Spatial Heterogeneity in Soil Microbial Communities Impacts Their Suitability as Bioindicators for Evaluating Productivity in Agricultural Practices
Abstract
1. Introduction
2. Materials and Methods
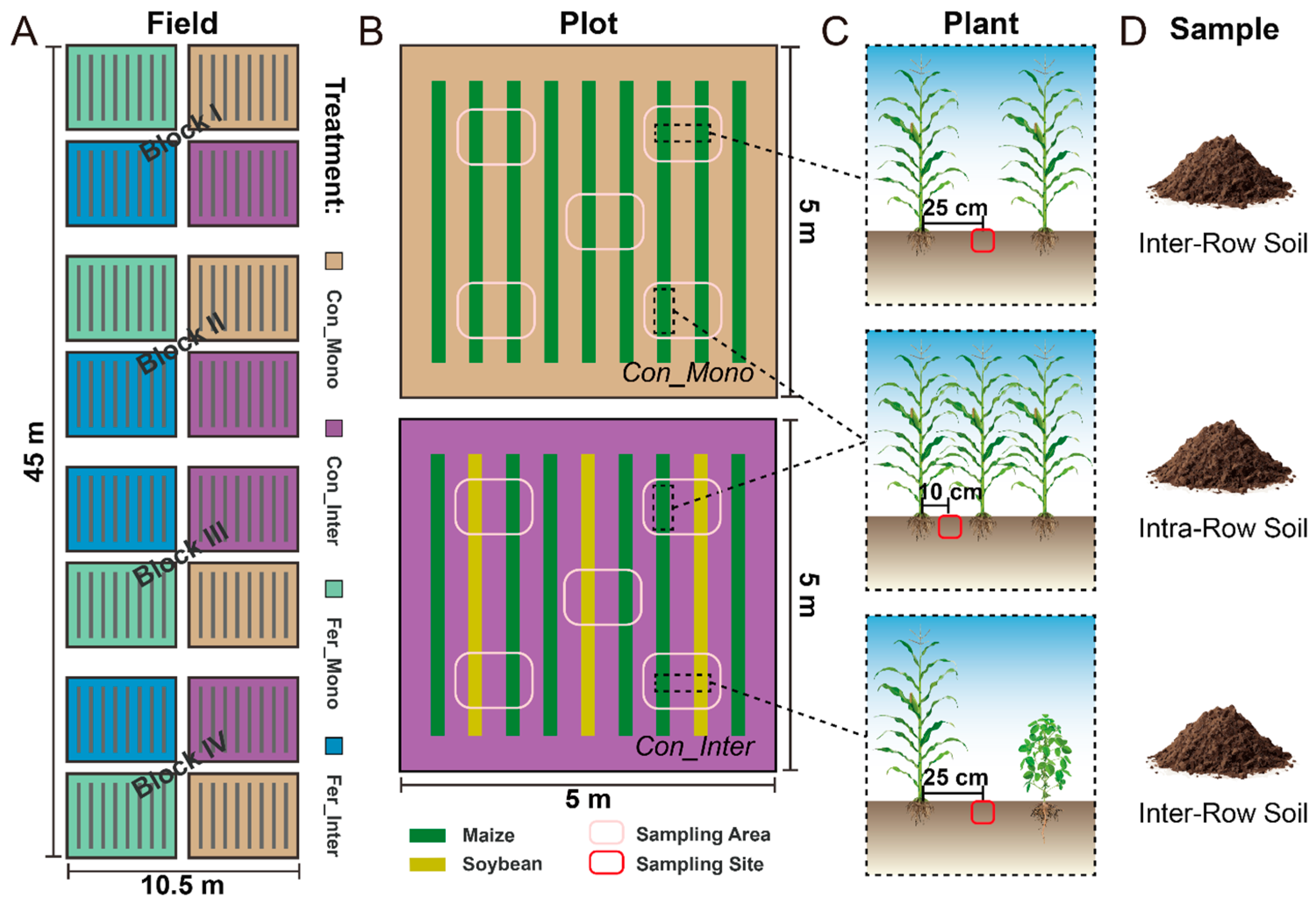
2.1. Site Description and Experimental Design
2.2. Fertilization and Management
2.3. Sample Collection and Processing
2.4. DNA Extraction, PCR, and High-Throughput Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
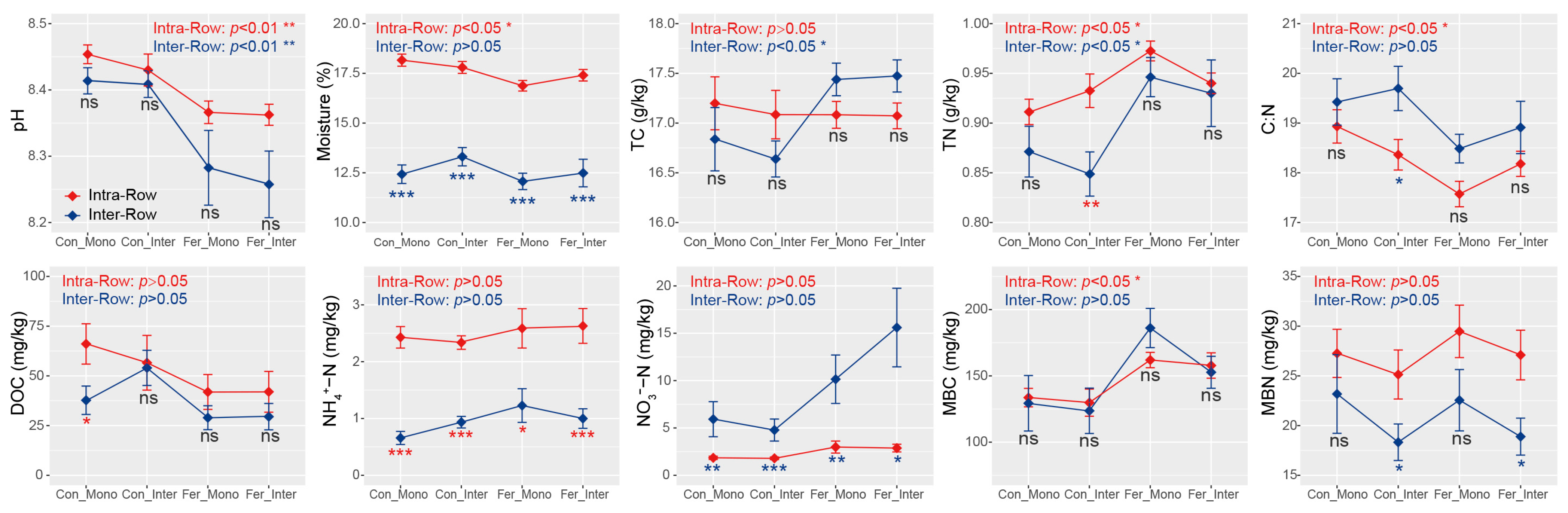
3.1. Maize Biomass and Soil Properties Across Treatments
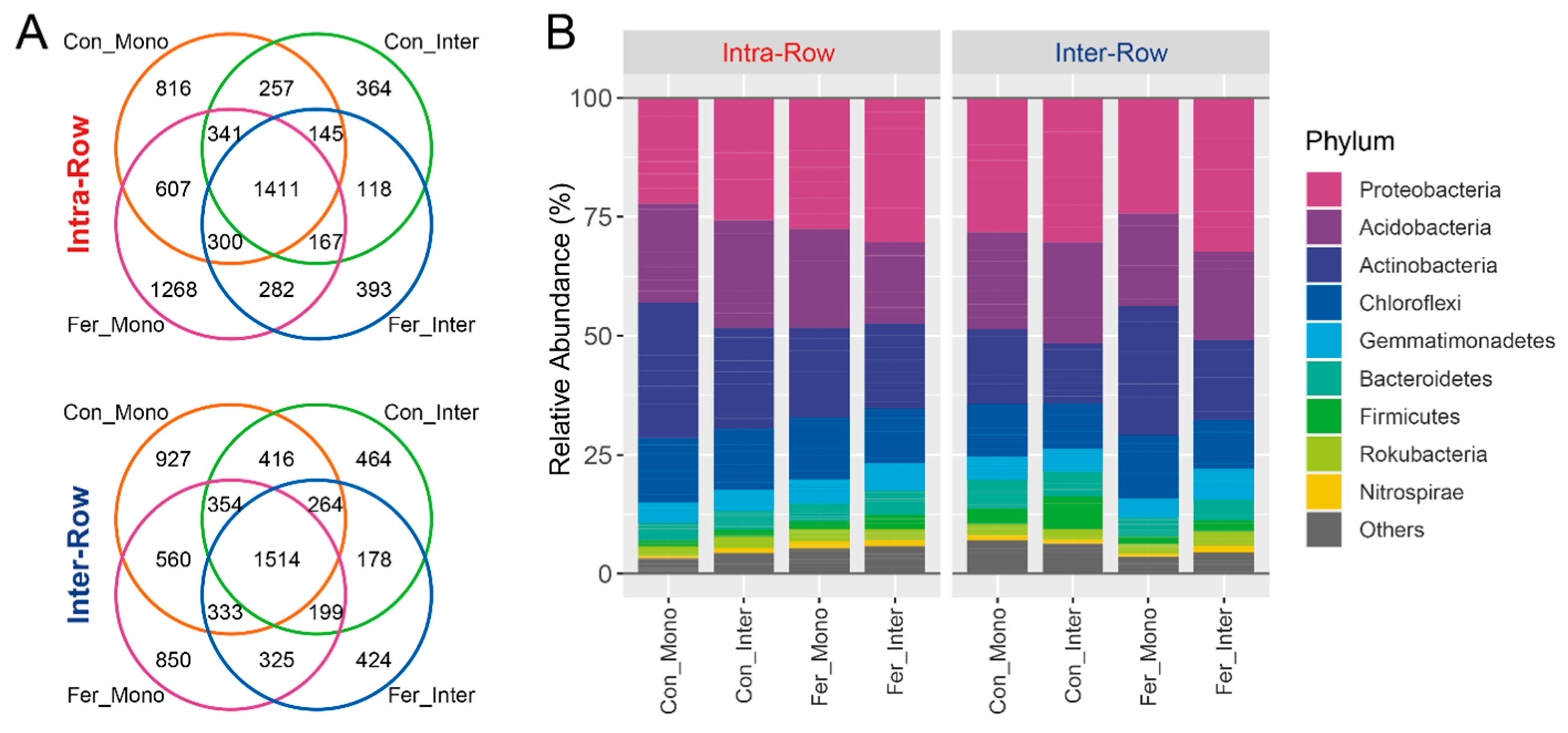
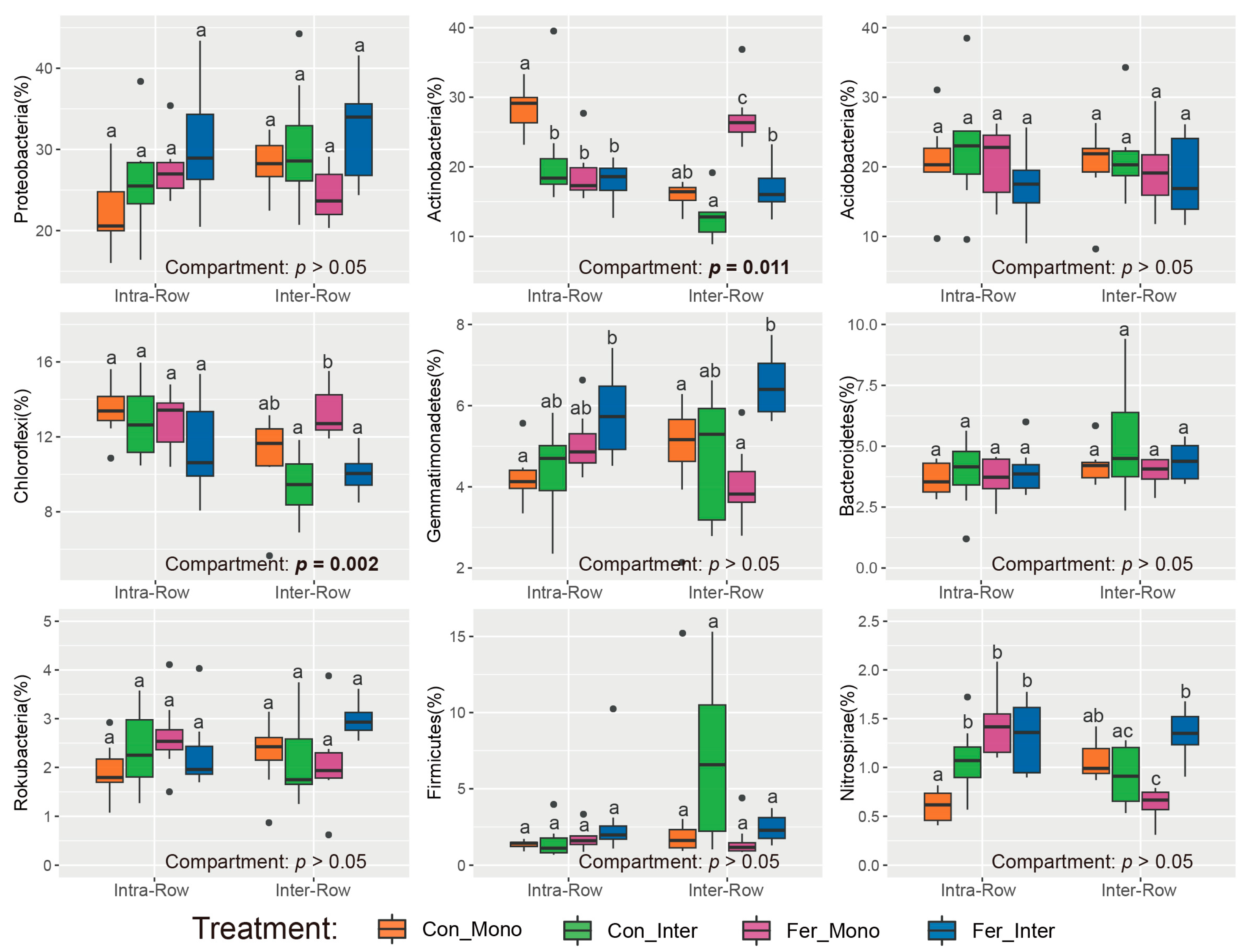
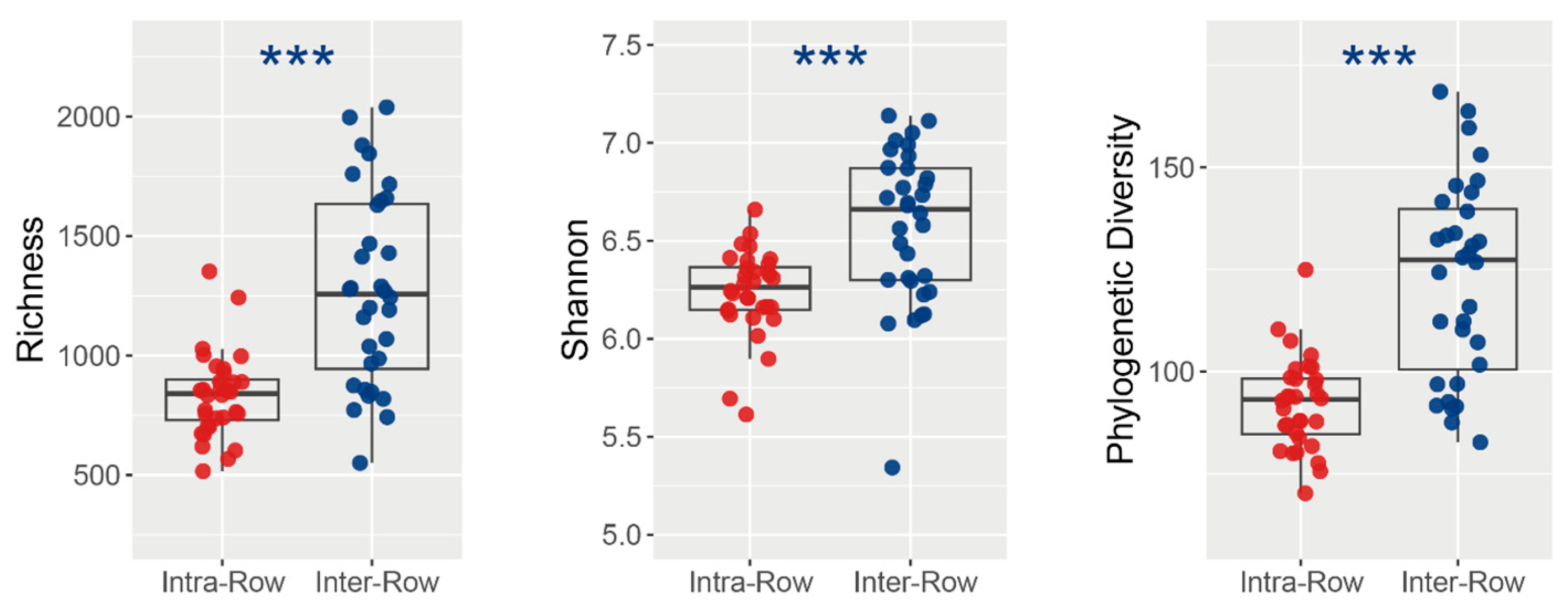
3.2. Composition and Alpha Diversity of Soil Bacterial Communities Across Treatments
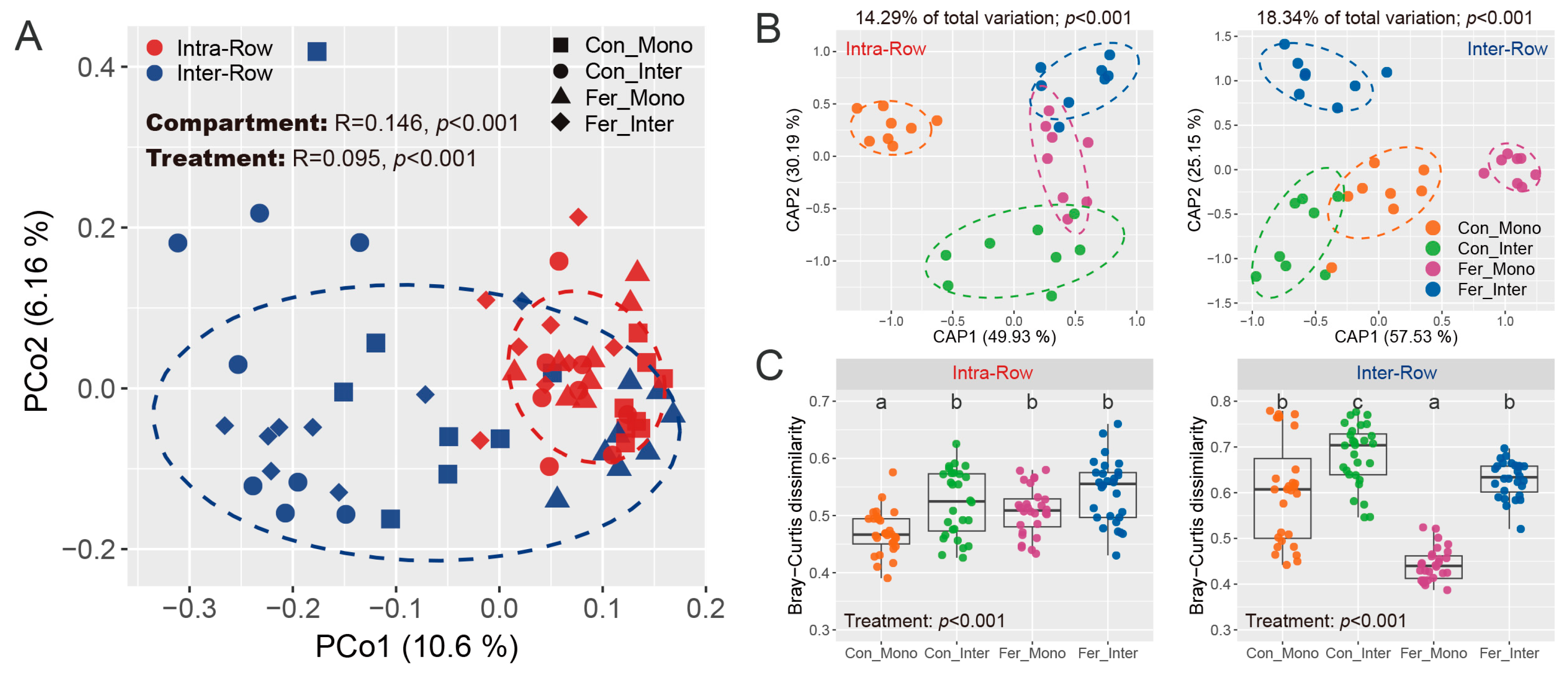
3.3. Bacterial Community Structure in Soils
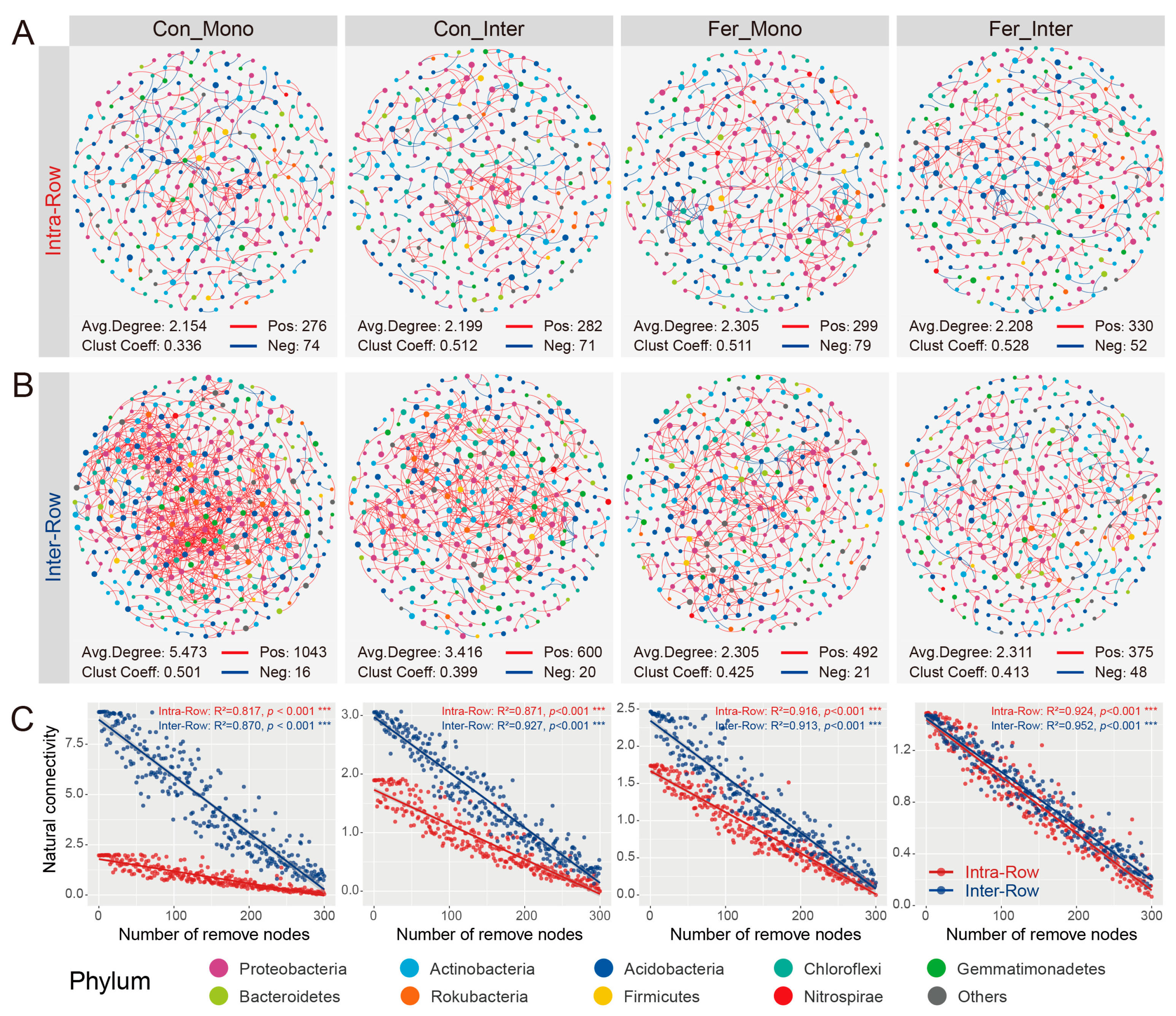
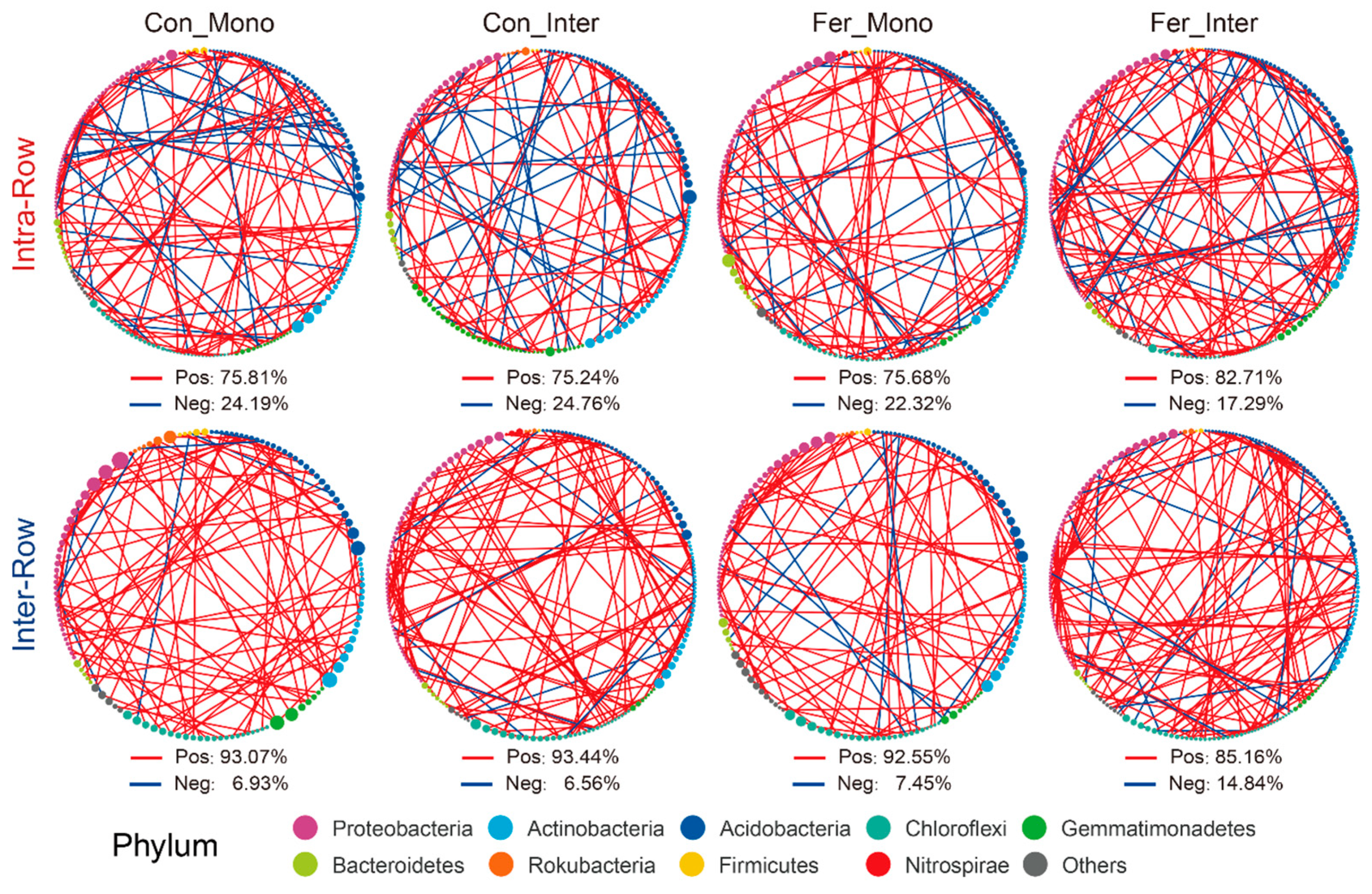
3.4. Co-Occurrence Patterns of Soil Bacterial Communities Across Treatments
3.5. Key Indicator Species and Their Relationships with Soil Properties and Maize Biomass Across Treatments
4. Discussion
4.1. Bacterial Communities Produce Significant Responses to Compartment Soils
4.2. Bacterial Communities Exhibit Significant Responses to Agricultural Practices in Different Soil Compartments
4.3. Co-Occurrence Networks Reflect the Effects of Agricultural Practices on Soil Bacteria Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellouze, W.; Taheri, A.E.; Bainard, L.D.; Yang, C.; Bazghaleh, N.; Navarro-Borrell, A.; Hanson, K.; Hamel, C. Soil Fungal Resources in Annual Cropping Systems and Their Potential for Management. Biomed. Res. Int. 2014, 2014, 531824. