The highest detection rate was achieved using PBS as buffer combined with real-time RT-PCR, where 19.2% of liver samples were identified as positive for HEV, and 23.1% of the same ultra-purified samples also tested positive [274].
Porea [303] sought to determine the prevalence of HEV in wild boars from eastern Romania, identifying circulating strains and correlating them with data from previous studies in the region. Fifty samples of liver (45) and spleen (5) from wild boars were collected, and RNA extraction was performed using the RNeasy Mini Kit (Qiagen) with β-mercaptoethanol. To identify HEV-positive samples, the Jothikumar protocol [302] was performed targeting the ORF3 gene. A standard HEV RNA, produced from the transcription of a pCDNA 3.1 ORF2-3 HEV plasmid, was used. Then, nested PCR was performed to amplify ORF2 fragments and allow sequencing analysis of the products. Positive amplicons were sequenced using an internal primer from Nested PCR. The authors found an 18% prevalence of HEV, and all isolates were GT3 [303].
Three of the cited studies employed the Jothikumar protocol [302], albeit with variations in extraction kits and internal controls. The discrepancies in results can be attributed to differences in the matrices sampled and the geographic locations of the studies. Establishing a standardized technique is essential to ensure the comparability of results for specific sample types while minimizing the risk of false-negative and false-positive outcomes. Furthermore, the prevalence of HEV varies between countries, reflecting the circulation of distinct genotypes in different regions.
Zhang [304] explored the prevalence of HEV in Tibetan pig populations in China using nested PCR. From bile samples, RNA extraction was performed with TRIzol™ Reagent (Thermo Fisher Scientific, Carlsbad, USA). cDNA was synthesized for the first round of PCR, along with Forward and Reverse primers targeting sequences of the ORF2 gene and Ex-Taq DNA enzyme. Nested PCR was performed with a set of internal primers and the product of the first PCR. The study revealed 11 positive HEV samples (4.35%), with sequencing revealing GT4 and subgenotype 4b [304].
Among the most modern methodologies based on the detection of genetic material, the RT-RPA (Reverse Transcription Recombinase Polymerase Amplification) technique stands out for its speed in obtaining results, high sensitivity and specificity, and relatively low cost due to the reduced need for equipment, due to the isothermal nature of the reaction [305]
Recent studies, as discussed by Wang [306], involve the development of reverse transcription recombinase polymerase amplification (RT-RPA) techniques targeting the HEV ORF2 gene, integrating fluorescence detection platforms (qRT-RPA) and lateral flow biosensor visible to the naked eye (LFB RT-RPA). The enzyme-based method consists of primer annealing and extension mediated by different enzymes at a constant temperature between 37 and 42 °C. During amplification, amplicons are conjugated to gold particles. Subsequently, the lateral flow test occurs where amplicons move by capillarity and positive samples are visually detected by comparison with controls. Compared to qRT-PCR, the qRT-RPA and LFB RT-RPA assays showed strong agreement, identifying 11 and 12 HEV RNA-positive samples, respectively, out of 14 confirmed positive cases. Their coincidence rates with qRT-PCR were 96.3% for qRT-RPA and 97.5% for LFB RT-RPA. The assays demonstrated resilience to mutations, successfully detecting HEV genotype 4d despite the presence of 4 to 9 nucleotide mismatches in the primers and probes. However, three samples with low HEV RNA levels (Ct values between 34.22 and 37.04) tested negative in the RT-RPA assays but were weakly positive by qRT-PCR. This highlights the slightly lower sensitivity of RT-RPA in detecting low-viral-load samples. The study concluded that RT-RPA assays are effective diagnostic tools for rapid HEV detection, particularly in resource-limited settings, although further validation is required for other HEV genotypes [306].
RPA technology offers several advantages. It operates at low temperatures, such as body temperature, or with simple heating equipment like water baths, making it suitable for use in diverse environments. Its ability to tolerate up to nine mismatches in primers and probes allows for flexibility in detecting viruses with significant genetic variability. Additionally, the reagents are freeze-dried, enabling storage at room temperature for up to six months, which is especially valuable in resource-limited or remote settings. These features make RPA a convenient and rapid alternative for point-of-care diagnostics. However, RPA also presents challenges. The requirement to open reaction tubes for lateral flow biosensor analysis increases the risk of aerosol contamination, particularly in field or laboratory settings with limited containment measures. To mitigate this risk, strict laboratory partitions and frequent use of UV irradiation or DNase treatment are necessary. Handling must also be performed with care, including frequent glove changes and proper tube management. Furthermore, RPA showed slightly lower sensitivity compared to qRT-PCR, as it failed to detect low viral RNA concentrations in some samples. Despite these drawbacks, the speed, ease of use, and low infrastructure requirements make RPA a promising diagnostic tool in various contexts [306].
Using the same technique, Li [307] tested 28 human samples (feces and serum) and 360 rabbit samples (feces and serum). The results showed that when compared to RT-qPCR, RT-RPA-LFS (37 °C for 30 min) was statistically equally sensitive and specific to HEV, with sequencing identifying GT3 and GT4 genotypes [307].
Other isothermal methods useful in HEV diagnosis, which can be applied in the field, include Transcription Mediated Amplification (TMA) and Loop-Mediated Isothermal Amplification (LAMP) [308].
Genetic heterogeneity of HEV is a complicating factor for molecular biology detection assays. Various genotypes could require special adjustments to PCR methodologies or the employment of more generic primers and probes. Genetic diversity can also affect immune responses, complicating the interpretation of serologic tests and may affect the accuracy of prevalence estimates. Furthermore, while being very sensitive and specific, RT-qPCR and nested PCR methods are susceptible to inhibitors in environmental samples such as organic matter or disinfectants used in water treatment. pH, salinity, and detergent concentration can lower the efficacy of RNA extraction and prevent amplification. Therefore, standardizing sample preprocessing and virus-concentration protocols is critical. The lack of standardized practice in laboratories complicates data comparison and the development of a global epidemiological overview.
A key question is the distinction between the detection of mere viral RNA and true infectivity. RT-qPCR is not capable of distinguishing between infectious and non-infectious viral particles and non-viable fragments. When the environmental sample indicates a high viral load, this may not always indicate an immediate risk of infection because most of the particles may be non-viable. Supportive methods such as cell culture or PMA-qPCR can help estimate the infectivity of HEV particles.
4.2. Cell-Culture Assays
Cell culture is the gold standard for assessing virus infectivity. However, for HEV, scientists face the absence of efficient cell culture systems and difficulties that require several complex and time-consuming adaptations for effective growth in cell cultures. As a result, the pathogenesis of HEV infection and the basic steps of its life cycle remain understudied [309]. HEV is very difficult to propagate in conventional cell lines and so, there are no standardized methodologies involving cell culture in recognizing HEV infectivity. Although various cell lines and HEV strains have been tested, viral replication is typically slow and results in low virion yields, often leading to non-productive infections [310]. Furthermore, studies on infection in cell cultures have not allowed visualization of viral cytopathic effect, necessitating molecular or immunofluorescence techniques for quantifying infectious HEV, which increases costs [299]. Here, we present some of the cell culture systems developed in the last few years.
Several cell culture systems have been identified for studying human HEV, including HEV isolated from patients, complementary DNA clones, cancer-derived cell lines, primary human hepatocytes, induced pluripotent stem cells, as well as cell culture systems for animal-derived HEV [310]
The HEV viruses used for in vitro and in vivo infection studies can be recovered from patients and are well documented in literature, including the strains Sar-55 (GT1) [53,311], MEX-14 (GT2) [312], 87A (GT1) [44], F23 (GT1) [100], JE03-1760F (GT3) [313], HE-JF5/15F (GT4) [314], TW6196E [315], Kernow-C1 (GT3) [316], and 47832 (GT3) [317]. However, these viruses cannot have their genomes easily altered, presenting a challenge for studying viral protein functions and non-coding regions. In such cases, cDNA clones are utilized.
A pSGI-HEV(I) (GT1) was constructed through subgenomic PCR amplification of hepatitis E outbreak isolates from India. However, in vitro-produced RNA alone failed to infect rhesus monkeys due to the absence of the 5′ cap, which was later identified as essential [318,319,320]. In another study, a pSK-HEV-2-derived was generated via RT-PCR to produce full-length cDNA clones of the HEV isolate Sar-55 (GT1) [319].
A system was also developed to generate swine HEV-derived cDNA clones (pSHEV-1 to -3) by amplifying eight overlapping fragments covering the entire genome, which were subsequently ligated into the pGEM-9zf(-) vector. These clones demonstrated replication competence in Huh-7 cells [321]. Another study synthesized a cDNA clone corresponding to the GT3 strain JE03-1760F (pJE03-1760F/wt) by amplifying three fragments covering the entire genome, which were then ligated into the pUC19ΔAatIISapI vector [322].
For GT4, a cDNA clone (pHEV-4TW) was derived from strain TW6196E by assembling three overlapping genomic fragments. This construct was transfected into a hepatocellular carcinoma cell line, and ORF2 expression confirmed its replication competence. The progeny of this clone also demonstrated the ability to infect HepG2/C3A cells. Notably, both GT3 and GT4 clones are infectious not only in vitro but also in vivo, retaining their ability to infect pigs, their natural hosts [315,323].
In addition to these, other studies have also reported the creation of cDNA clones, including a cDNA clone derived from the wild type and adapted GT3 strain Kernow-C1 and a full-length cDNA clone based on the strain G3-HEV83-2-27 [6,324]. Other cDNA clones have been used to construct intergenotypic chimeras to study HEV’s species tropism [323,325].
HEV can infect various immortalized cell lines, including those derived from the colon: Caco-2 [326], Caco-2 subclone C25j [327]; Kidney: BHK-21 (golden hamster) [324], FRhK-4 (rhesus macaque) [325], FRhK-4 (rhesus macaque) in co-culture with infected primary monkey kidney cells [328], LLC-PK1 [316], 2BS [44,329]; Liver: Hepa-RG [330], HepG2/C3A [327,331], Huh-7 [326], Huh-7 subclone S10-3 [332], Huh-7.5 [316], OHH1.Li [316], PLC/PRF/5 [100]; Lung: A549 [329,333], A549/D3 [334]; and Neuron: M03.13, DBRTG, SK-N-MC, DAOY [335], U87 [336], JEG-3, and BeWo [337]. Although tumor-derived cell lines have contributed significantly to advances in HEV research, they present some important limitations. These include their inability to accurately replicate the physiological environment of primary hepatocytes. Additionally, critical cellular factors and pathways that influence authentic virus infection, RNA replication, and progeny virus release may be absent. That said, primary cells, including monkey cells [328,338,339], primary human hepatocytes [340], mouse embryonic fibroblasts [341], stromal cells and decidual and placental explants [342], as well as induced pluripotent stem cells or embryonic stem cells [343] can be utilized to overcome these limitations.
Several characteristics of HEV contribute to its difficulty in cultivation and replication. As previously mentioned, HEV can naturally exist as neHEV in bile and feces or as eHEV in blood, where it evades neutralizing antibodies. The neHEV is more infectious than eHEV, which explains the difficulty of isolating HEV from blood samples during the acute phase of infection [26]. Furthermore, the different HEV genotypes have varying influences on cell culture systems, which can impact research outcomes and our understanding of the virus. Genotypes 1 and 2 are primarily human-specific and have been more challenging to culture in vitro, showing limited replication in some non-primate cell lines, such as swine kidney cells, albeit with lower efficiency than primate cells [325,344]. Since genotypes 3 and 4 are zoonotic, they have a broader host range and are generally easier to cultivate in cell culture systems. GT3, in particular, has been successfully cultured in various human cell lines and several hepatocarcinoma cell lines, including PLC/PRF/5 and HepG2/C3A, as well as on A549 lung carcinoma cells [310].
To enhance in vitro replication, several modifications have been made to optimize the achievement of reliable results, such as supplementing culture media with MgCl2, dimethyl sulfoxide (DMSO), amphotericin B, and FBS, which have increased HEV replication and adaptation in vitro [345,346].
Locus [347] tested different cell lines, including human hepatocellular carcinoma, human lung adenocarcinoma, rat neuroblastoma, and porcine liver cells, to determine which was most susceptible to GT3 infection. The supernatant was collected for HEV RNA detection by RT-qPCR seven days post-infection, and cells were conjugated with an anti-HEV capsid antibodies. The A549-D3 cell line showed the highest viral titer in the supernatant, followed by HuH7 and HuH7-S10-3 cell lines, which exhibited the highest percentage of infected cells. Additionally, it was observed that culture in MMEM-10-D medium and the presence of fetal bovine serum (FBS) along with DMSO contributed to viral propagation. In comparative terms, the authors performed molecular techniques aiming at the indirect identification of infectivity by RT-qPCR and achieved better results in terms of assay time, cost, and test sensitivity for methods using PtCl4 and RNAse A to assess capsid integrity, followed by Long-Range RT-qPCR targeting genome integrity [347].
Similarly, Chew [346] investigated the influence of amphotericin B, MgCl2, progesterone, and DMSO on Paslahepevirus species balayani (HEV-A) and Rocahepevirus species ratti (HEV-C1) production in PLC/PRF/5 cells. Improved production was observed when amphotericin B, MgCl2, and DMSO were used from fecal samples with high viral load, with HEV-A genotype 4 remaining infectious for over 18 months when the medium was supplemented with amphotericin B and MgCl2 [346]
In the context of food sample detection, the complex matrix of components further complicates the development of standardization in cell cultures. Stunnenberg [239] implemented a HEV cell culture for the detection of infectious GT3c and GT3e adding dry sausage and liver homogenate inoculation to A549/D3 culture. The method was applied to study the effect of different food processing temperatures on HEV infectivity in pork meat products, demonstrating positive viral detection under different circumstances [239].
In the study by Berto [348], a 3D cell culture methodology was developed using the Rotating Wall Vessel (RVW) tool for detecting infectious HEV. The RVW simulates a more favorable environment for growth and replication in cell cultures, with the authors working with the human hepatocellular carcinoma cell line PLC/PRF/5. Using an HEV-positive pig liver sample, successful HEV replication and high viral titers were observed over 5 months, confirmed by real-time RT-PCR and transmission electron microscopy [348].
The difficulty in isolating HEV via cell culture varies depending on the sample type, genotype, subgenotype, and strain; acute infections tend to hinder isolation due to the low viral titer found. Schilling-Loeffler [349] isolated HEV using an established protocol, with GT3-positive wild boar serum and liver samples in PLC/PRF/5 cell cultures. The first viral passage lasted 3 months, while the second viral passage lasted 6 weeks, with infectivity monitored by RT-qPCR, immunofluorescence, and electron microscopy, as the virus did not exhibit cytopathic effect. The authors successfully isolated two liver samples that originally had high viral titers [349].
In cell culture-based assays, the low viral loads in food samples necessitate maximizing detection potential with minimal processing, emphasizing the need to determine if food homogenates are cytotoxic to HEV-susceptible cells [350]. Developing efficient cell culture systems for HEV is challenging, often requiring months of adaptation for successful in vitro replication, and difficulties in detecting viral infectivity often requiring molecular or fluorescence techniques, thereby prolonging result delivery times [351].
Considering the challenges related to the cell culture system for HEV, animal models provide a valuable alternative for assessing infectivity and transmission risk of environmental HEV strains. Several studies have demonstrated the susceptibility of Mongolian gerbils (Meriones unguiculatus) to a broad range of HEV genotypes, including GT1 [352], GT3 [353,354], GT4 [355], GT5, GT7, and rat HEV [356]. The results observed in these studies indicate that the Mongolia gerbil can be a valuable tool for studying the fundamental aspects of HEV. It can provide rapid propagation of wild-type viruses, pathogenesis and mechanisms of HEV replication studies, and potential for preclinical evaluation of antiviral therapies and vaccine candidates.
5. Challenges in HEV Surveillance
In Argentina, raw sewage samples (besides clinical samples) were collected between 2016 and 2017 and tested by both real-time and nested PCR to investigate the circulation of hepatitis A and E viruses. HEV was detected in 22.5% of sewage samples, while in 15.9% of clinical samples [357]. Another study in Argentina found HEV RNA (GT3) in 1.6% (3/189) of water samples from the Arias-Arenales River by nested PCR [358]. In Colombia, between 2012 and 2014, samples from drinking water sources and wastewater systems of eight municipalities and two villages was tested for HEV RNA by RT-PCR. HEV genome was present in 23.3% (7/30) of the samples from drinking water sources and in 16.7% (5/30) from sewage, while positive sewage samples were not detected in cell culture [359]. In Brazil, 68 sediment samples, 250 water samples, and 50 samples of pork products (pâté and blood sausage) were tested for HEV genome with a positivity of 36 % for HEV (GT3) for all food tested [360].
Moving to other places in the world, in Italy, a nine-year nationwide environmental surveillance approach (2011–2019) was conducted on 1374 sewage samples. By real-time RT-qPCR, HEV RNA was detected in 74 urban sewage samples (5.4%), while 56 samples were characterized as GT3 strains and 18 as GT1 [361]. In Germany, wastewater from 21 treatment plants and two rivers was collected between 2020–2021 and 605 samples were analyzed for the presence of HEV RNA by real-time RT-qPCR. About 73% of all samples tested positive for HEV RNA and GT3c was the most prevalent variant [362]. In another study, 68/155 (43.9%) of sewage samples collected from 14 wastewater treatment plants in Italy were positive for HEV RNA. In addition, sixty-eight strains exhibited 79.0–91.6% identity to reference sequences of rodent and human origin, clustering into distinct genetic groups with a clear pattern associated with geographic location and specific wastewater treatment plants [94].
