Transcriptomic and Immunopathological Profiles of Inflammasomes in Different Clinical Forms of American Cutaneous Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Histopathology
2.3. Immunohistochemistry
2.4. Quantitative Analysis of Immunostained Cells
2.5. Transcriptome
3. Results
3.1. Histopathological Analysis
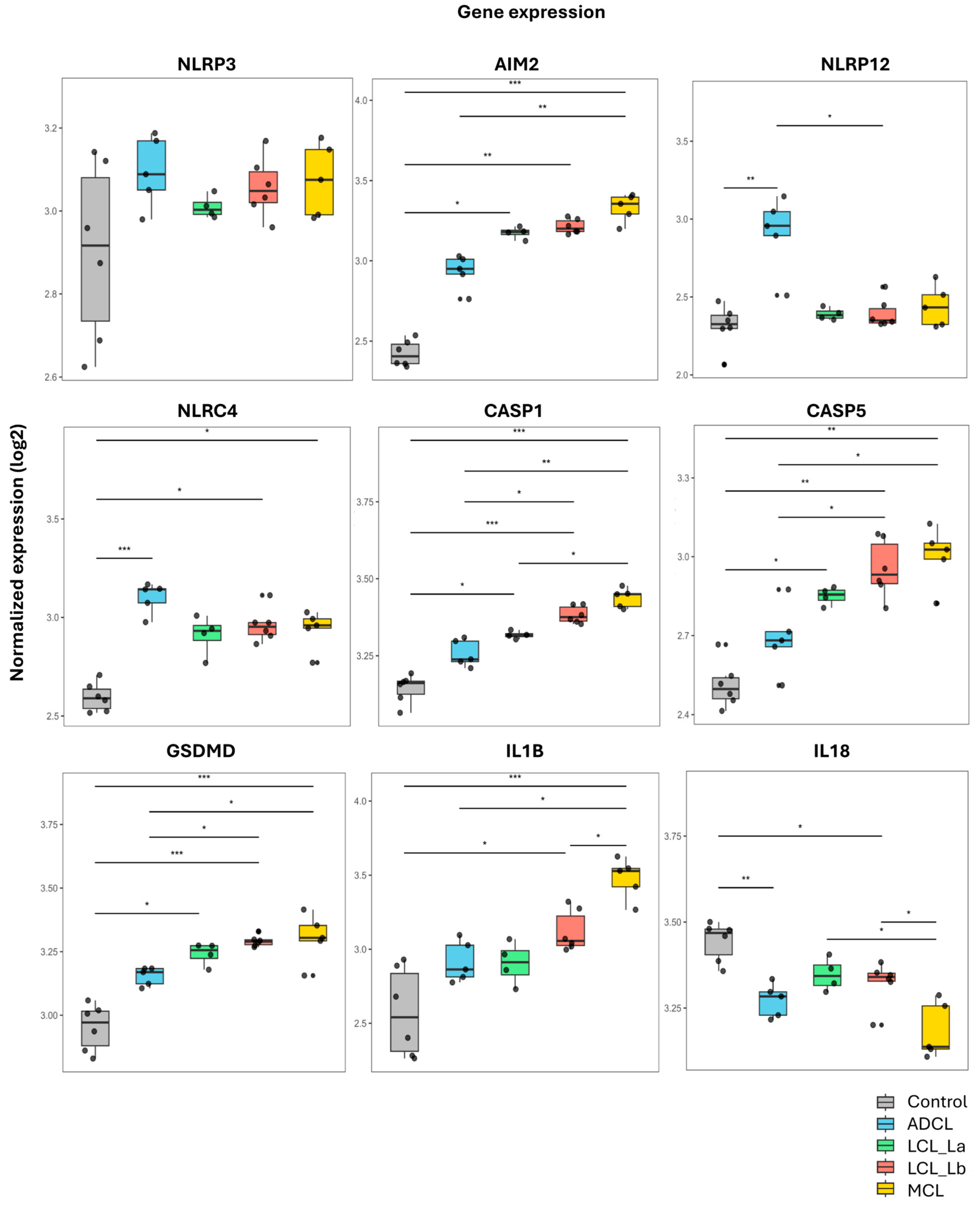
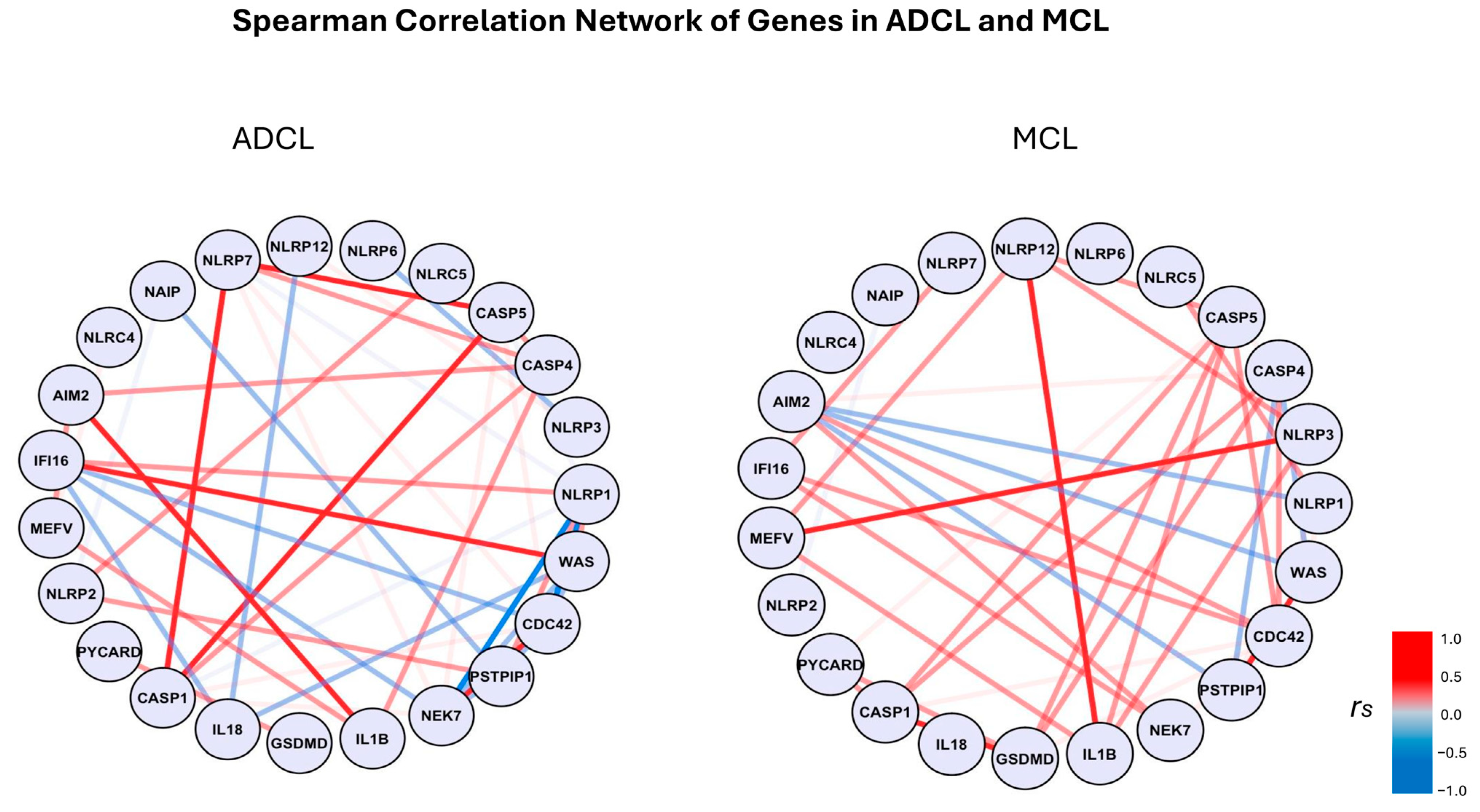
3.2. Transcriptome Analysis
3.3. Immunohistochemistry Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maslov, D.A.; Votýpka, J.; Yurchenko, V.; Lukeš, J. Diversity and phylogeny of insect trypanosomatids: All that is hidden shall be revealed. Trends Parasitol. 2013, 29, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Spinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 2018, 145, 430–442. [Google Scholar] [CrossRef]
- Silveira, F.T. What makes mucosal and anergic diffuse cutaneous leishmaniases so clinically and immunopathogically different? A review in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Lainson, R.; Corbett, C.E. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: A review. Mem. Inst. Oswaldo Cruz 2004, 99, 239–251. [Google Scholar] [CrossRef]
- Silveira, F.T.; Müller, S.R.; de Souza, A.A.; Lainson, R.; Gomes, C.; Laurenti, M.D.; Corbett, C.E. Revisão sobre a patogenia da Leishmaniose Tegumentar Americana na Amazônia, com ênfase à doença causada por Leishmania (V.) braziliensis e Leishmania (L.) amazonensis. Rev. Para. Med. 2008, 22, 9–20. [Google Scholar]
- Zamboni, D.S.; Lima-Junior, D.S. Inflammasomes in host response to protozoan parasites. Immunol. Rev. 2015, 265, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Lima Júnior, D.D.S.; Zamboni, D.S. NLRP3 Inflamassoma: Uma Plataforma Molecular Importante no Contrle da Infecção por Leishmania spp.; University of Sao Paulo: Sao Paulo, Brazil, 2013. [Google Scholar]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef]