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, M.M.; Feng, H.J.; Wei, M.; Song, F.P.; Lou, Y.H.; Cui, X.M.; Wang, H.; Zhuge, Y.P. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Till. Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Mukhtar, H.; Wunderlich, R.F.; Muzaffar, A.; Ansari, A.; Shipin, O.; Cao, T.N.D.; Lin, Y.P. Soil microbiome feedback to climate change and options for mitigation. Sci. Total Environ. 2023, 882, 163412. [Google Scholar] [CrossRef]
- Nicolas, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.E.; Hobbie, S.E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Wu, H.W.; Cui, H.L.; Fu, C.X.; Li, R.; Qi, F.Y.; Liu, Z.L.; Yang, G.; Xiao, K.Q.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Meng, S.S.; Peng, T.; Liu, X.B.; Wang, H.; Huang, T.W.; Gu, J.D.; Hu, Z. Ecological Role of Bacteria Involved in the Biogeochemical Cycles of Mangroves Based on Functional Genes Detected through GeoChip 5.0. Msphere 2022, 7, e00936-00921. [Google Scholar] [CrossRef]
- Teixeira, P.J.P.L.; Colaianni, N.R.; Fitzpatrick, C.R.; Dangl, J.L. Beyond pathogens: Microbiota interactions with the plant immune system. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef]
- Ji, L.; Yang, X.R.; Qi, F.F. Distinct Responses to Pathogenic and Symbionic Microorganisms: The Role of Plant Immunity. Int. J. Mol. Sci. 2022, 23, 10427. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Gao, Y.X.; Song, X.; Zheng, W.K.; Wu, L.; Chen, Q.; Yu, X.J.; Li, Z.L.; Li, R.C.; Gao, F.; Tian, H.Y.; et al. The controlled-release nitrogen fertilizer driving the symbiosis of microbial communities to improve wheat productivity and soil fertility. Field Crops Res. 2022, 289, 108712. [Google Scholar] [CrossRef]
- Chen, Q.X.; Song, Y.J.; An, Y.X.; Lu, Y.L.; Zhong, G.H. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liu, Q.S.; Li, Z.Y.; Cheng, W.; Sun, J.M.; Guo, Z.H.; Li, Y.M.; Zhou, J.Q.; Meng, D.L.; Li, H.B.; et al. Environmental factors shaping the diversity of bacterial communities that promote rice production. BMC Microbiol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Kumar, U.; Nayak, A.K.; Shahid, M.; Gupta, V.V.S.R.; Panneerselvam, P.; Mohanty, S.; Kaviraj, M.; Kumar, A.; Chatterjee, D.; Lal, B.; et al. Continuous application of inorganic and organic fertilizers over 47 years in paddy soil alters the bacterial community structure and its influence on rice production. Agric. Ecosyst. Environ. 2018, 262, 65–75. [Google Scholar] [CrossRef]
- Jia, J.Y.; de Goede, R.; Li, Y.Z.; Zhang, J.Z.; Wang, G.Z.; Zhang, J.L.; Creamer, R. Unlocking soil health: Are microbial functional genes effective indicators? Soil Biol. Biochem. 2025, 204, 109768. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.X.; Tian, L.; Zhang, Q. Conservation agriculture practices increase soil microbial biomass carbon and nitrogen in agricultural soils: A global meta-analysis. Soil Biol. Biochem. 2018, 121, 50–58. [Google Scholar] [CrossRef]
- Wang, Z.T.; Liu, L.; Chen, Q.; Wen, X.X.; Liao, Y.C. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef]