Regarding animals, in Spain, serum samples from 425 zoo animals belonging to 109 animal species, including artiodactyls, carnivores, perissodactyls, proboscideans, and rodents were collected from 11 different zoological parks. Anti-HEV antibodies were detected in 36 out of 425. Using a Western blot assay, specific antibodies against GT3 and HEV-C1 antigens were confirmed as ELISA positive. In addition, HEV RNA was not detected in any of the 262 animals tested by RT-PCR [363]. In another study conducted in Italy, serum and fecal specimens from sheep, goats, red deer, roe deer, chamois, and Alpine ibex were collected and screened molecularly and serologically. HEV antibodies were found in sheep (21.6%), goats (11.4%), red deer (2.6%), roe deer (3.1%), and Alpine ibex (6.3%), while HEV RNA was detected in four fecal specimens (3.0%) of sheep [364]. Yoon [365] investigated the prevalence of HEV infection in 283 blood and 114 fecal samples from 397 horses via sandwich enzyme-linked immunosorbent assay and nested RT-PCR. The results revealed positivity for anti-HEV antibodies in 35 serum samples; however, HEV RNA was not detected in any samples [365].
These studies underscore the global circulation of HEV and its presence in diverse environmental and animal reservoirs. Wastewater and environmental surveillance have consistently identified HEV RNA in sewage, rivers, and water sources across Latin America, Europe, and Asia, demonstrating its persistence in various ecosystems. Furthermore, the different detection methods and sampling strategies highlight the need for standardized approaches in surveillance.
Wastewater surveillance serves as a valuable epidemiological tool, offering insights into pathogen circulation. It has been employed to assess community infection trends, evaluate public health interventions, and support timely, evidence-based decisions to reduce the impact of epidemics or outbreaks. Establishing a surveillance system requires collaboration between different agencies, including health agencies, water treatment and management authorities, and environmental agencies for rapid reporting and exchange of information, which can prevent the spread of diseases [366]. In the same way, animal surveillance can also serve as a tool to provide a more comprehensive understanding of HEV circulation and potential sources of zoonotic transmission of HEV.
Regarding clinical cases, in high-income countries, HEV cases are associated mainly with HEV GT3, which is recognized as a zoonotic infection attributed to the consumption of contaminated pork or shellfish products and traced back to contaminated blood products or infected organs [236,367,368]. Based on that, HEV cases have continued to increase, with about 20.000 notified cases from 2005 to 2015 in the European Union/European Economic Area [369].
In Europe, there are published studies and high heterogeneity of the surveillance systems across EU/EEA Member States (e.g., distinct levels of awareness, testing, and surveillance efforts). However, there is emerging evidence that HEV has been an under-recognized pathogen in high-income countries over the last decade [370].
In 2017, the WHO Regional Office for Europe published an action plan for dealing with hepatitis in the WHO European Region [371]. This action plan, adapted to the WHO global health sector strategy on viral hepatitis, together with resolution EUR/RC66/R10, was endorsed and signed on 14 September 2016 and aims to eliminate viral hepatitis as a public health threat in the WHO European Region by 2030. One of the main goals was to harmonize surveillance objectives and case definitions by 2018. The goal for 2020 for the Member States was to establish a national hepatitis infection surveillance program to detect outbreaks promptly, assess trends in incidence, inform disease burden estimates, and do effective real-time tracking of viral hepatitis diagnosis, treatment, and care cascade, including vulnerable populations. Based on that, the European Centre for Disease Prevention and Control (ECDC) is supporting EU/EEA countries in implementing the WHO European action plan to enhance or adapt their capacity for HEV surveillance and control [371].
The program offers options for the implementation or adjustment of national HEV surveillance and covers criteria derived from the European Association for the Study of the Liver (EASL) for clinical testing, case definitions for acute and chronic HEV infections and reporting schemes, helping countries achieve the goals proposed. These achievements will further improve the knowledge of the epidemiology of HEV infections in the EU/EEA, provide evidence for risk assessment and the implementation of public health measures, and keep noted animal health and food safety authorities. Furthermore, it will support preventive and control measures to reduce the risk of transmission to humans [371].
Implementing surveillance programs for HEV poses several challenges. The first challenge is effective data management since this process is laborious, requiring the transformation from the data collection system to analytical files, appropriate inferences, and interpretation of data. The second major challenge is related to early detection. Complex and diverse matrices, such as blood, organs, and feces, complicate the standardization of techniques due to the need for specific methodologies [372]. Factors such as low viremia in infections and the lack of standardized concentration, extraction, and detection techniques hinder effective HEV control in public health contexts [373]. Therefore, developing new methods and technologies is necessary to harmonize methodology and detection robustness, improving public health emergency responses. A third point is the need for human resources to accomplish analytic data management, statistical analysis, and visualization. It is necessary to train professionals for positions, such as public health data managers and analysts [374].
The widespread detection of HEV in animals, environmental sources, and clinical samples emphasizes the importance of a One Health approach in managing HEV infections.
6. One Health Approach
Besides ribavirin, a key drug for chronic HEV infection [375], no other efficient treatment or global vaccination, apart from those implemented in China and Pakistan [376], is well established for Hepatitis E prevention and control. Still, the fact that HEV is vastly disseminated in several hosts besides humans makes managing the disease quite complex. Additionally, possible forestalling actions may vary depending on the geographic region, where in more prevalent areas the infection normally spreads via poor sanitation and hygiene; while in regions with low HEV prevalence, the transmission is mostly zoonotic [377].
From an environmental standpoint, water and swine products present themselves as the biggest disseminators of HEV, where viral particles can be transmitted through direct contact with contaminated water or by consuming contaminated or undercooked pork products, as well as other foods exposed to the virus. Even though more studies are required to evaluate the effectiveness of preventive measures globally, managing both water and porcine derivates would go a long way in lessening the transmission rate of HEV infections [36,38,236,377].
One of the main proposed prevention actions is personal hygiene. Actions as simple as washing your hands and following biosafety guidelines can help interrupt the transmission chain of HEV. Viral particles can be inactivated by either using chlorine solutions or heating them at 90 °C or higher, so treating water and wastewater, as well as cleaning and thoroughly cooking foods following the above-mentioned criteria also fit within the suggested forestalling strategies for HEV infections. Vaccination would also fit within these actions, but as previously mentioned to there is still no available vaccine for HEV; yet, WHO proposes additional research to achieve a safe and effective vaccine for both affected animals and humans [377,378].
7. Conclusions
Different HEV genotypes have been detected in a vast range of mammals, including the main source of HEV transmission to humans from meat products or water-supply sources. Once infected with HEV, humans can develop an acute, but self-limiting hepatitis, yet risk groups such as pregnant people and immunocompromised patients can develop a more hazardous disease possibly leading to death. So, monitoring HEV in humans, animal hosts, and the environment is essential for managing the virus. While hygiene barriers can help prevent dissemination, several limitations remain.
To study HEV in animal samples, firstly, efficient and cost-effective standardized viral concentration methods are needed, since the viral load can be minimal within these samples, as they can be complex matrices with high concentrations of inhibitors. Once concentrated, sensitive, specific, and standardized HEV detection techniques are still required, since the available technologies including molecular biology, serology, and cell-culture-based assays have flaws.
Molecular techniques are limited in their inability to assess infectious viruses, as they only detect genetic material, which complicates studies on HEV survival and inactivation, for example. On the other hand, while cell culture remains the gold standard method for evaluating virus infectivity, it has several limitations. These include specific limitations inherent to each cell-culture system. For example, some cultures are not susceptible to wild-type viruses isolated from patients, as they are often permissive to specific HEV strains. When using tumor-derived cells, an additional challenge lies in accurately replicating the physiological environment of primary hepatocytes.
The main trend of this study is the heterogeneity of surveillance systems, where notification, testing strategy, and report-back systems differ, weakening the ability to measure the actual epidemiological impact of HEV. In addition, surveillance operations are constrained by technical and logistic limitations, primarily the non-harmonization of standards for concentration, extraction, and detection methods, further complicated by the sample matrix heterogeneity—varying from clinical matrices (e.g., organs, blood) to environmental and food matrices. This heterogeneity contributes to variable detection rates and hinders the generation of comparable international datasets. So, although surveillance studies have been conducted globally across different sample types, there is a clear need for the development of new methods and technologies to standardize and harmonize detection approaches, thereby enhancing the robustness of virus monitoring and improving public health emergency responses.
Author Contributions
Conceptualization, Supervision, G.F. and D.R.-L.; Writing—original draft, tables M.A.E., C.P.P., Y.F.S.H.J., G.V.T.P., L.Z., H.B.d.S.G. and N.G.; Review and editing, M.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil, protocol 88887.910938/2023-00), and the research projects PID2021-123532OB-C31 and BU220P24 from the Spanish Ministry of Science and Innovation and Junta de Castilla y Leon, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HEV | Hepatitis E |
| HAV | Hepatitis A |
| ORFs | Open reading frames |
| GT1 | Genotype 1 |
| GT2 | Genotype 2 |
| GT3 | Genotype 3 |
| GT4 | Genotype 4 |
| WHO | World Health Organization |
| PEG | Polyethylene glycol |
| DEUF | Dead-end ultrafiltration |
| TFUF | Tangential flow ultrafiltration |
| IU | International Units |
| UC | Ultracentrifugation-based method |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| RT-RPA | Reverse Transcription Recombinase Polymerase Amplification |
| qRT-RPA | Reverse transcription recombinase polymerase amplification |
| LFB RT-RPA | Lateral flow biosensor |
| TMA | Transcription Mediated Amplification |
| LAMP | Loop-Mediated Isothermal Amplification |
| neHEV | Non-enveloped virus |
| eHEV | Quasi-enveloped virus |
| RVW | Rotating Wall Vessel |
| ECDC | European Centre for Disease Prevention and Control |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
References
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a Virus in Non-A, Non-B Hepatitis Transmitted via the Fecal-Oral Route. Intervirology 1983, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.S.Y.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the Hepatitis E Virus-like Particle Suggests Mechanisms for Virus Assembly and Receptor Binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992–12997. [Google Scholar] [CrossRef]
- Zhai, L.; Dai, X.; Meng, J. Hepatitis E Virus Genotyping Based on Full-Length Genome and Partial Genomic Regions. Virus Res. 2006, 120, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, M.G.; Johnson, J.E. Icosahedral Rna Virus Structure. Annu. Rev. Biochem. 1989, 58, 533–569. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Li, T.-C.; Mayazaki, N.; Simon, M.N.; Wall, J.S.; Moore, M.; Wang, C.-Y.; Takeda, N.; Wakita, T.; Miyamura, T.; et al. Structure of Hepatitis E Virion-Sized Particle Reveals an RNA-Dependent Viral Assembly Pathway. J. Biol. Chem. 2010, 285, 33175–33183. [Google Scholar] [CrossRef]
- Shiota, T.; Li, T.-C.; Yoshizaki, S.; Kato, T.; Wakita, T.; Ishii, K. The Hepatitis E Virus Capsid C-Terminal Region Is Essential for the Viral Life Cycle: Implication for Viral Genome Encapsidation and Particle Stabilization. J. Virol. 2013, 87, 6031–6036. [Google Scholar] [CrossRef]
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on Hepatitis E Virology: Implications for Clinical Practice. J. Hepatol. 2016, 65, 200–212. [Google Scholar] [CrossRef]
- Lhomme, S.; Magne, S.; Perelle, S.; Vaissière, E.; Abravanel, F.; Trelon, L.; Hennechart-Collette, C.; Fraisse, A.; Martin-Latil, S.; Izopet, J.; et al. Clustered Cases of Waterborne Hepatitis E Virus Infection, France. Viruses 2023, 15, 1149. [Google Scholar] [CrossRef]
- Peron, J.M.; Larrue, H.; Izopet, J.; Buti, M. The Pressing Need for a Global HEV Vaccine. J. Hepatol. 2023, 79, 876–880. [Google Scholar] [CrossRef]
- Wang, B.; Meng, X.J. Hepatitis E Virus: Host Tropism and Zoonotic Infection. Curr. Opin. Microbiol. 2021, 59, 8–15. [Google Scholar] [CrossRef]
- Capai, L.; Charrel, R.; Falchi, A. Hepatitis E in High-Income Countries: What Do We Know? And What Are the Knowledge Gaps? Viruses 2018, 10, 285. [Google Scholar] [CrossRef]
- Iaconelli, M.; Purpari, G.; Della Libera, S.; Petricca, S.; Guercio, A.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Fratini, M.; et al. Hepatitis A and E Viruses in Wastewaters, in River Waters, and in Bivalve Molluscs in Italy. Food Environ. Virol. 2015, 7, 316–324. [Google Scholar] [CrossRef]
- Fenaux, H.; Chassaing, M.; Berger, S.; Gantzer, C.; Bertrand, I.; Schvoerer, E. Transmission of Hepatitis E Virus by Water: An Issue Still Pending in Industrialized Countries. Water Res. 2019, 151, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Lodder, W.J.; Lodder-Verschoor, F.; Van Den Berg, H.H.J.L.; Vennema, H.; Duizer, E.; Koopmans, M.; De Husman, A.M.R. Sources of Hepatitis E Virus Genotype 3 in The Netherlands. Emerg. Infect. Dis. 2009, 15, 381–387. [Google Scholar] [CrossRef]
- CDC Hepatitis E | CDC Yellow Book 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/hepatitis-e (accessed on 24 November 2024).