- Bryson, K.J.; Wei, X.-Q.; Alexander, J. Interleukin-18 enhances a Th2 biased response and susceptibility to Leishmania mexicana in BALB/c mice. Microbes Infect. 2008, 10, 834–839. [Google Scholar] [CrossRef]
- Sousa, L.M.; Carneiro, M.B.; dos Santos, L.M.; Natale, C.C.; Resende, M.E.; Mosser, D.M.; Vieira, L.Q. IL-18 contributes to susceptibility to Leishmania amazonensis infection by macrophage-independent mechanisms. Cytokine 2015, 74, 327–330. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- de Sá, K.S.; Amaral, L.A.; Rodrigues, T.S.; Ishimoto, A.Y.; de Andrade, W.A.; de Almeida, L.; Freitas-Castro, F.; Batah, S.S.; Oliveira, S.C.; Pastorello, M.T.; et al. Gasdermin-D activation promotes NLRP3 activation and host resistance to Leishmania infection. Nat. Commun. 2023, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Harrington, V.; Gurung, P. Reconciling protective and pathogenic roles of the NLRP3 inflammasome in leishmaniasis. Immunol. Rev. 2020, 297, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Karki, R.; Vogel, P.; Watanabe, M.; Bix, M.; Lamkanfi, M.; Kanneganti, T.-D. An NLRP3 inflammasome–triggered Th2-biased adaptive immune response promotes leishmaniasis. J. Clin. Investig. 2015, 125, 1329–1338. [Google Scholar] [CrossRef]
- Tuladhar, S.; Kanneganti, T.-D. NLRP12 in innate immunity and inflammation. Mol. Asp. Med. 2020, 76, 100887. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.O.; Ketelut-Carneiro, N.; Milanezi, C.M.; Faccioli, L.H.; Gardinassi, L.G.; Silva, J.S. NLRC4 inhibits NLRP3 inflammasome and abrogates effective antifungal CD8+ T cell responses. iScience 2021, 24, 102548. [Google Scholar] [CrossRef]