- Gil, S.V.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; March, G.J. Response of soil microbial communities to different management practices in surface soils of a soybean agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, D.; Liu, J.; Qiao, P.; Zhou, X.; Lu, H.; Wu, X.; Liu, D.; Jin, X.; Wu, F. Changes in rhizosphere soil microbial communities in a continuously monocropped cucumber (Cucumis sativus L.) system. Eur. J. Soil Biol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Pascual, J.; Blanco, S.; Ramos, J.L.; van Dillewijn, P. Responses of bulk and rhizosphere soil microbial communities to thermoclimatic changes in a Mediterranean ecosystem. Soil Biol. Biochem. 2018, 118, 130–144. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Till. Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef]
- Shen, H.J.; Wang, B.X.; Jiao, Y.; Zhang, X.; Zhang, Q.Q.; Xiong, Z.Q. Bacteria are more sensitive than fungi to soil fertility in an intensive vegetable field. Appl. Soil Ecol. 2023, 190, 105003. [Google Scholar] [CrossRef]
- Travis, S.E.; Simon, M.R.; Zogg, G.P. Environmental Sensitivity of Soil Microbial Communities is Altered in Association with Plant Roots in Saltmarsh Ecosystems. Northeast. Nat. 2024, 31, 146–162. [Google Scholar] [CrossRef]
- Kraft, E.; Alexandre, D.; de Oliveira, L.C.I.; Baretta, C.R.D.M.; Klauberg, O.; Baretta, D. Is there a relationship between enchytraeids diversity and community with soybean (Glycine max L.) productivity in no-till system in subtropical soils of Brazil? Ann. Appl. Biol. 2023, 183, 159–169. [Google Scholar] [CrossRef]