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The Global Epidemiology of Hepatitis E Virus Infection: A Systematic Review and Meta-Analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic Analysis of Global Hepatitis E Virus Sequences: Genetic Diversity, Subtypes and Zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Van Cauteren, D.; Le Strat, Y.; Sommen, C.; Bruyand, M.; Tourdjman, M.; Jourdan-Da Silva, N.; Couturier, E.; Fournet, N.; de Valk, H.; Desenclos, J.C. Estimated Annual Numbers of Foodborne Pathogen–Associated Illnesses, Hospitalizations, and Deaths, France, 2008–2013. Emerg. Infect. Dis. 2017, 23, 1486. [Google Scholar] [CrossRef]
- Péron, J.M.; Bureau, C.; Poirson, H.; Mansuy, J.M.; Alric, L.; Selves, J.; Dupuis, E.; Izopet, J.; Vinel, J.P. Fulminant Liver Failure from Acute Autochthonous Hepatitis E in France: Description of Seven Patients with Acute Hepatitis E and Encephalopathy. J. Viral Hepat. 2007, 14, 298–303. [Google Scholar] [CrossRef]
- Manka, P.; Bechmann, L.P.; Coombes, J.D.; Thodou, V.; Schlattjan, M.; Kahraman, A.; Syn, W.K.; Saner, F.; Gerken, G.; Baba, H.; et al. Hepatitis E Virus Infection as a Possible Cause of Acute Liver Failure in Europe. Clin. Gastroenterol. Hepatol. 2015, 13, 1836–1842.e2. [Google Scholar] [CrossRef] [PubMed]
- Aherfi, S.; Borentain, P.; Raissouni, F.; Le Goffic, A.; Guisset, M.; Renou, C.; Grimaud, J.C.; Hardwigsen, J.; Garcia, S.; Botta-Fridlund, D.; et al. Liver Transplantation for Acute Liver Failure Related to Autochthonous Genotype 3 Hepatitis E Virus Infection. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Haim-Boukobza, S.; Coilly, A.; Sebagh, M.; Bouamoud, M.; Antonini, T.; Roche, B.; Yordanova, O.; Savary, J.; Saliba, F.; Duclos-Vallee, J.C.; et al. Hepatitis E Infection in Patients with Severe Acute Alcoholic Hepatitis. Liver Int. 2015, 35, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Perrin, H.; Madden, R.G.; Stanley, A.; Crossan, C.; Hunter, J.G.; Vine, L.; Lane, K.; Devooght-Johnson, N.; McLaughlin, C.; Petrik, J.; et al. Hepatitis E Virus in Patients with Decompensated Chronic Liver Disease: A Prospective UK/French Study. Aliment. Pharmacol. Ther. 2015, 42, 574–581. [Google Scholar] [CrossRef]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z. Distinct Entry Mechanisms for Nonenveloped and Quasi-Enveloped Hepatitis E Viruses. J. Virol. 2016, 90, 4232. [Google Scholar] [CrossRef]
- Kapur, N.; Thakral, D.; Durgapal, H.; Panda, S.K. Hepatitis E Virus Enters Liver Cells through Receptor-Dependent Clathrin-Mediated Endocytosis. J. Viral Hepat. 2012, 19, 436–448. [Google Scholar] [CrossRef]
- Holla, P.; Ahmad, I.; Ahmed, Z.; Jameel, S. Hepatitis E Virus Enters Liver Cells through a Dynamin-2, Clathrin and Membrane Cholesterol-Dependent Pathway. Traffic 2015, 16, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Kapur, N.; Durgapal, H.; Panda, S.K. Subcellular Localization of Hepatitis E Virus (HEV) Replicase. Virology 2008, 370, 77–92. [Google Scholar] [CrossRef]
- Perttilä, J.; Spuul, P.; Ahola, T. Early Secretory Pathway Localization and Lack of Processing for Hepatitis E Virus Replication Protein PORF1. J. Gen. Virol. 2013, 94, 807–816. [Google Scholar] [CrossRef]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, Antigenicity, and Function of a Secreted Form of ORF2 in Hepatitis E Virus Infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef]
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.-J. The Hepatitis E Virus Open Reading Frame 3 Product Interacts with Microtubules and Interferes with Their Dynamics. J. Virol. 2009, 83, 6375–6382. [Google Scholar] [CrossRef] [PubMed]
- Zafrullah, M.; Ozdener, M.H.; Panda, S.K.; Jameel, S. The ORF3 Protein of Hepatitis E Virus Is a Phosphoprotein That Associates with the Cytoskeleton. J. Virol. 1997, 71, 9045–9053. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanaka, T.; Nishizawa, T.; Yasuda, J.; Okamoto, H. Tumour Susceptibility Gene 101 and the Vacuolar Protein Sorting Pathway Are Required for the Release of Hepatitis E Virions. J. Gen. Virol. 2011, 92, 2838–2848. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, 22. [Google Scholar] [CrossRef] [PubMed]
- Takuissu, G.R.; Kenmoe, S.; Ndip, L.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Bowo-Ngandji, A.; Oyono, M.G.; Kenfack-Momo, R.; Tchatchouang, S.; et al. Hepatitis E Virus in Water Environments: A Systematic Review and Meta-Analysis. Food Environ. Virol. 2022, 14, 223–235. [Google Scholar] [CrossRef]
- Salvador, D.; Neto, C.; Benoliel, M.J.; Filomena Caeiro, M. Assessment of the Presence of Hepatitis E Virus in Surface Water and Drinking Water in Portugal. Microorganisms 2020, 8, 761. [Google Scholar] [CrossRef]
- Van Der Poel, W.H. Food and Environmental Routes of Hepatitis E Virus Transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef]
- Gentry-Shields, J.; Myers, K.; Pisanic, N.; Heaney, C.; Stewart, J. Hepatitis E Virus and Coliphages in Waters Proximal to Swine Concentrated Animal Feeding Operations. Sci. Total Environ. 2015, 505, 487–493. [Google Scholar] [CrossRef]
- Steyer, A.; Naglič, T.; Močilnik, T.; Poljšak-Prijatelj, M.; Poljak, M. Hepatitis E Virus in Domestic Pigs and Surface Waters in Slovenia: Prevalence and Molecular Characterization of a Novel Genotype 3 Lineage. Infect. Genet. Evol. 2011, 11, 1732–1737. [Google Scholar] [CrossRef]
- Bouseettine, R.; Hassou, N.; Bessi, H.; Ennaji, M.M. Waterborne Transmission of Enteric Viruses and Their Impact on Public Health. In Emerging and Reemerging Viral Pathogens Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens; Academic Press: Cambridge, MA, USA, 2020; pp. 907–932. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Saune, K.; Rech, H.; Abravanel, F.; Mengelle, C.; L’Homme, S.; Destruel, F.; Kamar, N.; Izopet, J. Seroprevalence in Blood Donors Reveals Widespread, Multi-Source Exposure to Hepatitis E Virus, Southern France, October 2011. Euro Surveill. 2015, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Gallian, P.; Dimeglio, C.; Saune, K.; Arnaud, C.; Pelletier, B.; Morel, P.; Legrand, D.; Tiberghien, P.; Izopet, J. A Nationwide Survey of Hepatitis E Viral Infection in French Blood Donors. Hepatology 2016, 63, 1145–1154. [Google Scholar] [CrossRef]
- Huang, R.T.; Li, D.R.; Wei, J.; Huang, X.R.; Yuan, X.T.; Tian, X. Isolation and Identification of Hepatitis E Virus in Xinjiang, China. J. Gen. Virol. 1992, 73, 1143–1148. [Google Scholar] [CrossRef]
- Lubis, I. Epidemik Virus Hepatitis Non-A Non-B Di Sintang, Kalbar. Epidemiol. Bull. 1989, 252, 3140. [Google Scholar]
- Corwin, A.; Jarot, K.; Lubis, I.; Nasution, K.; Suparmawo, S.; Sumardiati, A.; Widodo, S.; Nazir, S.; Orndorff, G.; Choi, Y.; et al. Two Years’ Investigation of Epidemic Hepatitis E Virus Transmission in West Kalimantan (Borneo), Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 262–265. [Google Scholar] [CrossRef]
- Sedyaningsih-Mamahit, E.R.; Larasati, R.P.; Laras, K.; Sidemen, A.; Sukri, N.; Sabaruddin, N.; Didi, S.; Saragih, J.M.; Myint, K.S.A.; Endy, T.P.; et al. First Documented Outbreak of Hepatitis E Virus Transmission in Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 398–404. [Google Scholar] [CrossRef]
- Uchida, T.; Aye, T.T.; Ma, X.; Iida, F.; Shikata, T.; Ichikawa, M.; Rikihisa, T.; Win, K.M. An Epidemic Outbreak of Hepatitis E in Yangon of Myanmar: Antibody Assay and Animal Transmission of the Virus. Acta Pathol. Jpn. 1993, 43, 94–98. [Google Scholar] [CrossRef]
- Corwin, A.L.; Khiem, H.B.; Clayson, E.T.; Sac, P.K.; Nhung, V.T.T.; Yen, V.T.; Cuc, C.T.T.; Vaughn, D.; Merven, J.; Richie, T.L.; et al. A Waterborne Outbreak of Hepatitis E Virus Transmission in Southwestern Vietnam. Am. J. Trop. Med. Hyg. 1996, 54, 559–562. [Google Scholar] [CrossRef]
- Aye, T.T.; Uchida, T.; Ma, X.-Z.; Lida, F.; Shikata, T.; Zhuang, H.; Win, K.M. Complete Nucleotide Sequence of a Hepatitis E Virus Isolated from the Xinjiang Epidemic (1986–1988) of China. Nucleic Acids Res. 1992, 20, 3512. [Google Scholar] [CrossRef]
- Gurley, E.S.; Hossain, M.J.; Paul, R.C.; Sazzad, H.M.S.; Islam, M.S.; Parveen, S.; Faruque, L.I.; Husain, M.; Ara, K.; Jahan, Y.; et al. Outbreak of Hepatitis E in Urban Bangladesh Resulting in Maternal and Perinatal Mortality. Clin. Infect. Dis. 2014, 59, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Akbar, S.M.F.; Takahashi, K.; Al-Mahtab, M.; Khan, M.S.I.; Alim, M.A.; Ekram, A.R.M.S.; Khan, M.M.R.; Arai, M.; Mishiro, S. Epidemiological and Molecular Analyses of a Non-seasonal Outbreak of Acute Icteric Hepatitis E in Bangladesh. J. Med. Virol. 2013, 85, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ahmed, A.; Qamar, A.; Dixon, K.; Duncan, J.F.; Islam, N.U.; Rauf, A.; Bryan, J.P.; Malik, I.A.; Legters, L.J. An Outbreak of Enterically Transmitted Non-A, Non-B Hepatitis in Pakistan. Am. J. Trop. Med. Hyg. 1989, 40, 438–443. [Google Scholar] [CrossRef]
- Bryan, J.P.; Iqbal, M.; Tsarev, S.; Malik, I.A.; Duncan, J.F.; Ahmed, A.; Khan, A.; Khan, A.; Rafiqui, A.R.; Purcell, R.H.; et al. Epidemic of Hepatitis E in a Military Unit in Abbotrabad, Pakistan. Am. J. Trop. Med. Hyg. 2002, 67, 662–668. [Google Scholar] [CrossRef][Green Version]
- Rab, M.A.; Bile, M.K.; Mubarik, M.M.; Asghar, H.; Sami, Z.; Siddiqi, S.; Dil, A.S.; Barzgar, M.A.; Chaudhry, M.A.; Burney, M.I. Water-Borne Hepatitis E Virus Epidemic in Islamabad, Pakistan: A Common Source Outbreak Traced to the Malfunction of a Modern Water Treatment Plant. Am. J. Trop. Med. Hyg. 1997, 57, 151–157. [Google Scholar] [CrossRef]
- Clayson, E.T.; Vaughn, D.W.; Innis, B.L.; Shrestha, M.P.; Pandey, R.; Malla, D.B. Association of Hepatitis E Virus with an Outbreak of Hepatitis at a Military Training Camp in Nepal. J. Med. Virol. 1998, 54, 178–182. [Google Scholar] [CrossRef]
- Naik, S.R.; Aggarwal, R.; Salunke, P.N.; Mehrotra, N.N. A Large Waterborne Viral Hepatitis E Epidemic in Kanpur, India. Bull. World Health Organ. 1992, 70, 597–604. [Google Scholar]
- Arankalle, V.A.; Chadha, M.S.; Tsarev, S.A.; Emerson, S.U.; Risbud, A.R.; Banerjee, K.; Purcell, R.H. Seroepidemiology of Water-Borne Hepatitis in India and Evidence for a Third Enterically-Transmitted Hepatitis Agent. Proc. Natl. Acad. Sci. USA 1994, 91, 3428–3432. [Google Scholar] [CrossRef]
- Martolia, H.C.S.; Hutin, Y.; Ramachandran, V.; Manickam, P.; Murhekar, M.; Gupte, M. An Outbreak of Hepatitis E Tracked to a Spring in the Foothills of the Himalayas, India, 2005. Indian J. Gastroenterol. 2009, 28, 99–101. [Google Scholar] [CrossRef]
- Shata, M.T.; Daef, E.A.; Zaki, M.E.; Abdelwahab, S.F.; Marzuuk, N.M.; Sobhy, M.; Rafaat, M.; Abdelbaki, L.; Nafeh, M.A.; Hashem, M.; et al. Protective Role of Humoral Immune Responses during an Outbreak of Hepatitis E in Egypt. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 613–618. [Google Scholar] [CrossRef]
- Mast, E.E.; Polish, L.B.; Favorov, M.O.; Khudyakova, N.S.; Collins, C.; Tukei, P.M.; Koptich, D.; Khudyakov, Y.E.; Fields, H.A.; Margolis, H.S. Hepatitis E Among Refugees in Kenya: Minimal Apparent Person-to-Person Transmission, Evidence for Age-Dependent Disease Expression, and New Serologic Assays. In Viral Hepatitis and Liver Disease; Nishioka, K., Suzuki, H., Mishiro, S., Oda, T., Eds.; Springer: Tokyo, Japan, 1994; pp. 375–378. [Google Scholar] [CrossRef]
- Ahmed, J.A.; Moturi, E.; Spiegel, P.; Schilperoord, M.; Burton, W.; Kassim, N.H.; Mohamed, A.; Ochieng, M.; Nderitu, L.; Navarro-Colorado, C.; et al. Hepatitis E Outbreak, Dadaab Refugee Camp, Kenya, 2012. Emerg. Infect. Dis. 2013, 19, 1010–1012. [Google Scholar] [CrossRef]
- Mc Carthy, M.C.; He, J.; Hyams, K.C.; El-Tigani, A.; Khalid, I.O. Acute Hepatitis E Infection during the 1988 Floods in Khartoum, Sudan. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 177. [Google Scholar] [CrossRef] [PubMed]
- Guthmann, J.P.; Klovstad, H.; Boccia, D.; Hamid, N.; Pinoges, L.; Nizou, J.Y.; Tatay, M.; Diaz, F.; Moren, A.; Grais, R.F.; et al. A Large Outbreak of Hepatitis E among a Displaced Population in Darfur, Sudan, 2004: The Role of Water Treatment Methods. Clin. Infect. Dis. 2006, 42, 1685–1691. [Google Scholar] [CrossRef]
- Boccia, D.; Guthmann, J.P.; Klovstad, H.; Hamid, N.; Tatay, M.; Ciglenecki, I.; Nizou, J.Y.; Nicand, E.; Guerin, P.J. High Mortality Associated with an Outbreak of Hepatitis E among Displaced Persons in Darfur, Sudan. Clin. Infect. Dis. 2006, 42, 1679–1684. [Google Scholar] [CrossRef]
- WHO. Hepatitis E: Chad, Sudan. Wkly. Epidemiol Rec. Wkly. Epidemiol. Rec. 2004, 39, 321–328. [Google Scholar]
- Rayis, D.A.; Jumaa, A.M.; Gasim, G.I.; Karsany, M.S.; Adam, I. An Outbreak of Hepatitis E and High Maternal Mortality at Port Sudan, Eastern Sudan. Pathog. Glob. Health 2013, 107, 66–68. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) Investigation of Hepatitis E Outbreak among Refugees-Upper Nile, South Sudan, 2012–2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 581–586.