- Valadares, D.G.; Clay, O.S.; Chen, Y.; Scorza, B.M.; Cassel, S.L.; Sutterwala, F.S.; Wilson, M.E. NLRP12-expressing dendritic cells mediate both dissemination of infection and adaptive immune responses in visceral leishmaniasis. iScience 2023, 26, 106163. [Google Scholar] [CrossRef]
- Moreira, R.B.; Pirmez, C.; de Oliveira-Neto, M.P.; Aguiar, L.S.; Gonçalves, A.J.S.; Pereira, L.O.R.; Abreu, L.; De Oliveira, M.P. AIM2 inflammasome is associated with disease severity in tegumentary leishmaniasis caused by Leishmania (V.) braziliensis. Parasite Immunol. 2017, 39, e12435. [Google Scholar] [CrossRef]
- Ridley, D.S.; Ridley, M.J. The evolution of the lesion in cutaneous leishmaniasis. J. Pathol. 1983, 141, 83–96. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 5 April 2025).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Fantecelle, C.H.; Covre, L.P.; Lopes, P.O.; Sarmento, I.V.; Decote-Ricardo, D.; Freire-De-Lima, C.G.; Guedes, H.L.d.M.; Pimentel, M.I.F.; Conceição-Silva, F.; Maretti-Mira, A.C.; et al. Senescence-related genes are associated with the immunopathology signature of American tegumentary leishmaniasis lesions and may predict progression to mucosal leishmaniasis. Clin. Exp. Immunol. 2024, 219, uxae088. [Google Scholar] [CrossRef]
- de Carvalho, R.V.H.; Zamboni, D.S. Inflammasome activation in response to intracellular protozoan parasites. Trends Parasitol. 2020, 36, 459–472. [Google Scholar] [CrossRef]
- Zhao, C.; Gu, Y.; Zeng, X.; Wang, J. NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis. Clin. Immunol. 2018, 197, 154–160. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur. J. Immunol. 2016, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Guo, Z.; Guo, H.; Chen, L.; Zhang, G.; Liang, K.; Xie, L.; Tan, X.; Gibson, S.A.; Rampanelli, E.; et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature 2021, 591, 300–305. [Google Scholar] [CrossRef]