- Wang, Z.T.; Li, Y.Z.; Li, T.; Zhao, D.Q.; Liao, Y.C. Tillage practices with different soil disturbance shape the rhizosphere bacterial community throughout crop growth. Soil Till. Res. 2020, 197, 104501. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 00187. [Google Scholar] [CrossRef]
- Zhu, X.C.; Sun, L.Y.; Song, F.B.; Liu, S.Q.; Liu, F.L.; Li, X.N. Soil microbial community and activity are affected by integrated agricultural practices in China. Eur. J. Soil Sci. 2018, 69, 924–935. [Google Scholar] [CrossRef]
- Liang, H.; Song, Y.; Dai, Z.; Liu, H.Q.; Zhong, K.Y.; Feng, H.L.; Xu, L.C. Soil total nitrogen content and pH value estimation method considering spatial heterogeneity: Based on GNNW-XGBoost model. Spectrochim. Acta A 2025, 330, 125716. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Gibbons, S.M.; Owens, S.M.; Hampton-Marcell, J.; Johnston, E.R.; Jastrow, J.D.; Gilbert, J.A.; Meyer, F.; Antonopoulos, D.A. Spatial scale drives patterns in soil bacterial diversity. Environ. Microbiol. 2016, 18, 2039–2051. [Google Scholar] [CrossRef]
- Endress, M.G.; Dehghani, F.; Blagodatsky, S.; Reitz, T.; Schlüter, S.; Blagodatskaya, E. Spatial substrate heterogeneity limits microbial growth as revealed by the joint experimental quantification and modeling of carbon and heat fluxes. Soil Biol. Biochem. 2024, 197, 109509. [Google Scholar] [CrossRef]
- Uritskiy, G.; Munn, A.; Dailey, M.; Gelsinger, D.R.; Getsin, S.; Davila, A.; McCullough, P.R.; Taylor, J.; DiRuggiero, J. Environmental Factors Driving Spatial Heterogeneity in Desert Halophile Microbial Communities. Front. Microbiol. 2020, 11, 578669. [Google Scholar] [CrossRef]