- Escribà, J.M.; Nakoune, E.; Recio, C.; Massamba, P.M.; Matsika-Claquin, M.D.; Goumba, C.; Rose, A.M.C.; Nicand, E.; García, E.; Leklegban, C.; et al. Hepatitis E, Central African Republic. Emerg. Infect. Dis. 2008, 14, 681. [Google Scholar] [CrossRef]
- Goumba, C.M.; Yandoko-Nakouné, E.R.; Komas, N.P. A Fatal Case of Acute Hepatitis e among Pregnant Women, Central African Republic. BMC Res. Notes 2010, 3, 103. [Google Scholar] [CrossRef]
- Goumba, A.I.; Konamna, X.; Komas, N.P. Clinical and Epidemiological Aspects of a Hepatitis E Outbreak in Bangui, Central African Republic. BMC Infect. Dis. 2011, 11, 93. [Google Scholar] [CrossRef]
- Teshale, E.H.; Howard, C.M.; Grytdal, S.P.; Handzel, T.R.; Barry, V.; Kamili, S.; Drobeniuc, J.; Okware, S.; Downing, R.; Tappero, J.W.; et al. Hepatitis E Epidemic, Uganda. Emerg. Infect. Dis. 2010, 16, 126–129. [Google Scholar] [CrossRef]
- Teshale, E.H.; Grytdal, S.P.; Howard, C.; Barry, V.; Kamili, S.; Drobeniuc, J.; Hill, V.R.; Okware, S.; Hu, D.J.; Holmberg, S.D. Evidence of Person-to-Person Transmission of Hepatitis e Virus during a Large Outbreak in Northern Uganda. Clin. Infect. Dis. 2010, 50, 1006–1010. [Google Scholar] [CrossRef]
- Howard, C.M.; Handzel, T.; Hill, V.R.; Grytdal, S.P.; Blanton, C.; Kamili, S.; Drobeniuc, J.; Hu, D.; Teshale, E. Novel Risk Factors Associated with Hepatitis E Virus Infection in a Large Outbreak in Northern Uganda: Results from a Case-Control Study and Environmental Analysis. Am. J. Trop. Med. Hyg. 2010, 83, 1170–1173. [Google Scholar] [CrossRef]
- Cummings, M.J.; Wamala, J.F.; Komakech, I.; Lukwago, L.; Malimbo, M.; Omeke, M.E.; Mayer, D.; Bakamutumaho, B. Hepatitis E in Karamoja, Uganda, 2009–2012: Epidemiology and Challenges to Control in a Setting of Semi-Nomadic Pastoralism. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 648–655. [Google Scholar] [CrossRef]
- Coursaget, P.; Krawczynski, K.; Buisson, Y.; Nizou, C.; Molinié, C. Hepatitis E and Hepatitis C Virus Infections among French Soldiers with Non-A, Non-B Hepatitis. J. Med. Virol. 1993, 39, 163–166. [Google Scholar] [CrossRef]
- Van Cuyck-Gandre, H.; Caudill, J.D.; Zhang, H.Y.; Longer, C.F.; Molinie, C.; Roue, R.; Deloince, R.; Coursaget, P.; Mamouth, N.N.; Buisson, Y. Short Report: Polymerase Chain Reaction Detection of Hepatitis E Virus in North African Fecal Samples. Am. J. Trop. Med. Hyg. 1996, 54, 134–135. [Google Scholar] [CrossRef]
- Molinié, C.; Roué, R.; Saliou, P.; Denée, J.M.; Farret, O.; Vergeau, B.; Vindrios, J.; Martin, D. Acute Epidemic Non-A, Non-B Hepatitis: Clinical Study of 38 Cases Seen in Chad. Gastroenterol. Clin. Biol. 1986, 10, 475–479. [Google Scholar]
- Coursaget, P.; Buisson, Y.; Enogat, N.; Bercion, R.; Baudet, J.M.; Delmaire, P.; Prigent, D.; Desramé, J. Outbreak of Enterically-Transmitted Hepatitis Due to Hepatitis A and Hepatitis E Viruses. J. Hepatol. 1998, 28, 745–750. [Google Scholar] [CrossRef]
- Grandadam, M.; Tebbal, S.; Caron, M.; Siriwardana, M.; Larouze, B.; Koeck, J.L.; Buisson, Y.; Enouf, V.; Nicand, E. Evidence for Hepatitis E Virus Quasispecies. J. Gen. Virol. 2004, 85, 3189–3194. [Google Scholar] [CrossRef]
- Isaäcson, M.; Frean, J.; He, J.; Seriwatana, J.; Innis, B.L. An Outbreak of Hepatitis E in Northern Namibia, 1983. Am. J. Trop. Med. Hyg. 2000, 62, 619–625. [Google Scholar] [CrossRef]
- Maila, H.T.; Bowyer, S.M.; Swanepoel, R. Identification of a New Strain of Hepatitis E Virus from an Outbreak in Namibia in 1995. J. Gen. Virol. 2004, 85, 89–95. [Google Scholar] [CrossRef]
- Benjelloun, S.; Bahbouhi, B.; Bouchrit, N.; Cherkaoui, L.; Hda, N.; Mahjour, J.; Benslimane, A. Seroepidemiological Study of an Acute Hepatitis E Outbreak in Morocco. Res. Virol. 1997, 148, 279–287. [Google Scholar] [CrossRef]
- Bile, K.; Isse, A.; Mohamud, P.; Allebeck, P.; Nilsson, L.; Norder, H.; Mushahwar, I.K.; Magnius, L.O. Contrasting Roles of Rivers and Wells as Sources of Drinking Water on Attack and Fatality Rates in a Hepatitis E Epidemic in Somalia. Am. J. Trop. Med. Hyg. 1994, 51, 466–474. [Google Scholar] [CrossRef]
- Mushahwar, I.K.; Dawson, G.J.; Bile, K.M.; Magnius, L.O. Serological Studies of an Enterically Transmitted Non-A, Non-B Hepatitis in Somalia. J. Med. Virol. 1993, 40, 218–221. [Google Scholar] [CrossRef]
- Maurice, D.; Abassora, M.; Marcelin, N.M.; Richard, N. First Documented Outbreak of Hepatitis e in Northern Cameroon. Ann. Trop. Med. Public Health 2013, 6, 682–683. [Google Scholar] [CrossRef]
- Velázquez, O.; Stetler, H.C.; Avila, C.; Ornelas, G.; Alvarez, C.; Hadler, S.C.; Bradley, D.W.; Sepúlveda, J. Epidemic Transmission of Enterically Transmitted Non-A, Non-B Hepatitis in Mexico, 1986–1987. JAMA 1990, 263, 3281–3285. [Google Scholar] [CrossRef]
- Villalba, M.d.l.C.M.; Lay, L.d.L.A.R.; Chandra, V.; Corredor, M.B.; Frometa, S.S.; Moreno, A.G.; Jameel, S. Hepatitis E Virus Genotype 1, Cuba. Emerg. Infect. Dis. 2008, 14, 1320–1322. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Perez, A.B.; Pineda, J.A.; Reina, G.; Fuentes-Lopez, A.; Freyre-Carrillo, C.; Ramirez-Arellano, E.; Alados, J.C.; Rivero, A. Orthohepevirus C Infection as an Emerging Cause of Acute Hepatitis in Spain: First Report in Europe. J. Hepatol. 2022, 77, 326–331. [Google Scholar] [CrossRef]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.; Wu, S.; Chew, N.F.; Leung, K.; Chan, J.F.; Zhao, P.S.; Chan, W.; Poon, R.W.; Tsoi, H.; et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef]
- Casares-Jimenez, M.; Rivero-Juarez, A.; Lopez-Lopez, P.; Montes, M.L.; Navarro-Soler, R.; Peraire, J.; Espinosa, N.; Alemán-Valls, M.R.; Garcia-Garcia, T.; Caballero-Gomez, J.; et al. Rat Hepatitis E Virus (Rocahepevirus ratti) in People Living with HIV. Emerg. Microbes Infect. 2024, 13, 2295389. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.-X.; Leung, K.-H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.-M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Palombieri, A.; Di Profio, F.; Sarchese, V.; Fruci, P.; Suffredini, E.; Martella, V.; Veneri, C.; Bonanno Ferraro, G.; Mancini, P.; La Rosa, G.; et al. Surveillance for Rat Hepatitis E in Wastewater Networks, Italy. Microbiol. Spectr. 2023, 11, e02675-23. [Google Scholar] [CrossRef]
- Casares-Jimenez, M.; Garcia-Garcia, T.; Suárez-Cárdenas, J.M.; Perez-Jimenez, A.B.; Martín, M.A.; Caballero-Gómez, J.; Michán, C.; Corona-Mata, D.; Risalde, M.A.; Perez-Valero, I.; et al. Correlation of Hepatitis E and Rat Hepatitis E Viruses Urban Wastewater Monitoring and Clinical Cases. Sci. Total Environ. 2024, 908, 168203. [Google Scholar] [CrossRef]
- Balayan, M.S.; Usmanov, R.K.; Zamyatina, N.A.; Djumalieva, D.I.; Karas, F.R. Experimental Hepatitis E Infection in Domestic Pigs. J. Med. Virol. 1990, 32, 58–59. [Google Scholar] [CrossRef]
- Meng, X.J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A Novel Virus in Swine Is Closely Related to the Human Hepatitis E Virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef]
- Meng, X.J. Zoonotic and Foodborne Transmission of Hepatitis E Virus. Semin. Liver Dis. 2013, 33, 41–49. [Google Scholar] [CrossRef]
- Treagus, S.; Wright, C.; Baker-Austin, C.; Longdon, B.; Lowther, J. The Foodborne Transmission of Hepatitis E Virus to Humans. Food Environ. Virol. 2021, 13, 127–145. [Google Scholar] [CrossRef]
- Meng, J.; Dubreuil, P.; Pillot, J. A New PCR-Based Seroneutralization Assay in Cell Culture for Diagnosis of Hepatitis E. J. Clin. Microbiol. 1997, 35, 1373–1377. [Google Scholar] [CrossRef]
- Di Cola, G.; Di Cola, G.; Fantilli, A.; Mamani, V.; Tamiozzo, P.; Martínez Wassaf, M.; Nates, S.V.; Ré, V.E.; Pisano, M.B. High Circulation of Hepatitis E Virus (HEV) in Pigs from the Central Region of Argentina without Evidence of Virus Occurrence in Pork Meat and Derived Products. Res. Vet. Sci. 2023, 164, 105000. [Google Scholar] [CrossRef]
- Paiva, H.H.; Tzaneva, V.; Haddad, R.; Yokosawa, J. Molecular Characterization of Swine Hepatitis E Virus from Southeastern Brazil. Braz. J. Microbiol. 2007, 38, 693–698. [Google Scholar] [CrossRef]
- Passos-Castilho, A.M.; Granato, C.F.H. High Frequency of Hepatitis E Virus Infection in Swine from South Brazil and Close Similarity to Human HEV Isolates. Braz. J. Microbiol. 2017, 48, 373–379. [Google Scholar] [CrossRef]
- de Oliveira-Filho, E.F.; Dos Santos, D.R.L.; Durães-Carvalho, R.; da Silva, A.; de Lima, G.B.; Filho, A.F.B.B.; Pena, L.J.; Gil, L.H.V.G. Evolutionary Study of Potentially Zoonotic Hepatitis E Virus Genotype 3 from Swine in Northeast Brazil. Mem. Inst. Oswaldo Cruz. 2019, 114, e180585. [Google Scholar] [CrossRef]
- Forero, J.E.; Gutiérrez-Vergara, C.; Parra Suescún, J.; Correa, G.; Rodríguez, B.; Gutiérrez, L.A.; Díaz, F.J.; López-Herrera, A. Phylogenetic Analysis of Hepatitis E Virus Strains Isolated from Slaughter-Age Pigs in Colombia. Infect. Genet. Evol. 2017, 49, 138–145. [Google Scholar] [CrossRef]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodríguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E Virus in Pork Production Chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef]
- Breum, S.; Hjulsager, C.K.; de Deus, N.; Segalés, J.; Larsen, L.E. Hepatitis E Virus Is Highly Prevalent in the Danish Pig Population. Vet. Microbiol. 2010, 146, 144–149. [Google Scholar] [CrossRef]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N.; Ois Madec, F. High Prevalence of Hepatitis E Virus in French Domestic Pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef]
- Choi, I.S.; Kwon, H.J.; Shin, N.R.; Yoo, H.S. Identification of Swine Hepatitis E Virus (HEV) and Prevalence of Anti-HEV Antibodies in Swine and Human Populations in Korea. J. Clin. Microbiol. 2003, 41, 3602–3608. [Google Scholar] [CrossRef]
- Milojević, L.; Velebit, B.; Teodorović, V.; Kirbiš, A.; Petrović, T.; Karabasil, N.; Dimitrijević, M. Screening and Molecular Characterization of Hepatitis E Virus in Slaughter Pigs in Serbia. Food Environ. Virol. 2019, 11, 410–419. [Google Scholar] [CrossRef]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of Hepatitis E Virus Infection and Its Prevalence in Pigs on Commercial Farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef]
- Pires, H.; Cardoso, L.; Lopes, A.P.; Fontes, M.d.C.; Santos-Silva, S.; Matos, M.; Pintado, C.; Figueira, L.; Matos, A.C.; Mesquita, J.R.; et al. Prevalence and Risk Factors for Hepatitis E Virus in Wild Boar and Red Deer in Portugal. Microorganisms 2023, 11, 2576. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Sarchese, V.; Palombieri, A.; Fruci, P.; Lanave, G.; Robetto, S.; Martella, V.; Di Martino, B. Current Knowledge of Hepatitis E Virus (HEV) Epidemiology in Ruminants. Pathogens 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Tsachev, I.; Gospodinova, K.; Pepovich, R.; Takova, K.; Kundurzhiev, T.; Zahmanova, G.; Kaneva, K.; Baymakova, M. First Insight into the Seroepidemiology of Hepatitis E Virus (HEV) in Dogs, Cats, Horses, Cattle, Sheep, and Goats from Bulgaria. Viruses 2023, 15, 1594. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.; Roche, S.J.; Sammin, D. Seroprevalence of Hepatitis e Virus Infection in the Irish Pig Population. Ir. Vet. J. 2015, 68, 8. [Google Scholar] [CrossRef] [PubMed]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of Hepatitis E Virus Infection in Pigs at the Time of Slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396. [Google Scholar] [CrossRef]
- Carpentier, A.; Chaussade, H.; Rigaud, E.; Rodriguez, J.; Berthault, C.; Boué, F.; Tognon, M.; Touzé, A.; Garcia-Bonnet, N.; Choutet, P.; et al. High Hepatitis E Virus Seroprevalence in Forestry Workers and in Wild Boars in France. J. Clin. Microbiol. 2012, 50, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Sachs, K.; Lierz, M.; Appel, B.; Jansen, A.; Johne, R. Detection of Hepatitis e Virus in Wild Boars of Rural and Urban Regions in Germany and Whole Genome Characterization of an Endemic Strain. Virol. J. 2009, 6, 58. [Google Scholar] [CrossRef]
- Adlhoch, C.; Wolf, A.; Meisel, H.; Kaiser, M.; Ellerbrok, H.; Pauli, G. High HEV Presence in Four Different Wild Boar Populations in East and West Germany. Vet. Microbiol. 2009, 139, 270–278. [Google Scholar] [CrossRef]
- Martelli, F.; Caprioli, A.; Zengarini, M.; Marata, A.; Fiegna, C.; Di Bartolo, I.; Ruggeri, F.M.; Delogu, M.; Ostanello, F. Detection of Hepatitis E Virus (HEV) in a Demographic Managed Wild Boar (Sus scrofa Scrofa) Population in Italy. Vet. Microbiol. 2008, 126, 74–81. [Google Scholar] [CrossRef]
- Caruso, C.; Modesto, P.; Bertolini, S.; Peletto, S.; Acutis, P.L.; Dondo, A.; Robetto, S.; Mignone, W.; Orusa, R.; Ru, G.; et al. Serological and Virological Survey of Hepatitis E Virus in Wild Boar Populations in Northwestern Italy: Detection of HEV Subtypes 3e and 3f. Arch. Virol. 2015, 160, 153–160. [Google Scholar] [CrossRef]
- Aprea, G.; Amoroso, M.G.; Di Bartolo, I.; D’Alessio, N.; Di Sabatino, D.; Boni, A.; Cioffi, B.; D’Angelantonio, D.; Scattolini, S.; De Sabato, L.; et al. Molecular Detection and Phylogenetic Analysis of Hepatitis E Virus Strains Circulating in Wild Boars in South-Central Italy. Transbound. Emerg. Dis. 2018, 65, e25–e31. [Google Scholar] [CrossRef]
- Ferri, G.; Lauteri, C.; Festino, A.R.; Piccinini, A.; Olivastri, A.; Vergara, A. Hepatitis E Virus Detection in Hunted Wild Boar Liver and Muscle Tissues in Central Italy. Microorganisms 2022, 10, 1628. [Google Scholar] [CrossRef]
- Abrantes, A.C.; Santos-Silva, S.; Mesquita, J.; Vieira-Pinto, M. Hepatitis E Virus in the Wild Boar Population: What Is the Real Zoonotic Risk in Portugal? Trop. Med. Infect. Dis. 2023, 8, 433. [Google Scholar] [CrossRef]
- Kukielka, D.; Rodriguez-Prieto, V.; Vicente, J.; Sánchez-Vizcaíno, J.M. Constant Hepatitis E Virus (HEV) Circulation in Wild Boar and Red Deer in Spain: An Increasing Concern Source of HEV Zoonotic Transmission. Transbound. Emerg. Dis. 2016, 63, e360–e368. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Risalde, M.A.; Frias, M.; García-Bocanegra, I.; Lopez-Lopez, P.; Cano-Terriza, D.; Camacho, A.; Jimenez-Ruiz, S.; Gomez-Villamandos, J.C.; Rivero, A. Prevalence of Hepatitis E Virus Infection in Wild Boars from Spain: A Possible Seasonal Pattern? BMC Vet. Res. 2018, 14, 54. [Google Scholar] [CrossRef]
- de Deus, N.; Peralta, B.; Pina, S.; Allepuz, A.; Mateu, E.; Vidal, D.; Ruiz-Fons, F.; Martín, M.; Gortázar, C.; Segalés, J. Epidemiological Study of Hepatitis E Virus Infection in European Wild Boars (Sus scrofa) in Spain. Vet. Microbiol. 2008, 129, 163–170. [Google Scholar] [CrossRef]
- Widén, F.; Sundqvist, L.; Matyi-Toth, A.; Metreveli, G.; Belák, S.; Hallgren, G.; Norder, H. Molecular Epidemiology of Hepatitis E Virus in Humans, Pigs and Wild Boars in Sweden. Epidemiol. Infect. 2011, 139, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic Transmission of Hepatitis E Virus from Deer to Human Beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Suzuki, M.; Yoshimatsu, K.; Arikawa, J.; Takashima, I.; Yokoyama, M.; Igota, H.; Yamauchi, K.; Ishida, S.; Fukui, D.; et al. Prevalence of Antibody to Hepatitis E Virus among Wild Sika Deer, Cervus Nippon, in Japan. Arch. Virol. 2007, 152, 1375–1381. [Google Scholar] [CrossRef]
- Sonoda, H.; Abe, M.; Sugimoto, T.; Sato, Y.; Bando, M.; Fukui, E.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Prevalence of Hepatitis E Virus (HEV) Infection in Wild Boars and Deer and Genetic Identification of a Genotype 3 HEV from a Boar in Japan. J. Clin. Microbiol. 2004, 42, 5371–5374. [Google Scholar] [CrossRef]
- Tomiyama, D.; Inoue, E.; Osawa, Y.; Okazaki, K. Serological Evidence of Infection with Hepatitis E Virus among Wild Yezo-Deer, Cervus Nippon Yesoensis, in Hokkaido, Japan. J. Viral Hepat. 2009, 16, 524–528. [Google Scholar] [CrossRef]