- Hiruma, J.; Harada, K.; Motoyama, A.; Okubo, Y.; Maeda, T.; Yamamoto, M.; Miyai, M.; Hibino, T.; Tsuboi, R. Key component of inflammasome, NLRC 4, was identified in the lesional epidermis of psoriatic patients. J. Dermatol. 2018, 45, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Kopfnagel, V.; Wittmann, M.; Werfel, T. Human keratinocytes express AIM2 and respond to dsDNA with IL-1β secretion. Exp. Dermatol. 2011, 20, 1027–1029. [Google Scholar] [CrossRef]
- Martins, T.V.; de Melo, B.M.S.; Toller-Kawahisa, J.E.; da Silva, G.V.L.; Silva, C.E.A.; Paiva, I.M.; Públio, G.A.; Rosa, M.H.; Souza, C.d.S.; Zamboni, D.S.; et al. The DNA sensor AIM2 mediates psoriasiform inflammation by inducing type 3 immunity. JCI Insight 2024, 9, e171894. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Chen, Y.; Chen, S.; Zhang, Y.; Chen, H.; Yang, Y.; Gu, H.; Chen, Q.; Li, M.; Chen, X. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023, 14, 595. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, T.; Li, X.; Luo, P.; Zhang, B. GSDMD suppresses keratinocyte differentiation by inhibiting FLG expression and attenuating KCTD6-mediated HDAC1 degradation in atopic dermatitis. PeerJ 2024, 12, e16768. [Google Scholar] [CrossRef]
- Perri, A. The NLRP3-Inflammasome in Health and Disease. Int. J. Mol. Sci. 2022, 23, 13103. [Google Scholar] [CrossRef]
- Novais, F.O.; Carvalho, A.M.; Clark, M.L.; Carvalho, L.P.; Beiting, D.P.; Brodsky, I.E.; Carvalho, E.M.; Scott, P. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog. 2017, 13, e1006196. [Google Scholar] [CrossRef]