- Tang, H.M.; Li, C.; Xiao, X.P.; Pan, X.C.; Tang, W.G.; Cheng, K.K.; Shi, L.H.; Li, W.Y.; Wen, L.; Wang, K. Functional diversity of rhizosphere soil microbial communities in response to different tillage and crop residue retention in a double-cropping rice field. PLoS ONE 2020, 15, e0233642. [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, T.; Zhao, D.Q.; Wang, Z.T.; Liao, Y.C. Different tillage practices change assembly, composition, and co-occurrence patterns of wheat rhizosphere diazotrophs. Sci. Total Environ. 2021, 767, 144252. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Molina, L.; Segura, A.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria. J. Plant Physiol. 2018, 228, 208–217. [Google Scholar] [CrossRef]
- Wu, Z.X.; Hao, Z.P.; Zeng, Y.; Guo, L.P.; Huang, L.Q.; Chen, B.D. Molecular characterization of microbial communities in the rhizosphere soils and roots of diseased and healthy. Anton Leeuw Int. J. G. 2015, 108, 1059–1074. [Google Scholar] [CrossRef]
- Song, Y.Q.; Liu, C.; Yang, K.M.; Sun, S.Q.; Wang, L.; Yuan, C.S.; Xu, Y.C.; Xiong, W.; Shen, Q.R.; Wei, Z. Predatory protist promotes disease suppression against bacterial wilt through enriching plant beneficial microbes at the early stage of plant growth. Plant Soil 2024. [Google Scholar] [CrossRef]
- Brisson, V.L.; Schmidt, J.E.; Northen, T.R.; Vogel, J.P.; Gaudin, A.C.M. Impacts of Maize Domestication and Breeding on Rhizosphere Microbial Community Recruitment from a Nutrient Depleted Agricultural Soil. Sci. Rep. 2019, 9, 15611. [Google Scholar] [CrossRef]