- Cancela, F.; Cravino, A.; Icasuriaga, R.; González, P.; Bentancor, F.; Leizagoyen, C.; Echaides, C.; Ferreiro, I.; Cabrera, A.; Arbiza, J.; et al. Co-Circulation of Hepatitis E Virus (HEV) Genotype 3 and Moose-HEV-Like Strains in Free-Ranging-Spotted Deer (Axis Axis) in Uruguay. Food Environ. Virol. 2023, 15, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Tialla, D.; Cissé, A.; Ouédraogo, G.A.; Hübschen, J.M.; Tarnagda, Z.; Snoeck, C.J. Prevalence of Hepatitis E Virus Antibodies in Cattle in Burkina Faso Associated with Swine Mixed Farming. J. Vet. Sci. 2022, 23, e33. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, L.; Gong, L.; Lv, J.; Feng, Y.; Liu, J.; Song, L.; Xu, Q.; Jiang, M.; Xu, A. Hepatitis E Virus in Yellow Cattle, Shandong, Eastern China. Emerg. Infect. Dis. 2016, 22, 2211. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Joshi, M.V.; Kulkarni, A.M.; Gandhe, S.S.; Chobe, L.P.; Rautmare, S.S.; Mishra, A.C.; Padbidri, V.S. Prevalence of Anti-Hepatitis E Virus Antibodies in Different Indian Animal Species. J. Viral Hepat. 2001, 8, 223–227. [Google Scholar] [CrossRef]
- El-Tras, W.F.; Tayel, A.A.; El-Kady, N.N. Seroprevalence of Hepatitis E Virus in Humans and Geographically Matched Food Animals in Egypt. Zoonoses Public Health 2013, 60, 244–251. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of Infectious Hepatitis E Virus into Milk in Cows Imposes High Risks of Zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef]
- Sarchese, V.; Di Profio, F.; Melegari, I.; Palombieri, A.; Sanchez, S.B.; Arbuatti, A.; Ciuffetelli, M.; Marsilio, F.; Martella, V.; Di Martino, B. Hepatitis E Virus in Sheep in Italy. Transbound. Emerg. Dis. 2019, 66, 1120–1125. [Google Scholar] [CrossRef]
- Peralta, B.; Casas, M.; de Deus, N.; Martín, M.; Ortuño, A.; Pérez-Martín, E.; Pina, S.; Mateu, E. Anti-HEV Antibodies in Domestic Animal Species and Rodents from Spain Using a Genotype 3-Based ELISA. Vet. Microbiol. 2009, 137, 66–73. [Google Scholar] [CrossRef]
- Wu, J.; Si, F.; Jiang, C.; Li, T.; Jin, M. Molecular Detection of Hepatitis E Virus in Sheep from Southern Xinjiang, China. Virus Genes. 2015, 50, 410–417. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Robetto, S.; Marsilio, F.; Martella, V. Detection of Hepatitis E Virus (HEV) in Goats. Virus Res. 2016, 225, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Yu, W.; Yang, C.; Wang, J.; Li, Y.; Li, Y.; Huang, F. High Prevalence of Hepatitis E Virus Infection in Goats. J. Med. Virol. 2017, 89, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Sanford, B.J.; Emerson, S.U.; Purcell, R.H.; Engle, R.E.; Dryman, B.A.; Cecere, T.E.; Buechner-Maxwell, V.; Sponenberg, D.P.; Meng, X.J. Serological Evidence for a Hepatitis e Virus-Related Agent in Goats in the United States. Transbound. Emerg. Dis. 2013, 60, 538–545. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Cong, J.; Zhou, Y.; Miao, Z. Detection and Characterization of Hepatitis E Virus in Goats at Slaughterhouse in Tai’an Region, China. BioMed. Res. Int. 2017, 2017, 3723650. [Google Scholar] [CrossRef]
- Lin, J.; Norder, H.; Uhlhorn, H.; Belák, S.; Widén, F. Novel Hepatitis E like Virus Found in Swedish Moose. J. Gen. Virol. 2014, 95, 557–570. [Google Scholar] [CrossRef]
- Lin, J.; Karlsson, M.; Olofson, A.S.; Belák, S.; Malmsten, J.; Dalin, A.M.; Widén, F.; Norder, H. High Prevalence of Hepatitis e Virus in Swedish Moose—A Phylogenetic Characterization and Comparison of the Virus from Different Regions. PLoS ONE 2015, 10, e0122102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; et al. A Novel Genotype of Hepatitis E Virus Prevalent among Farmed Rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zeng, H.; Liu, L.; Zhang, Y.; Liu, P.; Geng, J.; Wang, L.; Wang, L.; Zhuang, H. Swine and Rabbits Are the Main Reservoirs of Hepatitis E Virus in China: Detection of HEV RNA in Feces of Farmed and Wild Animals. Arch. Virol. 2015, 160, 2791–2798. [Google Scholar] [CrossRef]
- Cossaboom, C.M.; Córdoba, L.; Dryman, B.A.; Meng, X.J. Hepatitis E Virus in Rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guérin, J.L. Hepatitis E Virus Strains in Rabbits and Evidence of a Closely Related Strain in Humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Caruso, C.; Modesto, P.; Prato, R.; Scaglione, F.E.; De Marco, L.; Bollo, E.; Acutis, P.L.; Masoero, L.; Peletto, S. Hepatitis E Virus: First Description in a Pet House Rabbit. A New Transmission Route for Human? Transbound. Emerg. Dis. 2015, 62, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, F.; Schwaiger, K.; Dähnert, L.; Vina-Rodriguez, A.; Höper, D.; Gareis, M.; Groschup, M.H.; Eiden, M. Hepatitis E Virus in Wild Rabbits and European Brown Hares in Germany. Zoonoses Public Health 2017, 64, 612–622. [Google Scholar] [CrossRef]
- Birke, L.; Cormier, S.A.; You, D.; Stout, R.W.; Clement, C.; Johnson, M.; Thompson, H. Hepatitis E Antibodies in Laboratory Rabbits from 2 US Vendors. Emerg. Infect. Dis. 2014, 20, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, L.; Wang, X.; Fu, H.; Bu, Q.; Zhu, Y.; Zhuang, H. Study on Prevalence and Genotype of Hepatitis E Virus Isolated from Rex Rabbits in Beijing, China. J. Viral Hepat. 2011, 18, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takahashi, K.; Taira, K.; Taira, M.; Ohno, A.; Sakugawa, H.; Arai, M.; Mishiro, S. Hepatitis E Virus Infection in Wild Mongooses of Okinawa, Japan: Demonstration of Anti-HEV Antibodies and a Full-Genome Nucleotide Sequence☆. Hepatol. Res. 2006, 34, 137–140. [Google Scholar] [CrossRef]
- Li, T.C.; Saito, M.; Ogura, G.; Ishibashi, O.; Miyamura, T.; Takeda, N. Serologic Evidence For Hepatitis E Virus Infection In Mongoose. Am. J. Trop. Med. Hyg. 2006, 74, 932–936. [Google Scholar] [CrossRef]
- Nidaira, M.; Takahashi, K.; Ogura, G.; Taira, K.; Okano, S.; Kudaka, J.; Itokazu, K.; Mishiro, S.; Nakamura, M. Detection and Phylogenetic Analysis of Hepatitis E Viruses from Mongooses in Okinawa, Japan. J. Vet. Med. Sci. 2012, 74, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Smits, S.L.; Pas, S.D.; Provacia, L.B.V.; Moorman-Roest, H.; Osterhaus, A.D.M.E.; Haagmans, B.L. Novel Hepatitis E Virus in Ferrets, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1369–1370. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Hemnani, M.; Lopez-Lopez, P.; Gonçalves, H.M.R.; Rivero-Juarez, A.; Van der Poel, W.H.M.; Nascimento, M.S.J.; Mesquita, J.R. A Systematic Review of Hepatitis E Virus Detection in Camels. Vet. Sci. 2023, 10, 323. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, M.; Wong, E.Y.M.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New Hepatitis E Virus Genotype in Camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef]
- Rasche, A.; Saqib, M.; Liljander, A.M.; Bornstein, S.; Zohaib, A.; Renneker, S.; Steinhagen, K.; Wernery, R.; Younan, M.; Gluecks, I.; et al. Hepatitis E Virus Infection in Dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983–2015. Emerg. Infect. Dis. 2016, 22, 1249–1252. [Google Scholar] [CrossRef]
- Guan, D.; Li, W.; Su, J.; Fang, L.; Takeda, N.; Wakita, T.; Li, T.C.; Ke, C. Asian Musk Shrew as a Reservoir of Rat Hepatitis E Virus, China. Emerg. Infect. Dis. 2013, 19, 1341–1343. [Google Scholar] [CrossRef]
- Li, W.; Guan, D.; Su, J.; Takeda, N.; Wakita, T.; Li, T.C.; Ke, C.W. High Prevalence of Rat Hepatitis E Virus in Wild Rats in China. Vet. Microbiol. 2013, 165, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Mulyanto; Suparyatmo, J.B.; Andayani, I.G.A.S.; Khalid; Takahashi, M.; Ohnishi, H.; Jirintai, S.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Marked Genomic Heterogeneity of Rat Hepatitis E Virus Strains in Indonesia Demonstrated on a Full-Length Genome Analysis. Virus Res. 2014, 179, 102–112. [Google Scholar] [CrossRef]
- Ryll, R.; Bernstein, S.; Heuser, E.; Schlegel, M.; Dremsek, P.; Zumpe, M.; Wolf, S.; Pépin, M.; Bajomi, D.; Müller, G.; et al. Detection of Rat Hepatitis E Virus in Wild Norway Rats (Rattus norvegicus) and Black Rats (Rattus rattus) from 11 European Countries. Vet. Microbiol. 2017, 208, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kabrane-Lazizi, Y.; Fine, J.B.; Elm, J.; Glass, G.E.; Higa, H.; Diwan, A.; Gibbs, C.J.; Meng, X.J.; Emerson, S.U.; Purcell, R.H. Evidence for Widespread Infection of Wild Rats with Hepatitis E Virus in the United States. Am. J. Trop. Med. Hyg. 1999, 61, 331–335. [Google Scholar] [CrossRef]
- Easterbrook, J.D.; Kaplan, J.B.; Vanasco, N.B.; Reeves, W.K.; Purcell, R.H.; Kosoy, M.Y.; Glass, G.E.; Watson, J.; Klein, S.L. A Survey of Zoonotic Pathogens Carried by Norway Rats in Baltimore, Maryland, USA. Epidemiol. Infect. 2007, 135, 1192. [Google Scholar] [CrossRef]
- Johne, R.; Heckel, G.; Plenge-Bönig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel Hepatitis E Virus Genotype in Norway Rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; Claire, M.S.; Emerson, S.U. Hepatitis E Virus in Rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef]
- He, W.; Wen, Y.; Xiong, Y.; Zhang, M.; Cheng, M.; Chen, Q. The Prevalence and Genomic Characteristics of Hepatitis E Virus in Murine Rodents and House Shrews from Several Regions in China. BMC Vet. Res. 2018, 14, 414. [Google Scholar] [CrossRef]
- Widén, F.; Ayral, F.; Artois, M.; Olofson, A.S.; Lin, J. PCR Detection and Analysis of Potentially Zoonotic Hepatitis E Virus in French Rats. Virol. J. 2014, 11, 90. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Kindler, E.; Schielke, A.; Plenge-Bönig, A.; Gregersen, H.; Wessels, U.; Schmidt, K.; Rietschel, W.; Groschup, M.H.; et al. Rat Hepatitis E Virus: Geographical Clustering within Germany and Serological Detection in Wild Norway Rats (Rattus norvegicus). Infect. Genet. Evol. 2012, 12, 947–956. [Google Scholar] [CrossRef]
- Kanai, Y.; Miyasaka, S.; Uyama, S.; Kawami, S.; Kato-Mori, Y.; Tsujikawa, M.; Yunoki, M.; Nishiyama, S.; Ikuta, K.; Hagiwara, K. Hepatitis E Virus in Norway Rats (Rattus norvegicus) Captured around a Pig Farm. BMC Res. Notes 2012, 5, 4. [Google Scholar] [CrossRef]
- Lack, J.B.; Volk, K.; Van Den Bussche, R.A. Hepatitis E Virus Genotype 3 in Wild Rats, United States. Emerg. Infect. Dis. 2012, 18, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, W.; Zhou, J.H.; Li, B.; Zhang, W.; Yang, W.H.; Pan, H.; Wang, L.X.; Bock, C.T.; Shi, Z.L.; et al. Chevrier’s Field Mouse (Apodemus chevrieri) and Père David’s Vole (Eothenomys melanogaster) in China Carry Orthohepeviruses That Form Two Putative Novel Genotypes Within the Species Orthohepevirus C. Virol. Sin. 2018, 33, 44–58. [Google Scholar] [CrossRef]
- de Souza, W.M.; Romeiro, M.F.; Sabino-Santos, G.; Maia, F.G.M.; Fumagalli, M.J.; Modha, S.; Nunes, M.R.T.; Murcia, P.R.; Figueiredo, L.T.M. Novel Orthohepeviruses in Wild Rodents from São Paulo State, Brazil. Virology 2018, 519, 12–16. [Google Scholar] [CrossRef]
- Kurucz, K.; Hederics, D.; Bali, D.; Kemenesi, G.; Horváth, G.; Jakab, F. Hepatitis E Virus in Common Voles (Microtus arvalis) from an Urban Environment, Hungary: Discovery of a Cricetidae-Specific Genotype of Orthohepevirus C. Zoonoses Public Health 2019, 66, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Krog, J.S.; Breum, S.; Jensen, T.H.; Larsen, L.E. Hepatitis E Virus Variant in Farmed Mink, Denmark. Emerg. Infect. Dis. 2013, 19, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Macdonald, R.E.; Tapscott, B.; Nagy, E.; Turner, P.V. Detection of Astrovirus, Rotavirus C, and Hepatitis E Viral RNA in Adult and Juvenile Farmed Mink (Neovison Vison). Front. Vet. Sci. 2018, 5, 132. [Google Scholar] [CrossRef]
- Bodewes, R.; van der Giessen, J.; Haagmans, B.L.; Osterhaus, A.D.M.E.; Smits, S.L. Identification of Multiple Novel Viruses, Including a Parvovirus and a Hepevirus, in Feces of Red Foxes. J. Virol. 2013, 87, 7758–7764. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.-L.; Li, W.; Zhu, Y.; Ge, X.Y.; Zhang, L.B.; Zhang, Y.Z.; Bock, C.T.; Shi, Z.L. Detection and Genome Characterization of Four Novel Bat Hepadnaviruses and a Hepevirus in China. Virol. J. 2017, 14, 40. [Google Scholar] [CrossRef]
- Kobayashi, T.; Murakami, S.; Yamamoto, T.; Mineshita, K.; Sakuyama, M.; Sasaki, R.; Maeda, K.; Horimoto, T. Detection of Bat Hepatitis E Virus RNA in Microbats in Japan. Virus Genes. 2018, 54, 599–602. [Google Scholar] [CrossRef]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Fumie Tateno, A.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats Worldwide Carry Hepatitis E Virus-Related Viruses That Form a Putative Novel Genus within the Family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef] [PubMed]
- Doceul, V.; Bagdassarian, E.; Demange, A.; Pavio, N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses 2016, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Cossaboom, C.M.; Meng, X.J. Naturally Occurring Animal Models of Human Hepatitis E Virus Infection. ILAR J. 2014, 55, 187–199. [Google Scholar] [CrossRef]
- Ticehurst, J.; Rhodes, L.L.; Krawczynski, K.; Asher, L.V.S.; Engler, W.F.; Mensing, T.L.; Caudill, J.D.; Sjogren, M.H.; Hoke, C.H.; LeDuc, J.W.; et al. Infection of Owl Monkeys (Aotus trivirgatus) and Cynomolgus Monkeys (Macaca fascicularis) with Hepatitis E Virus from Mexico. J. Infect. Dis. 1992, 165, 835–845. [Google Scholar] [CrossRef]
- Tsarev, S.A.; Tsareva, T.S.; Emerson, S.U.; Govindarajan, S.; Shapiro, M.; Gerin, J.L.; Purcell, R.H. Successful Passive and Active Immunization of Cynomolgus Monkeys against Hepatitis E. Proc. Natl. Acad. Sci. USA 1994, 91, 10198–10202. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Meng, J.; Dai, X.; Liang, J.H.; Feagins, A.R.; Meng, X.J.; Belfiore, N.M.; Bradford, C.; Corn, J.L.; Cray, C.; et al. Restricted Enzooticity of Hepatitis E Virus Genotypes 1 to 4 in the United States. J. Clin. Microbiol. 2011, 49, 4164–4172. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Q.; Mou, J.; Yang, Z.B.; Yuan, C.L.; Cui, L.; Zhu, J.G.; Hua, X.G.; Xu, C.M.; Hu, J. Cross-Species Infection of Hepatitis E Virus in a Zoo-like Location, Including Birds. Epidemiol. Infect. 2008, 136, 1020–1026. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Goverdhan, M.K.; Banerjee, K. Antibodies against Hepatitis E Virus in Old World Monkeys. J. Viral Hepat. 1994, 1, 125–129. [Google Scholar] [CrossRef]
- Bradley, D.W.; Krawczynski, K.; Cook, E.H.; McCaustland, K.A.; Humphrey, C.D.; Spelbring, J.E.; Myint, H.; Maynard, J.E. Enterically Transmitted Non-A, Non-B Hepatitis: Serial Passage of Disease in Cynomolgus Macaques and Tamarins and Recovery of Disease-Associated 27- to 34-Nm Viruslike Particles. Proc. Natl. Acad. Sci. USA 1987, 84, 6277–6281. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kamili, S.; Spelbring, J.; Krawczynski, K. Experimental Studies on Subclinical Hepatitis E Virus Infection in Cynomolgus Macaques. J. Infect. Dis. 2001, 184, 1380–1385. [Google Scholar] [CrossRef]
- de Carvalho, L.G.; Marchevsky, R.S.; dos Santos, D.R.L.; de Oliveira, J.M.; de Paula, V.S.; Lopes, L.M.; Van der Poel, W.H.M.; González, J.E.; Munné, M.S.; Moran, J.; et al. Infection by Brazilian and Dutch Swine Hepatitis E Virus Strains Induces Haematological Changes in Macaca Fascicularis. BMC Infect. Dis. 2013, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Teng, J.L.L.; Lau, S.K.P.; Sridhar, S.; Fu, H.; Gong, W.; Li, M.; Xu, Q.; He, Y.; Zhuang, H.; et al. Transmission of a Novel Genotype of Hepatitis E Virus from Bactrian Camels to Cynomolgus Macaques. J. Virol. 2019, 93, 7. [Google Scholar] [CrossRef]
- Li, T.C.; Bai, H.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Doan, Y.H.; Takahashi, K.; Mishiro, S.; Takeda, N.; Wakita, T. Genotype 5 Hepatitis E Virus Produced by a Reverse Genetics System Has the Potential for Zoonotic Infection. Hepatol. Commun. 2018, 3, 160–172. [Google Scholar] [CrossRef]