- Lugrin, J.; Martinon, F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol. Rev. 2018, 281, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.F.; Alcântara, L.S.; Barros, J.P.B.; de Lima, A.C.S.; Campos, M.B.; Moraes, C.; Ferreira, A.F.; Matta, V.L.R.; Laurenti, M.D.; Corbett, C.E.P.; et al. In situ expression of Th17 immunologic mediators in American cutaneous leishmaniasis caused by Leishmania (V.) braziliensis and Leishmania (L.) amazonensis in the Brazilian Amazon. Front. Trop. Dis. 2023, 4, 1067595. [Google Scholar] [CrossRef]
- Cai, S.; Batra, S.; Del Piero, F.; Jeyaseelan, S. NLRP12 modulates host defense through IL-17A–CXCL1 axis. Mucosal Immunol. 2016, 9, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Papale, A.; Kummer, E.; Galbiati, V.; Marinovich, M.; Galli, C.L.; Corsini, E. Understanding chemical allergen potency: Role of NLRP12 and Blimp-1 in the induction of IL-18 in human keratinocytes. Arch. Toxicol. 2017, 91, 1783–1794. [Google Scholar] [CrossRef]
- Galbiati, V.; Cornaghi, L.; Papale, A.; Donetti, E.; Marinovich, M.; Corsini, E. Study on the inflammasome nlrp3 and blimp-1/nlrp12 after keratinocyte exposure to contact allergens. Toxicol. Lett. 2019, 313, 130–136. [Google Scholar] [CrossRef]
- Lukens, J.R.; Gurung, P.; Shaw, P.J.; Barr, M.J.; Zaki, H.; Brown, S.A.; Vogel, P.; Chi, H.; Kanneganti, T.-D. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity 2015, 42, 654–664. [Google Scholar] [CrossRef]
- Fernández-Figueroa, E.A.; Rangel-Escareño, C.; Espinosa-Mateos, V.; Carrillo-Sánchez, K.; Salaiza-Suazo, N.; Carrada-Figueroa, G.; March-Mifsut, S.; Becker, I. Disease Severity in Patients Infected with Leishmania mexicana Relates to IL-1β. PLoS Neglected Trop. Dis. 2012, 6, e1533. [Google Scholar] [CrossRef]