- Du, P.H.; Cao, Y.; Yin, B.Y.; Zhou, S.S.; Li, Z.Y.; Zhang, X.Y.; Xu, J.Z.; Liang, B.W. Improved tolerance of apple plants to drought stress and nitrogen utilization by modulating the rhizosphere microbiome melatonin and dopamine. Front. Microbiol. 2022, 13, 980327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Harnessing phosphate-solubilizing microorganisms for mitigation of nutritional and environmental stresses, and sustainable crop production. Planta 2025, 261, 95. [Google Scholar] [CrossRef]
- Byers, A.K.; Wakelin, S.A.; Condron, L.; Black, A. Land Use Change Disrupts the Network Complexity and Stability of Soil Microbial Carbon Cycling Genes Across an Agricultural Mosaic Landscape. Microb. Ecol. 2024, 87, 167. [Google Scholar] [CrossRef]
- Gao, H.; Song, Y.; Li, M.Y.; Gao, M.; Peng, Z.H.; Pan, H.B.; Qi, J.J.; Chen, S.; Liu, Y.; Wang, Y.; et al. Nutrient Availability Shapes the Resistance of Soil Bacterial Community and Functions to Disturbances in Desert Ecosystem. Environ. Microbiol. 2025, 27, e70081. [Google Scholar] [CrossRef]
- Xu, X.P.; Liu, X.Y.; He, P.; Johnston, A.M.; Zhao, S.C.; Qiu, S.J.; Zhou, W. Yield Gap, Indigenous Nutrient Supply and Nutrient Use Efficiency for Maize in China. PLoS ONE 2015, 10, e0140767. [Google Scholar] [CrossRef]
- Ma, Y.H.; Fu, S.L.; Zhang, X.P.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Chaoui, R.; Boudsocq, S.; Taschen, E.; Sentenac, H.; Farissi, M.; Lazali, M. Intercropping durum wheat and chickpea increases nutrient availability and use efficiency under low phosphorus soils. J. Plant Nutr. 2023, 46, 4125–4139. [Google Scholar] [CrossRef]