- Yamamoto, H.; Suzuki, J.; Matsuda, A.; Ishida, T.; Ami, Y.; Suzaki, Y.; Adachi, I.; Wakita, T.; Takeda, N.; Li, T.C. Hepatitis E Virus Outbreak in Monkey Facility, Japan. Emerg. Infect. Dis. 2012, 18, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-J.; Halbur, P.G.; Shapiro, M.S.; Govindarajan, S.; Bruna, J.D.; Mushahwar, I.K.; Purcell, R.H.; Emerson, S.U. Genetic and Experimental Evidence for Cross-Species Infection by Swine Hepatitis E Virus. J. Virol. 1998, 72, 9714–9721. [Google Scholar] [CrossRef]
- Huang, F.; Yu, W.; Hua, X.; Jing, S.; Zeng, W.; He, Z. Seroepidemiology and Molecular Characterization of Hepatitis E Virus in Macaca Mulatta from a Village in Yunnan, China, Where Infection with This Virus Is Endemic. Hepat. Mon. 2011, 11, 745–749. [Google Scholar] [CrossRef]
- Liang, H.; Chen, J.; Xie, J.; Sun, L.; Ji, F.; He, S.; Zheng, Y.; Liang, C.; Zhang, G.; Su, S.; et al. Hepatitis E Virus Serosurvey among Pet Dogs and Cats in Several Developed Cities in China. PLoS ONE 2014, 9, e98068. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Ouchi, A.; Kawakami, K.; Ishida, T.; Li, T.-C.; Takeda, N.; Ikeda, H.; Tsunemitsu, H. Epidemiological Study of Hepatitis E Virus Infection of Dogs and Cats in Japan. Vet. Rec. 2006, 159, 853–854. [Google Scholar] [CrossRef]
- Yu, C.; Boon, D.; McDonald, S.L.; Myers, T.G.; Tomioka, K.; Nguyen, H.; Engle, R.E.; Govindarajan, S.; Emerson, S.U.; Purcell, R.H. Pathogenesis of Hepatitis E Virus and Hepatitis C Virus in Chimpanzees: Similarities and Differences. J. Virol. 2010, 84, 11264–11278. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Sreenivasan, M.A.; Popper, H.; Ticehurst, J.; Kapikian, A.Z.; Pavri, K.M.; Purcell, R.H. Aetiological Association of a Virus-like Particle with Enterically Transmitted Non-A, Non-B Hepatitis. Lancet 1988, 1, 550–554. [Google Scholar] [CrossRef]
- Zhou, C.; Li, W.; Yang, S. Analysis of Hepatitis e Virus-like Sequence in Chimpanzee. Hepat. Mon. 2014, 14, e19473. [Google Scholar] [CrossRef] [PubMed]
- Spahr, C.; Knauf-Witzens, T.; Vahlenkamp, T.; Ulrich, R.G.; Johne, R. Hepatitis E Virus and Related Viruses in Wild, Domestic and Zoo Animals: A Review. Zoonoses Public Health 2018, 65, 11–29. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Shen, Q.; Yang, S.; Huang, F.; Li, P.; Guo, X.; Yang, Z.; Cui, L.; Zhu, J.; et al. Prevalence of Antibody to Hepatitis E Virus among Pet Dogs in the Jiang-Zhe Area of China. Scand. J. Infect. Dis. 2009, 41, 291–295. [Google Scholar] [CrossRef] [PubMed]
- McElroy, A.; Hiraide, R.; Bexfield, N.; Jalal, H.; Brownlie, J.; Goodfellow, I.; Caddy, S.L. Detection of Hepatitis E Virus Antibodies in Dogs in the United Kingdom. PLoS ONE 2015, 10, e0128703. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Rivero, A.; Caballero-Gómez, J.; López-López, P.; Cano-Terriza, D.; Frías, M.; Jiménez-Ruiz, S.; Risalde, M.A.; Gómez-Villamandos, J.C.; Rivero-Juarez, A. Hepatitis E Virus Infection in Equines in Spain. Transbound. Emerg. Dis. 2019, 66, 66–71. [Google Scholar] [CrossRef]
- Saad, M.D.; Hussein, H.A.; Bashandy, M.M.; Kamel, H.H.; Earhart, K.C.; Fryauff, D.J.; Younan, M.; Mohamed, A.H. Hepatitis E Virus Infection in Work Horses in Egypt. Infect. Genet. Evol. 2007, 7, 368–373. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Q.; Mou, J.; Gong, G.; Yang, Z.; Cui, L.; Zhu, J.; Ju, G.; Hua, X. Hepatitis E Virus Infection among Domestic Animals in Eastern China. Zoonoses Public Health 2008, 55, 291–298. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, P.; Li, R.; She, R.; Tian, J.; Wang, J.; Mao, J.; Yin, J.; Shi, R. Increased Mast Cell Activation in Mongolian Gerbils Infected by Hepatitis E Virus. Front. Microbiol. 2018, 9, 2226. [Google Scholar] [CrossRef]
- Dähnert, L.; Conraths, F.J.; Reimer, N.; Groschup, M.H.; Eiden, M. Molecular and Serological Surveillance of Hepatitis E Virus in Wild and Domestic Carnivores in Brandenburg, Germany. Transbound. Emerg. Dis. 2018, 65, 1377–1380. [Google Scholar] [CrossRef]
- Villalba, M.C.M.; Martínez, D.C.; Ahmad, I.; Lay, L.A.R.; Corredor, M.B.; March, C.G.; Martínez, L.S.; Martínez-Campo, L.S.; Jameel, S. Hepatitis E Virus in Bottlenose Dolphins Tursiops Truncatus. Dis. Aquat. Organ. 2017, 123, 13–18. [Google Scholar] [CrossRef]
- Forgách, P.; Nowotny, N.; Erdélyi, K.; Boncz, A.; Zentai, J.; Szucs, G.; Reuter, G.; Bakonyi, T. Detection of Hepatitis E Virus in Samples of Animal Origin Collected in Hungary. Vet. Microbiol. 2010, 143, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef]
- Reuter, G.; Fodor, D.; Forgách, P.; Kátai, A.; Szucs, G. Characterization and Zoonotic Potential of Endemic Hepatitis E Virus (HEV) Strains in Humans and Animals in Hungary. J. Clin. Virol. 2009, 44, 277–281. [Google Scholar] [CrossRef]
- Wiratsudakul, A.; Sariya, L.; Prompiram, P.; Tantawet, S.; Suraruangchai, D.; Sedwisai, P.; Sangkachai, N.; Suksai, P.; Ratanakorn, P. Detection and Phylogenetic Characterization of Hepatitis E Virus Genotype 3 in a Captive Wild Boar in Thailand. J. Zoo. Wildl. Med. 2012, 43, 640–644. [Google Scholar] [CrossRef]
- Kaci, S.; Nöckler, K.; Johne, R. Detection of Hepatitis E Virus in Archived German Wild Boar Serum Samples. Vet. Microbiol. 2008, 128, 380–385. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Sato, H.; Sato, Y.; Jirintai; Nagashima, S.; Okamoto, H. Analysis of the Full-Length Genome of a Hepatitis E Virus Isolate Obtained from a Wild Boar in Japan That Is Classifiable into a Novel Genotype. J. Gen. Virol. 2011, 92, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K.; et al. Molecular Characterization of a Novel Hepatitis E Virus (HEV) Strain Obtained from a Wild Boar in Japan That Is Highly Divergent from the Previously Recognized HEV Strains. Virus Res. 2014, 180, 59–69. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K.; Jabłoński, A.; Kesik, J.; Bednarski, M.; Rola, J. Hepatitis E Virus Antibody Prevalence in Wildlife in Poland. Zoonoses Public Health 2015, 62, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Pavoni, E.; Filogari, D.; Ferrari, N.; Chiari, M.; Canelli, E.; Lombardi, G. Hepatitis E Virus in Wild Boar in the Central Northern Part of Italy. Transbound. Emerg. Dis. 2015, 62, 217–222. [Google Scholar] [CrossRef]
- Xu, F.; Pan, Y.; Baloch, A.R.; Tian, L.; Wang, M.; Na, W.; Ding, L.; Zeng, Q. Hepatitis E Virus Genotype 4 in Yak, Northwestern China. Emerg. Infect. Dis. 2014, 20, 2182–2184. [Google Scholar] [CrossRef]
- Li, T.C.; Miyamura, T.; Takeda, N. Detection Of Hepatitis E Virus Rna From The Bivalve Yamato-Shijimi (Corbicula Japonica) In Japan. Am. J. Trop. Med. Hyg. 2007, 76, 170–172. [Google Scholar] [CrossRef]
- O’Hara, Z.; Crossan, C.; Craft, J.; Scobie, L. First Report of the Presence of Hepatitis E Virus in Scottish-Harvested Shellfish Purchased at Retail Level. Food Environ. Virol. 2018, 10, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Krog, J.S.; Larsen, L.E.; Schultz, A.C. Enteric Porcine Viruses in Farmed Shellfish in Denmark. Int. J. Food Microbiol. 2014, 186, 105–109. [Google Scholar] [CrossRef]
- Diez-Valcarce, M.; Kokkinos, P.; Söderberg, K.; Bouwknegt, M.; Willems, K.; de Roda-Husman, A.M.; von Bonsdorff, C.H.; Bellou, M.; Hernández, M.; Maunula, L.; et al. Occurrence of Human Enteric Viruses in Commercial Mussels at Retail Level in Three European Countries. Food Environ. Virol. 2012, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Haqshenas, G.; Shivaprasad, H.L.; Woolcock, P.R.; Read, D.H.; Meng, X.J. Genetic Identification and Characterization of a Novel Virus Related to Human Hepatitis E Virus from Chickens with Hepatitis-Splenomegaly Syndrome in the United States. J. Gen. Virol. 2001, 82, 2449–2462. [Google Scholar] [CrossRef]
- Liu, B.; Fan, M.; Zhang, B.; Chen, Y.; Sun, Y.; Du, T.; Nan, Y.; Zhou, E.M.; Zhao, Q. Avian Hepatitis E Virus Infection of Duck, Goose, and Rabbit in Northwest China. Emerg. Microbes Infect. 2018, 7, 1–3. [Google Scholar] [CrossRef]
- Li, H.; Zhu, R.; She, R.; Zhang, C.; Shi, R.; Li, W.; Du, F.; Wu, Q.; Hu, F.; Zhang, Y.; et al. Case Report Associated with Aspergillosis and Hepatitis E Virus Coinfection in Himalayan Griffons. BioMed. Res. Int. 2015, 2015, 287315. [Google Scholar] [CrossRef]
- Zhang, X.; Bilic, I.; Troxler, S.; Hess, M. Evidence of Genotypes 1 and 3 of Avian Hepatitis E Virus in Wild Birds. Virus Res. 2017, 228, 75–78. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, Á.; Mátics, R.; Kapusinszky, B.; Delwart, E.; Pankovics, P. A Novel Avian-like Hepatitis E Virus in Wild Aquatic Bird, Little Egret (Egretta garzetta), in Hungary. Infect. Genet. Evol. 2016, 46, 74–77. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Shen, H.; Zheng, Y.; Gauger, P.C.; Chen, Q.; Zhang, J.; Yoon, K.J.; Harmon, K.M.; Main, R.G.; et al. Detection and Genomic Characterization of New Avian-like Hepatitis E Virus in a Sparrow in the United States. Arch. Virol. 2018, 163, 2861–2864. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.F.; Larsen, C.T.; Huang, F.F.; Billam, P.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Generation and Infectivity Titration of an Infectious Stock of Avian Hepatitis E Virus (HEV) in Chickens and Cross-Species Infection of Turkeys with Avian HEV. J. Clin. Microbiol. 2004, 42, 2658–2662. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public Health Risks Associated with Hepatitis E Virus (HEV) as a Food-Borne Pathogen. EFSA J. 2017, 15, e04886. [Google Scholar] [CrossRef]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne Transmission of Hepatitis A and Hepatitis E Viruses: A Literature Review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of Infectious Hepatitis E Virus Present in Commercial Pig Livers Sold in Local Grocery Stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Stunnenberg, M.; van Huizen, S.C.; Swart, A.; Lodder, W.J.; Boxman, I.L.A.; Rutjes, S.A. Thermal Inactivation of Hepatitis E Virus in Pork Products Estimated with a Semiquantitative Infectivity Assay. Microorganisms 2023, 11, 2451. [Google Scholar] [CrossRef]
- Matsuda, H.; Okada, K.; Takahashi, K.; Mishiro, S. Severe Hepatitis E Virus Infection after Ingestion of Uncooked Liver from a Wild Boar. J. Infect. Dis. 2003, 188, 944. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Yano, K.; Yatsuhashi, H.; Inoue, O.; Mawatari, F.; Ishibashi, H. Consumption of Wild Boar Linked to Cases of Hepatitis E. J. Hepatol. 2004, 40, 869–870. [Google Scholar] [CrossRef]
- Li, T.C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; et al. Hepatitis E Virus Transmission from Wild Boar Meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef]
- Inoue, G.; Michitaka, K.; Takahashi, K.; Abe, N.; Oka, K.; Nunoi, H.; Ueda, A.; Shimase, K.; Hiasa, Y.; Horiike, N.; et al. A case of acute hepatitis E developed in a housewife who had cooked and eaten wild boar meat a month before. Kanzo 2006, 47, 459–464. [Google Scholar] [CrossRef][Green Version]
- Miyashita, K.; Kang, J.H.; Saga, A.; Takahashi, K.; Shimamura, T.; Yasumoto, A.; Fukushima, H.; Sogabe, S.; Konishi, K.; Uchida, T.; et al. Three Cases of Acute or Fulminant Hepatitis E Caused by Ingestion of Pork Meat and Entrails in Hokkaido, Japan: Zoonotic Food-Borne Transmission of Hepatitis E Virus and Public Health Concerns. Hepatol. Res. 2012, 42, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Deest, G.; Zehner, L.; Nicand, E.; Gaudy-Graffin, C.; Goudeau, A.; Bacq, Y. Hépatite Virale E Autochtone En France et Consommation de Viande de Porc Séchée. Gastroenterol. Clin. Biol. 2007, 31, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig Liver Sausage as a Source of Hepatitis E Virus Transmission to Humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, S.H.; Kim, J.Y.; Song, J.C.; Lee, J.H.; Kim, J.W.; Yun, H.; Kim, J.S. The First Case of Genotype 4 Hepatitis E Related to Wild Boar in South Korea. J. Clin. Virol. 2011, 50, 253–256. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Mínguez, B.; Gironés, R.; Rodriguez-Frías, F.; Quer, J.; Buti, M. Phylogenetic Demonstration of Hepatitis E Infection Transmitted by Pork Meat Ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Chi-Yuan Teo, E.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Kim Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef]
- Renou, C.; Afonso, A.M.R.; Pavio, N. Foodborne Transmission of Hepatitis E Virus from Raw Pork Liver Sausage, France. Emerg. Infect. Dis. 2014, 20, 1945–1947. [Google Scholar] [CrossRef]
- Guillois, Y.; Abravanel, F.; Miura, T.; Pavio, N.; Vaillant, V.; Lhomme, S.; Le Guyader, F.S.; Rose, N.; Le Saux, J.C.; King, L.A.; et al. High Proportion of Asymptomatic Infections in an Outbreak of Hepatitis E Associated With a Spit-Roasted Piglet, France, 2013. Clin. Infect. Dis. 2016, 62, 351–357. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; García-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial Hepatitis E Outbreak Linked to Wild Boar Meat Consumption. Zoonoses Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef]
- Yin, W.; Han, Y.; Xin, H.; Liu, W.; Song, Q.; Li, Z.; Gao, S.; Jiang, F.; Cao, J.; Bi, S.; et al. Hepatitis E Outbreak in a Mechanical Factory in Qingdao City, China. Int. J. Infect. Dis. 2019, 86, 191–196. [Google Scholar] [CrossRef]
- Moor, D.; Liniger, M.; Baumgartner, A.; Felleisen, R. Screening of Ready-to-Eat Meat Products for Hepatitis E Virus in Switzerland. Food Environ. Virol. 2018, 10, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ferrando, E.; Randazzo, W.; Pérez-Cataluña, A.; Sánchez, G. HEV Occurrence in Waste and Drinking Water Treatment Plants. Front. Microbiol. 2020, 10, 501698. [Google Scholar] [CrossRef]
- Randazzo, W.; Vásquez-García, A.; Bracho, M.A.; Alcaraz, M.J.; Aznar, R.; Sánchez, G. Hepatitis E Virus in Lettuce and Water Samples: A Method-Comparison Study. Int. J. Food Microbiol. 2018, 277, 34–40. [Google Scholar] [CrossRef]
- Othman, I.; Helmi, A.; Slama, I.; Hamdi, R.; Mastouri, M.; Aouni, M. Evaluation of Three Viral Concentration Methods for Detection and Quantification of SARS-CoV-2 in Wastewater. J. Water Health 2023, 21, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Pellett, C.; Alex-Sanders, N.; Bridgman, M.T.P.; Corbishley, A.; Grimsley, J.M.S.; Kasprzyk-Hordern, B.; Kevill, J.L.; Pântea, I.; Richardson-O’Neill, I.S.; et al. Comparative Assessment of Filtration- and Precipitation-Based Methods for the Concentration of SARS-CoV-2 and Other Viruses from Wastewater. Microbiol. Spectr. 2022, 10, 4. [Google Scholar] [CrossRef]
- Stoufer, S.; Soorneedi, A.R.; Kim, M.; Moore, M.D. Sample Processing and Concentration Methods for Viruses from Foods and the Environment Prior to Detection. Annu. Rev. Food Sci. Technol. 2024, 15, 455–472. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Oglesbee, S.E.; Wait, D.A. Evaluation of Methods for Concentrating Hepatitis A Virus from Drinking Water. Appl. Environ. Microbiol. 1985, 50, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Perelle, S.; Cavellini, L.; Burger, C.; Blaise-Boisseau, S.; Hennechart-Collette, C.; Merle, G.; Fach, P. Use of a Robotic RNA Purification Protocol Based on the NucliSens® EasyMAGTM for Real-Time RT-PCR Detection of Hepatitis A Virus in Bottled Water. J. Virol. Methods 2009, 157, 80–83. [Google Scholar] [CrossRef]
- Schultz, A.C.; Perelle, S.; Di Pasquale, S.; Kovac, K.; De Medici, D.; Fach, P.; Sommer, H.M.; Hoorfar, J. Collaborative Validation of a Rapid Method for Efficient Virus Concentration in Bottled Water. Int. J. Food Microbiol. 2011, 145, S158–S166. [Google Scholar] [CrossRef]
- Martin-Latil, S.; Hennechart-Collette, C.; Guillier, L.; Perelle, S. Method for HEV Detection in Raw Pig Liver Products and Its Implementation for Naturally Contaminated Food. Int. J. Food Microbiol. 2014, 176, 1–8. [Google Scholar] [CrossRef]
- EN ISO 15216-1:2017; Microbiology of the Food Chain–Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part1: Method for Quantification. International Organization for Standardization: Geneva, Switzerland, 2017.