- Gonzalez, K.; Calzada, J.E.; Corbett, C.E.P.; Saldaña, A.; Laurenti, M.D. Involvement of the Inflammasome and Th17 Cells in Skin Lesions of Human Cutaneous Leishmaniasis Caused by Leishmania (Viannia) panamensis. Mediat. Inflamm. 2020, 2020, 9278931. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Schmauch, E.; Severin, Y.; Xing, X.; Mangold, A.; Conrad, C.; Johannsen, P.; Kahlenberg, J.M.; Mellett, M.; Navarini, A.; Nobbe, S.; et al. Targeting IL-1 controls refractory pityriasis rubra pilaris. Sci. Adv. 2024, 10, eado2365. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, J.; Wu, J.; Yang, X. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome in systemic lupus erythematosus. Biomed. Pharmacother. 2024, 172, 116261. [Google Scholar] [CrossRef] [PubMed]
- Fetter, T.; de Graaf, D.M.; Claus, I.; Wenzel, J. Aberrant inflammasome activation as a driving force of human autoimmune skin disease. Front. Immunol. 2023, 14, 1190388. [Google Scholar] [CrossRef] [PubMed]

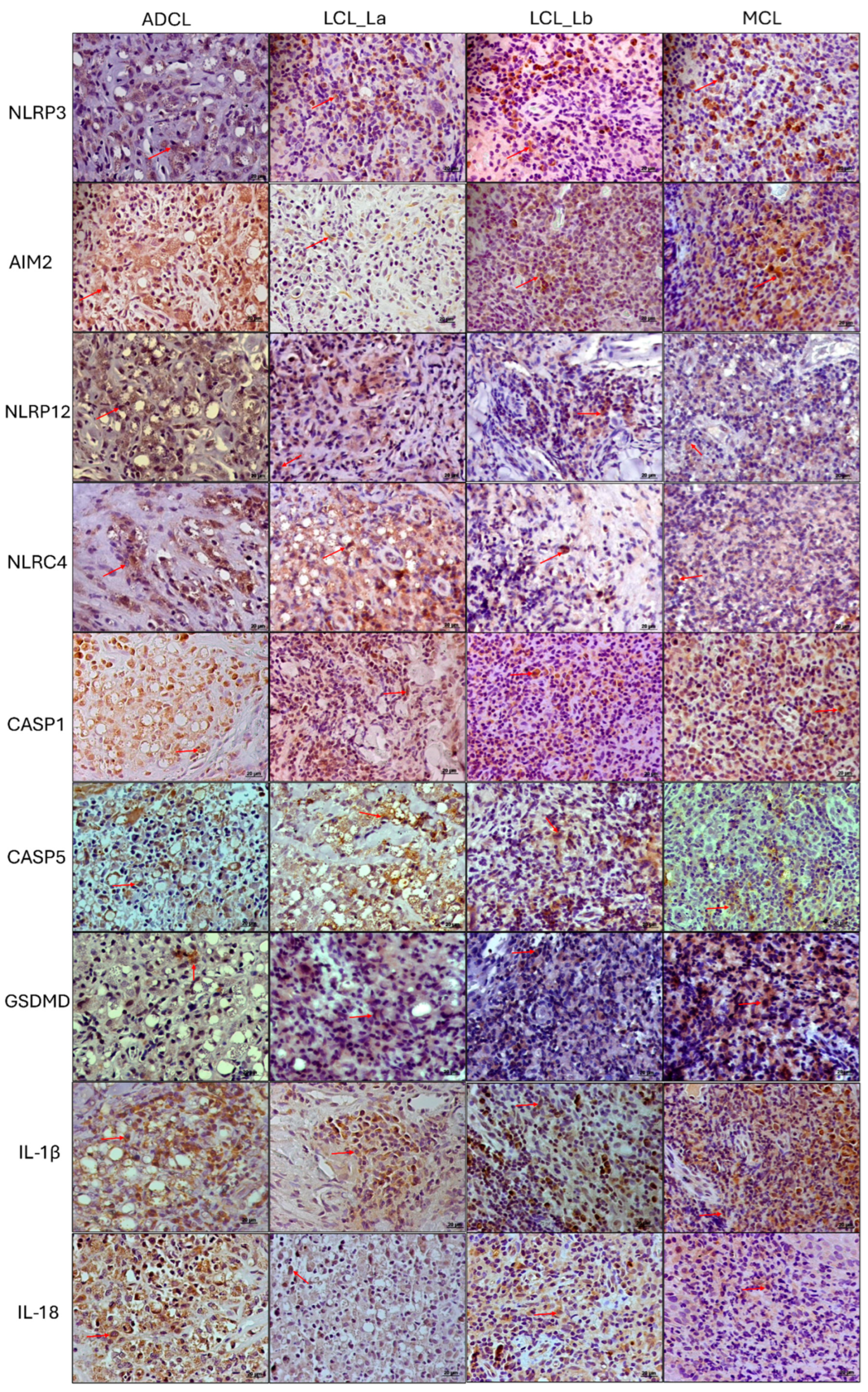

| Cell Density (Cells/mm2 ± Standard Error) | |||||
|---|---|---|---|---|---|
| Antibody (Marker) | Control | ADCL | LCL (La) | LCL (Lb) | MCL |
| NLRP3 | 13.56 ± 3.32 | 332.04 ± 29.90 | 427.13 ± 44.03 | 414.95 ± 28.22 | 636.91 ± 55.30 |
| AIM2 | 8.47 ± 3.10 | 941.21 ± 27.05 | 551.05 ± 33.93 | 467.56 ± 47.55 | 869.97 ± 64.49 |
| NLRC4 | 5.27 ± 1.11 | 827.94 ± 108.18 | 995.73 ± 221.50 | 541.94 ± 83.74 | 509.61 ± 30.17 |
| NLRP12 | 8.29 ± 2.20 | 945.57 ± 76.09 | 531.88 ± 148.16 | 525.15 ± 137.03 | 695.60 ± 86.49 |
| CASP1 | 6.03 ± 2.73 | 914.23 ± 53.76 | 448.16 ± 15.73 | 396.48 ± 33.85 | 728.57 ± 40.20 |
| CASP5 | 3.01 ± 3.49 | 689.12 ± 91.74 | 789.98 ± 35.11 | 526.65 ± 57.55 | 487.35 ± 108.99 |
| GSDMD | 10.61 ± 2.65 | 258.10 ± 90.10 | 440.73 ± 68.97 | 620.52 ± 108.68 | 447.27 ± 87.23 |
| IL1-β | 2.26 ± 1.11 | 1194.13 ± 71.32 | 587.19 ± 7.61 | 563.49 ± 31.39 | 1028.96 ± 76.73 |