- Hennechart-Collette, C.; Dehan, O.; Laurentie, M.; Fraisse, A.; Martin-Latil, S.; Perelle, S. Method for Detecting Norovirus, Hepatitis A and Hepatitis E Viruses in Tap and Bottled Drinking Water. Int. J. Food Microbiol. 2022, 377, 109757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sikora, P.; Rutgersson, C.; Lindh, M.; Brodin, T.; Björlenius, B.; Larsson, D.G.J.; Norder, H. Differential Removal of Human Pathogenic Viruses from Sewage by Conventional and Ozone Treatments. Int. J. Hyg. Environ. Health 2018, 221, 479–488. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; Ng, K.M.D.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef]
- Maletskyi, Z. Advances in Membrane Materials and Processes for Water and Wastewater Treatment. ACS Symp. Ser. 2020, 1348, 3–35. [Google Scholar] [CrossRef]
- Mohammed, T.A.; Birima, A.H.; Noor, M.J.M.M.; Muyibi, S.A.; Idris, A. Evaluation of Using Membrane Bioreactor for Treating Municipal Wastewater at Different Operating Conditions. Desalination 2008, 221, 502–510. [Google Scholar] [CrossRef]
- Lewis, G.D.; Metcalf, T.G. Polyethylene Glycol Precipitation for Recovery of Pathogenic Viruses, Including Hepatitis A Virus and Human Rotavirus, from Oyster, Water, and Sediment Samples. Appl. Environ. Microbiol. 1988, 54, 1983–1988. [Google Scholar] [CrossRef]
- Goldring, J.P.D. Concentrating Proteins by Salt, Polyethylene Glycol, Solvent, SDS Precipitation, Three-Phase Partitioning, Dialysis, Centrifugation, Ultrafiltration, Lyophilization, Affinity Chromatography, Immunoprecipitation or Increased Temperature for Protein Isolation, Drug Interaction, and Proteomic and Peptidomic Evaluation. Methods Mol. Biol. 2019, 1855, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.C.; Ramos, T.D.M.; Wu, X.; DiCaprio, E. Presence of Hepatitis E Virus in Commercially Available Pork Products. Int. J. Food Microbiol. 2021, 339, 109033. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors–Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Son, N.R.; Seo, D.J.; Lee, M.H.; Seo, S.; Wang, X.; Lee, B.H.; Lee, J.S.; Joo, I.S.; Hwang, I.G.; Choi, C. Optimization of the Elution Buffer and Concentration Method for Detecting Hepatitis E Virus in Swine Liver Using a Nested Reverse Transcription-Polymerase Chain Reaction and Real-Time Reverse Transcription-Polymerase Chain Reaction. J. Virol. Methods 2014, 206, 99–104. [Google Scholar] [CrossRef]
- Beyer, S.; Szewzyk, R.; Gnirss, R.; Johne, R.; Selinka, H.C. Detection and Characterization of Hepatitis E Virus Genotype 3 in Wastewater and Urban Surface Waters in Germany. Food Environ. Virol. 2020, 12, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Rzezutka, A.; D’Agostino, M.; Cook, N. An Ultracentrifugation-Based Approach to the Detection of Hepatitis A Virus in Soft Fruits. Int. J. Food Microbiol. 2006, 108, 315–320. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, Y.; Xu, X.; Li, S.; Zhang, Y.; Ding, J.; On, H.Y.; Lai, J.C.C.; In Yau, C.; Chin, A.W.H.; et al. Comparison of Virus Concentration Methods and RNA Extraction Methods for SARS-CoV-2 Wastewater Surveillance. Sci. Total Environ. 2022, 824, 153687. [Google Scholar] [CrossRef] [PubMed]
- Winona, L.J.; Ommani, A.W.; Olszewski, J.; Nuzzo, J.B.; Oshima, K.H. Efficient and Predictable Recovery of Viruses from Water by Small Scale Ultrafiltration Systems. Can. J. Microbiol. 2011, 47, 1033–1041. [Google Scholar] [CrossRef]
- Tahk, H.; Lee, K.B.; Lee, M.H.; Seo, D.J.; Cheon, D.S.; Choi, C. Development of Reverse Transcriptase Polymerase Chain Reaction Enzyme-Linked Immunosorbent Assay for the Detection of Hepatitis A Virus in Vegetables. Food Control 2012, 23, 210–214. [Google Scholar] [CrossRef]
- Forés, E.; Bofill-Mas, S.; Itarte, M.; Martínez-Puchol, S.; Hundesa, A.; Calvo, M.; Borrego, C.M.; Corominas, L.L.; Girones, R.; Rusiñol, M. Evaluation of Two Rapid Ultrafiltration-Based Methods for SARS-CoV-2 Concentration from Wastewater. Sci. Total Environ. 2021, 768, 144786. [Google Scholar] [CrossRef]
- Ye, Y.; Ellenberg, R.M.; Graham, K.E.; Wigginton, K.R. Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef]
- Smith, C.M.; Hill, V.R. Dead-End Hollow-Fiber Ultrafiltration for Recovery of Diverse Microbes from Water. Appl. Environ. Microbiol. 2009, 75, 5284–5289. [Google Scholar] [CrossRef]
- Gunnarsdottir, M.J.; Gardarsson, S.M.; Figueras, M.J.; Puigdomènech, C.; Juárez, R.; Saucedo, G.; Arnedo, M.J.; Santos, R.; Monteiro, S.; Avery, L.; et al. Water Safety Plan Enhancements with Improved Drinking Water Quality Detection Techniques. Sci. Total Environ. 2020, 698, 134185. [Google Scholar] [CrossRef]
- Forés, E.; Rusiñol, M.; Itarte, M.; Martínez-Puchol, S.; Calvo, M.; Bofill-Mas, S. Evaluation of a Virus Concentration Method Based on Ultrafiltration and Wet Foam Elution for Studying Viruses from Large-Volume Water Samples. Sci. Total Environ. 2022, 829, 154431. [Google Scholar] [CrossRef]
- Pascual-Benito, M.; Emiliano, P.; Casas-Mangas, R.; Dacal-Rodríguez, C.; Gracenea, M.; Araujo, R.; Valero, F.; García-Aljaro, C.; Lucena, F. Assessment of Dead-End Ultrafiltration for the Detection and Quantification of Microbial Indicators and Pathogens in the Drinking Water Treatment Processes. Int. J. Hyg. Environ. Health 2020, 230, 113628. [Google Scholar] [CrossRef]
- Mull, B.; Hill, V.R. Recovery of Diverse Microbes in High Turbidity Surface Water Samples Using Dead-End Ultrafiltration. J. Microbiol. Methods 2012, 91, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Yang, Y.; Jiao, N.; Zhang, R. Evaluation of Tangential Flow Filtration for the Concentration and Separation of Bacteria and Viruses in Contrasting Marine Environments. PLoS ONE 2015, 10, e0136741. [Google Scholar] [CrossRef]
- Rhodes, E.R.; Huff, E.M.; Hamilton, D.W.; Jones, J.L. The Evaluation of Hollow-Fiber Ultrafiltration and Celite Concentration of Enteroviruses, Adenoviruses and Bacteriophage from Different Water Matrices. J. Virol. Methods 2016, 228, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.G.; Gin, K.Y.-H.; Goh, S.G.; Te, S.H. Sample Preparation of Microbial Contaminants in Water. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 723–742. [Google Scholar]
- Cashdollar, J.L.; Wymer, L. Methods for Primary Concentration of Viruses from Water Samples: A Review and Meta-Analysis of Recent Studies. J. Appl. Microbiol. 2013, 115, 1–11. [Google Scholar] [CrossRef]
- Babler, K.M.; Amirali, A.; Sharkey, M.E.; Williams, S.L.; Boone, M.M.; Cosculluela, G.A.; Currall, B.B.; Grills, G.S.; Laine, J.; Mason, C.E.; et al. Comparison of Electronegative Filtration to Magnetic Bead-Based Concentration and V2G-QPCR to RT-QPCR for Quantifying Viral SARS-CoV-2 RNA from Wastewater. ACS EST Water 2022, 2, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lee, B.E.; Ruecker, N.J.; Neumann, N.; Ashbolt, N.; Pang, X. A One-Step Centrifugal Ultrafiltration Method to Concentrate Enteric Viruses from Wastewater. J. Virol. Methods 2016, 237, 150–153. [Google Scholar] [CrossRef]
- EN ISO/DIS 16140-4:2018; Microbiology of the Food Chain—Method Validation—Part 4: Protocol for Single-Laboratory (In-House) Method Validation. International Organization for Standardization: Geneva, Switzerland, 2017.
- Wang, H.; Kjellberg, I.; Sikora, P.; Rydberg, H.; Lindh, M.; Bergstedt, O.; Norder, H. Hepatitis E Virus Genotype 3 Strains and a Plethora of Other Viruses Detected in Raw and Still in Tap Water. Water Res. 2020, 168, 115141. [Google Scholar] [CrossRef]
- Martínez Wassaf, M.G.; Pisano, M.B.; Barril, P.A.; Elbarcha, O.C.; Pinto, M.A.; Mendes de Oliveira, J.; DiGiusto, P.; Nates, S.V.; Ré, V.E. First Detection of Hepatitis E Virus in Central Argentina: Environmental and Serological Survey. J. Clin. Virol. 2014, 61, 334–339. [Google Scholar] [CrossRef]
- Miura, T.; Lhomme, S.; Le Saux, J.C.; Le Mehaute, P.; Guillois, Y.; Couturier, E.; Izopet, J.; Abranavel, F.; Le Guyader, F.S. Detection of Hepatitis E Virus in Sewage After an Outbreak on a French Island. Food Environ. Virol. 2016, 8, 194–199. [Google Scholar] [CrossRef]
- Chatonnat, E.; Julien, M.; Jubinville, E.; Goulet-Beaulieu, V.; Pavio, N.; Jean, J. High Occurrence of Hepatitis E Virus in Raw Pork Liver and Pork Liver Pâté Produced in the Canadian Province of Quebec. Front. Sustain. Food Syst. 2023, 7, 1163507. [Google Scholar] [CrossRef]
- Modesto, P.; Maniaci, M.G.; Cavallazzi, U.; Acutis, P.L.; Peletto, S. Evaluation of a Molecular Method for Hepatitis E Virus (HEV) Detection in Pancreatin of Porcine Origin. J. Virol. Methods 2020, 276, 113790. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; D’agostino, M.; Wood, A.; Scobie, L. Real-Time PCR-Based Methods for Detection of Hepatitis E Virus in Pork Products: A Critical Review. Microorganisms 2022, 10, 428. [Google Scholar] [CrossRef]
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of Hepatitis E Virus RNA in Raw Sausages and Liver Sausages from Retail in Germany Using an Optimized Method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef]
- Marziali, F.; Acosta, J.; Bolatti, E.; Mirazo, S.; Skejich, P.; Silva, P.; Brassard, J.; Costaguta, A.; Gardiol, D.; Cavatorta, A.L. Detection of HEV in Naturally Infected Swine from Central Argentina by an Optimized HEV/MS2 Duplex RT-QPCR. Zoonoses Public Health 2019, 66, 729–738. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A Broadly Reactive One-Step Real-Time RT-PCR Assay for Rapid and Sensitive Detection of Hepatitis E Virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Porea, D.; Anita, A.; Demange, A.; Raileanu, C.; Oslobanu (Ludu), L.; Anita, D.; Savuta, G.; Pavio, N. Molecular Detection of Hepatitis E Virus in Wild Boar Population in Eastern Romania. Transbound. Emerg. Dis. 2018, 65, 527–533. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Li, K.; Rehman, M.U.; Jiang, X.; Tong, X.; Zhang, H.; Iqbal, M.K.; Mehmood, K.; Liu, S.; et al. Molecular Detection of Indigenous Hepatitis E Virus (HEV) from Tibetan Pigs in Tibet, China. Food Environ. Virol. 2018, 10, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sui, X.; Ma, Y.; Li, M.; Zhang, X.; Fei, D.; Ma, M. Real-Time Reverse Transcription Recombinase Polymerase Amplification for Rapid Detection of Murine Hepatitis Virus. Front. Microbiol. 2022, 13, 1067694. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhou, C.; Sun, X.; Liu, L.; Xu, X.; Wang, J. Rapid and Direct Detection of Hepatitis E Virus in Raw Pork Livers by Recombinase Polymerase Amplification Assays. Front. Cell Infect. Microbiol. 2022, 12, 958990. [Google Scholar] [CrossRef]
- Li, M.; Li, T.; Hao, X.; Liu, Y.; Lan, H.; Zhou, C. Preliminary Investigation of Hepatitis E Virus Detection by a Recombinase Polymerase Amplification Assay Combined with a Lateral Flow Strip. J. Vet. Diagn. Investig. 2023, 35, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Pallerla, S.R.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E: An Update on One Health and Clinical Medicine. Liver Int. 2021, 41, 1462–1473. [Google Scholar] [CrossRef]
- Fu, R.M.; Decker, C.C.; Dao Thi, V.L. Cell Culture Models for Hepatitis E Virus. Viruses 2019, 11, 608. [Google Scholar] [CrossRef]
- Meister, T.L.; Bruening, J.; Todt, D.; Steinmann, E. Cell Culture Systems for the Study of Hepatitis E Virus. Antivir. Res. 2019, 163, 34–49. [Google Scholar] [CrossRef]
- Tsarev, S.A.; Emerson, S.U.; Reyes, G.R.; Tsareva, T.S.; Legters, L.J.; Malik, I.A.; Iqbal, M.; Purcell, R.H. Characterization of a Prototype Strain of Hepatitis E Virus. Proc. Natl. Acad. Sci. USA 1992, 89, 559–563. [Google Scholar] [CrossRef]
- Huang, C.C.; Nguyen, D.; Fernandez, J.; Yun, K.Y.; Fry, K.E.; Bradley, D.W.; Tam, A.W.; Reyes, G.R. Molecular Cloning and Sequencing of the Mexico Isolate of Hepatitis E Virus (HEV). Virology 1992, 191, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, M.; Kusano, E.; Okamoto, H. Development and Evaluation of an Efficient Cell-Culture System for Hepatitis E Virus. J. Gen. Virol. 2007, 88, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, M.; Takahashi, H.; Ichiyama, K.; Hoshino, Y.; Nagashima, S.; Mizuo, H.; Okamoto, H. Development and Characterization of a Genotype 4 Hepatitis E Virus Cell Culture System Using a HE-JF5/15F Strain Recovered from a Fulminant Hepatitis Patient. J. Clin. Microbiol. 2009, 47, 1906–1910. [Google Scholar] [CrossRef]
- Feagins, A.R.; Córdoba, L.; Sanford, B.J.; Dryman, B.A.; Huang, Y.W.; LeRoith, T.; Emerson, S.U.; Meng, X.J. Intergenotypic Chimeric Hepatitis E Viruses (HEVs) with the Genotype 4 Human HEV Capsid Gene in the Backbone of Genotype 3 Swine HEV Are Infectious in Pigs. Virus Res. 2011, 156, 141–146. [Google Scholar] [CrossRef]
- Shukla, P.; Nguyen, H.T.; Torian, U.; Engle, R.E.; Faulk, K.; Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Purcell, R.H.; Emerson, S.U. Cross-Species Infections of Cultured Cells by Hepatitis E Virus and Discovery of an Infectious Virus-Host Recombinant. Proc. Natl. Acad. Sci. USA 2011, 108, 2438–2443. [Google Scholar] [CrossRef]
- Johne, R.; Reetz, J.; Ulrich, R.G.; Machnowska, P.; Sachsenröder, J.; Nickel, P.; Hofmann, J. An ORF1-Rearranged Hepatitis E Virus Derived from a Chronically Infected Patient Efficiently Replicates in Cell Culture. J. Viral Hepat. 2014, 21, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Ansari, I.H.; Durgapal, H.; Agrawal, S.; Jameel, S. The In Vitro-Synthesized RNA from a CDNA Clone of Hepatitis E Virus Is Infectious. J. Virol. 2000, 74, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Zhang, M.; Meng, X.J.; Nguyen, H.; St. Claire, M.; Govindarajan, S.; Huang, Y.K.; Purcell, R.H. Recombinant Hepatitis E Virus Genomes Infectious for Primates: Importance of Capping and Discovery of a Cis-Reactive Element. Proc. Natl. Acad. Sci. USA 2001, 98, 15270–15275. [Google Scholar] [CrossRef]
- Zhang, M.; Purcell, R.H.; Emerson, S.U. Identification of the 5′ Terminal Sequence of the SAR-55 and MEX-14 Strains of Hepatitis E Virus and Confirmation That the Genome Is Capped. J. Med. Virol. 2001, 65, 293–295. [Google Scholar] [CrossRef]
- Huang, Y.W.; Haqshenas, G.; Kasorndorkbua, C.; Halbur, P.G.; Emerson, S.U.; Meng, X.J. Capped RNA Transcripts of Full-Length CDNA Clones of Swine Hepatitis E Virus Are Replication Competent When Transfected into Huh7 Cells and Infectious When Intrahepatically Inoculated into Pigs. J. Virol. 2005, 79, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takahashi, M.; Hoshino, Y.; Takahashi, H.; Ichiyama, K.; Tanaka, T.; Okamoto, H. Construction of an Infectious CDNA Clone of Hepatitis E Virus Strain JE03-1760F That Can Propagate Efficiently in Cultured Cells. J. Gen. Virol. 2009, 90, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, L.; Feagins, A.R.; Opriessnig, T.; Cossaboom, C.M.; Dryman, B.A.; Huang, Y.W.; Meng, X.J. Rescue of a Genotype 4 Human Hepatitis E Virus from Cloned CDNA and Characterization of Intergenotypic Chimeric Viruses in Cultured Human Liver Cells and in Pigs. J. Gen. Virol. 2012, 93, 2183–2194. [Google Scholar] [CrossRef]
- Shukla, P.; Nguyen, H.T.; Faulk, K.; Mather, K.; Torian, U.; Engle, R.E.; Emerson, S.U. Adaptation of a Genotype 3 Hepatitis E Virus to Efficient Growth in Cell Culture Depends on an Inserted Human Gene Segment Acquired by Recombination. J. Virol. 2012, 86, 5697–5707. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Shukla, P.; Torian, U.; Faulk, K.; Emerson, S.U. Hepatitis E Virus Genotype 1 Infection of Swine Kidney Cells In Vitro Is Inhibited at Multiple Levels. J. Virol. 2014, 88, 868–877. [Google Scholar] [CrossRef]
- Emerson, S.U.; Nguyen, H.; Graff, J.; Stephany, D.A.; Brockington, A.; Purcell, R.H. In Vitro Replication of Hepatitis E Virus (HEV) Genomes and of an HEV Replicon Expressing Green Fluorescent Protein. J. Virol. 2004, 78, 4838–4846. [Google Scholar] [CrossRef]
- Emerson, S.U.; Nguyen, H.T.; Torian, U.; Burke, D.; Engle, R.; Purcell, R.H. Release of Genotype 1 Hepatitis E Virus from Cultured Hepatoma and Polarized Intestinal Cells Depends on Open Reading Frame 3 Protein and Requires an Intact PXXP Motif. J. Virol. 2010, 84, 9059–9069. [Google Scholar] [CrossRef]
- Kazachkov, Y.A.; Balayan, M.S.; Ivannikova, T.A.; Panina, L.I.; Orlova, T.M.; Zamyatina, N.A.; Kusov, Y.Y. Hepatitis E Virus in Cultivated Cells. Arch. Virol. 1992, 127, 399–402. [Google Scholar] [CrossRef]
- Huang, R.; Li, D.; Wei, S.; Li, Q.; Yuan, X.; Geng, L.; Li, X.; Liu, M. Cell Culture of Sporadic Hepatitis E Virus in China. Clin. Diagn. Lab. Immunol. 1999, 6, 729–733. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Li, Y.; Zhou, X.; Yin, Y.; Wang, Y.; de Man, R.A.; van der Laan, L.J.W.; Huang, F.; Kamar, N.; et al. RIG-I Is a Key Antiviral Interferon-Stimulated Gene against Hepatitis E Virus Regardless of Interferon Production. Hepatology 2017, 65, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Arankalle, V.A.; Purcell, R.H. Thermal Stability of Hepatitis E Virus. J. Infect. Dis. 2005, 192, 930–933. [Google Scholar] [CrossRef]
- Emerson, S.U.; Nguyen, H.; Torian, U.; Purcell, R.H. ORF3 Protein of Hepatitis E Virus Is Not Required for Replication, Virion Assembly, or Infection of Hepatoma Cells In Vitro. J. Virol. 2006, 80, 10457–10464. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Nakazono, N.; Ishii, K.; Li, D.; Kawamata, O.; Kawaguchi, R.; Tsukada, Y.I. Hepatitis E Virus (87A Strain) Propagated in A549 Cells. J. Med. Virol. 1995, 47, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Trojnar, E.; Filter, M.; Hofmann, J. Thermal Stability of Hepatitis E Virus as Estimated by a Cell Culture Method. Appl. Environ. Microbiol. 2016, 82, 4225–4231. [Google Scholar] [CrossRef]
- Drave, S.A.; Debing, Y.; Walter, S.; Todt, D.; Engelmann, M.; Friesland, M.; Wedemeyer, H.; Neyts, J.; Behrendt, P.; Steinmann, E. Extra-Hepatic Replication and Infection of Hepatitis E Virus in Neuronal-Derived Cells. J. Viral Hepat. 2016, 23, 512–521. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, F.; Xu, L.; Lin, Z.; De Vrij, F.M.S.; Ayo-Martin, A.C.; Van Der Kroeg, M.; Zhao, M.; Yin, Y.; Wang, W.; et al. Hepatitis E Virus Infects Neurons and Brains. J. Infect. Dis. 2017, 215, 1197–1206. [Google Scholar] [CrossRef]
- Knegendorf, L.; Drave, S.A.; Dao Thi, V.L.; Debing, Y.; Brown, R.J.P.; Vondran, F.W.R.; Resner, K.; Friesland, M.; Khera, T.; Engelmann, M.; et al. Hepatitis E Virus Replication and Interferon Responses in Human Placental Cells. Hepatol. Commun. 2018, 2, 173–187. [Google Scholar] [CrossRef]
- Tam, A.W.; White, R.; Reed, E.; Short, M.; Zhang, Y.; Fuerst, T.R.; Lanford, R.E. In VitroPropagation and Production of Hepatitis E Virus Fromin Vivo-Infected Primary Macaque Hepatocytes. Virology 1996, 215, 1–9. [Google Scholar] [CrossRef]
- Tam, A.W.; White, R.; Yarbough, P.O.; Murphy, B.J.; McAtee, C.P.; Lanford, R.E.; Fuerst, T.R. In VitroInfection and Replication of Hepatitis E Virus in Primary Cynomolgus Macaque Hepatocytes. Virology 1997, 238, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L.; Wang, Y.; Wang, W.; Sprengers, D.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Requirement of the Eukaryotic Translation Initiation Factor 4F Complex in Hepatitis E Virus Replication. Antivir. Res. 2015, 124, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, X.; Ambardekar, C.; Hu, Z.; Lhomme, S.; Feng, Z. Hepatitis E Virus Persists in the Presence of a Type III Interferon Response. PLoS Pathog. 2017, 13, e1006417. [Google Scholar] [CrossRef] [PubMed]
- Gouilly, J.; Chen, Q.; Siewiera, J.; Cartron, G.; Levy, C.; Dubois, M.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N.; El Costa, H. Genotype Specific Pathogenicity of Hepatitis E Virus at the Human Maternal-Fetal Interface. Nature Commun. 2018, 9, 4748. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Wu, X.; Rice, C.M. Stem Cell–Derived Culture Models of Hepatitis E Virus Infection. Cold Spring Harb. Perspect. Med. 2019, 9, a031799. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E Virus: Advances and Challenges. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 96. [Google Scholar] [CrossRef]
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized Hepatitis E Virus (HEV) Culture and Its Application to Measurements of HEV Infectivity. Viruses 2020, 12, 139. [Google Scholar] [CrossRef]
- Chew, N.; Situ, J.; Wu, S.; Yao, W.; Sridhar, S. Independent Evaluation of Cell Culture Systems for Hepatitis E Virus. Viruses 2022, 14, 1254. [Google Scholar] [CrossRef]
- Locus, T.; Lambrecht, E.; Lamoral, S.; Willems, S.; Van Gucht, S.; Vanwolleghem, T.; Peeters, M. A Multifaceted Approach for Evaluating Hepatitis E Virus Infectivity In Vitro: Cell Culture and Innovative Molecular Methods for Integrity Assessment. Vet. Sci. 2023, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Van der Poel, W.H.M.; Hakze-van der Honing, R.; Martelli, F.; La Ragione, R.M.; Inglese, N.; Collins, J.; Grierson, S.; Johne, R.; Reetz, J.; et al. Replication of Hepatitis E Virus in Three-Dimensional Cell Culture. J. Virol. Methods 2013, 187, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Schilling-Loeffler, K.; Viera-Segura, O.; Corman, V.M.; Schneider, J.; Gadicherla, A.K.; Schotte, U.; Johne, R. Cell Culture Isolation and Whole Genome Characterization of Hepatitis E Virus Strains from Wild Boars in Germany. Microorganisms 2021, 9, 2302. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y. HEV Cell Culture. Adv. Exp. Med. Biol. 2023, 1417, 119–131. [Google Scholar] [CrossRef]
- Semaan, D.; Scobie, L. Feasibility Study for in Vitro Analysis of Infectious Foodborne HEV. 2022, completed. [CrossRef]
- Liu, T.; He, Q.; Yang, X.; Li, Y.; Yuan, D.; Lu, Q.; Tang, T.; Guan, G.; Zheng, L.; Zhang, H.; et al. An Immunocompetent Mongolian Gerbil Model for Hepatitis E Virus Genotype 1 Infection. Gastroenterology 2024, 167, 750–763.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sakai, Y.; Ami, Y.; Suzaki, Y.; Isogawa, M. Strain- and Subtype-Specific Replication of Genotype 3 Hepatitis E Viruses in Mongolian Gerbils. Viruses 2024, 16, 1605. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Yin, X.; Lu, F.; Zhao, H.; Wang, L.; Qin, C.-F. Establishment of Enterically Transmitted Hepatitis Virus Animal Models Using Lipid Nanoparticle-Based Full-Length Viral Genome RNA Delivery System. Gut 2025, 74, 467–476. [Google Scholar] [CrossRef]
- Xu, L.-D.; Zhang, F.; Chen, C.; Peng, L.; Luo, W.-T.; Chen, R.; Xu, P.; Huang, Y.-W. Revisiting the Mongolian Gerbil Model for Hepatitis E Virus by Reverse Genetics. Microbiol. Spectr. 2022, 10, 2. [Google Scholar] [CrossRef]
- Zhang, W.; Ami, Y.; Suzaki, Y.; Doan, Y.H.; Muramatsu, M.; Li, T.-C. Mongolia Gerbils Are Broadly Susceptible to Hepatitis E Virus. Viruses 2022, 14, 1125. [Google Scholar] [CrossRef]
- Lo Castro, I.; Espul, C.; de Paula, V.S.; Altabert, N.R.; Gonzalez, J.E.; Lago, B.V.; Villar, L.M. High Prevalence of Hepatitis A and E Viruses in Environmental and Clinical Samples from West Argentina. Braz. J. Infect. Dis. 2023, 27, 102738. [Google Scholar] [CrossRef]
- Pisano, M.B.; Lugo, B.C.; Poma, R.; Cristóbal, H.A.; Raskovsky, V.; Wassaf, M.G.M.; Rajal, V.B.; Ré, V.E. Environmental Hepatitis E Virus Detection Supported by Serological Evidence in the Northwest of Argentina. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Baez, P.A.; Lopez, M.C.; Duque-Jaramillo, A.; Pelaez, D.; Molina, F.; Navas, M.C. First Evidence of the Hepatitis E Virus in Environmental Waters in Colombia. PLoS ONE 2017, 12, 177525. [Google Scholar] [CrossRef]
- Heldt, F.H.; Staggmeier, R.; Gularte, J.S.; Demoliner, M.; Henzel, A.; Spilki, F.R. Hepatitis E Virus in Surface Water, Sediments, and Pork Products Marketed in Southern Brazil. Food Environ. Virol. 2016, 8, 200–205. [Google Scholar] [CrossRef]
- Iaconelli, M.; Ferraro, G.B.; Mancini, P.; Suffredini, E.; Veneri, C.; Ciccaglione, A.R.; Bruni, R.; Della Libera, S.; Bignami, F.; Brambilla, M.; et al. Nine-Year Nationwide Environmental Surveillance of Hepatitis E Virus in Urban Wastewaters in Italy (2011–2019). Int. J. Environ. Res. Public Health 2020, 17, 2059. [Google Scholar] [CrossRef] [PubMed]
- Rau, F.; Elsner, C.; Meister, T.L.; Gömer, A.; Kallies, R.; Dittmer, U.; Steinmann, E.; Todt, D. Monitoring of Hepatitis E Virus in Wastewater Can Identify Clinically Relevant Variants. Liver Int. 2024, 44, 637–643. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; García-Bocanegra, I.; Cano-Terriza, D.; Beato-Benítez, A.; Ulrich, R.G.; Martínez, J.; Guerra, R.; Martínez-Valverde, R.; Martínez-Nevado, E.; Ángel Quevedo-Muñoz, M.; et al. Monitoring of Hepatitis E Virus in Zoo Animals from Spain, 2007–2021. Transbound. Emerg. Dis. 2022, 69, 3992–4001. [Google Scholar] [CrossRef] [PubMed]
- Palombieri, A.; Robetto, S.; Di Profio, F.; Sarchese, V.; Fruci, P.; Bona, M.C.; Ru, G.; Orusa, R.; Marsilio, F.; Martella, V.; et al. Surveillance Study of Hepatitis E Virus (HEV) in Domestic and Wild Ruminants in Northwestern Italy. Animals 2020, 10, 2351. [Google Scholar] [CrossRef]
- Yoon, J.; Park, T.; Sohn, Y.; Lee, S.K.; Park, B.J.; Ahn, H.S.; Go, H.J.; Kim, D.H.; Lee, J.B.; Park, S.Y.; et al. Surveillance of Hepatitis E Virus in the Horse Population of Korea: A Serological and Molecular Approach. Infect. Genet. Evol. 2022, 103, 105317. [Google Scholar] [CrossRef]
- Bleotu, C.; Matei, L.; Dragu, L.D.; Necula, L.G.; Pitica, I.M.; Chivu-Economescu, M.; Diaconu, C.C. Viruses in Wastewater—A Concern for Public Health and the Environment. Microorganisms 2024, 12, 1430. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E Virus Infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Chand, M.A.; Kafatos, G.; Tedder, R.; Morgan, D. Hepatitis E Virus in England and Wales: Indigenous Infection Is Associated with the Consumption of Processed Pork Products. Epidemiol. Infect. 2014, 142, 1467–1475. [Google Scholar] [CrossRef]
- Aspinall, E.J.; Couturier, E.; Faber, M.; Said, B.; Ijaz, S.; Tavoschi, L.; Takkinen, J.; Adlhoch, C. Hepatitis E Virus Infection in Europe: Surveillance and Descriptive Epidemiology of Confirmed Cases, 2005 to 2015. Eurosurveillance 2017, 22, 30561. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Avellon, A.; Baylis, S.A.; Ciccaglione, A.R.; Couturier, E.; de Sousa, R.; Epštein, J.; Ethelberg, S.; Faber, M.; Fehér, Á.; et al. Hepatitis E Virus: Assessment of the Epidemiological Situation in Humans in Europe, 2014/15. J. Clin. Virol. 2016, 82, 9–16. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Action Plan for the Health Sector Response to Viral Hepatitis in the WHO European Region; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2017; pp. 1–45. [Google Scholar]
- Zhao, M.Y.; Li, D. Optimization and Implementation of the Virus Extraction Method for Hepatitis E Virus Detection from Raw Pork Liver. Food Environ. Virol. 2021, 13, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Majdalawieh, A.F.; Mesleh, A.G.; Abdalla, O.M.; Nasrallah, G.K. Laboratory Challenges in the Diagnosis of Hepatitis E Virus. J. Med. Microbiol. 2018, 67, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Jayatilleke, K. Challenges in Implementing Surveillance Tools of High-Income Countries (HICs) in Low Middle Income Countries (LMICs). Curr. Treat. Options Infect. Dis. 2020, 12, 191. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef]
- WHO. Hepatitis E Vaccines Background Paper. In Proceedings of the Strategic Advisory Group of Experts (SAGE) on Immunization Meeting, 23–26 September 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Kamani, L.; Padhani, Z.A.; Das, J.K. Hepatitis E: Genotypes, Strategies to Prevent and Manage, and the Existing Knowledge Gaps. JGH Open 2021, 5, 1127. [Google Scholar] [CrossRef]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The Global Burden of Hepatitis E Virus Genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).