| IL-18 | 5.27 ± 2.12 | 816.65 ± 80.10 | 455.57 ± 30.08 | 456.13 ± 58.10 | 310.93 ± 32.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcântara, L.d.S.; Campos, M.B.; Lima, A.C.S.; Pontillo, A.; Souza, K.B.d.S.; Ferreira, A.F.; Camargo, C.P.; Oba-Shinjo, S.M.; Laurenti, M.D.; Corbett, C.E.P.; et al. Transcriptomic and Immunopathological Profiles of Inflammasomes in Different Clinical Forms of American Cutaneous Leishmaniasis. Microorganisms 2025, 13, 980. https://doi.org/10.3390/microorganisms13050980
Alcântara LdS, Campos MB, Lima ACS, Pontillo A, Souza KBdS, Ferreira AF, Camargo CP, Oba-Shinjo SM, Laurenti MD, Corbett CEP, et al. Transcriptomic and Immunopathological Profiles of Inflammasomes in Different Clinical Forms of American Cutaneous Leishmaniasis. Microorganisms. 2025; 13(5):980. https://doi.org/10.3390/microorganisms13050980
Chicago/Turabian StyleAlcântara, Larissa dos Santos, Marliane Batista Campos, Ana Carolina Stocco Lima, Alessandra Pontillo, Kamilla Batista da Silva Souza, Aurea Favero Ferreira, Cristina Pires Camargo, Sueli Mieko Oba-Shinjo, Márcia Dalastra Laurenti, Carlos Eduardo Pereira Corbett, and et al. 2025. "Transcriptomic and Immunopathological Profiles of Inflammasomes in Different Clinical Forms of American Cutaneous Leishmaniasis" Microorganisms 13, no. 5: 980. https://doi.org/10.3390/microorganisms13050980
APA StyleAlcântara, L. d. S., Campos, M. B., Lima, A. C. S., Pontillo, A., Souza, K. B. d. S., Ferreira, A. F., Camargo, C. P., Oba-Shinjo, S. M., Laurenti, M. D., Corbett, C. E. P., da Matta, V. L. R., Nakaya, H., Silveira, F. T., & Gomes, C. M. d. C. (2025). Transcriptomic and Immunopathological Profiles of Inflammasomes in Different Clinical Forms of American Cutaneous Leishmaniasis. Microorganisms, 13(5), 980. https://doi.org/10.3390/microorganisms13050980