- García-Orenes, F.; Roldán, A.; Mataix-Solera, J.; Cerdà, A.; Campoy, M.; Arcenegui, V.; Caravaca, F. Soil structural stability and erosion rates influenced by agricultural management practices in a semi-arid Mediterranean agro-ecosystem. Soil Use Manag. 2012, 28, 571–579. [Google Scholar] [CrossRef]
- Charaslertrangsi, T.; Arunrat, N.; Sereenonchai, S.; Wongkamhang, P.R. Comparison of microbial diversity and metabolic activities in organic and conventional rice farms in Thailand. Microbiol. Spectr. 2024, 12, e03071-23. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.K.; Zhang, J.D.; Gao, H.N.; Zhao, Y.M.; Cheng, M.M.; Meng, X.Q.; An, L.Z.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, L.; Zhang, J.T.; Li, D.M.; Yu, J.; Gao, H.; Li, H.K.; Zhao, Z.Y. Soil phytoremediation reveals alteration in soil microbial metabolic activities along time gradient of cover crop mulching. Environ. Res. 2022, 209, 112884. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.B.; Qiu, W.W.; Sun, J.P.; Müller, C.; Lei, B.K. Effects of wheat/faba bean intercropping on soil nitrogen transformation processes. J. Soils Sediments 2019, 19, 1724–1734. [Google Scholar] [CrossRef]
- Kiani, M.; Hernandez-Ramirez, G.; Quideau, S.; Smith, E.; Janzen, H.; Larney, F.J.; Puurveen, D. Quantifying sensitive soil quality indicators across contrasting long-term land management systems: Crop rotations and nutrient regimes. Agric. Ecosyst. Environ. 2017, 248, 123–135. [Google Scholar] [CrossRef]
- Scaini, A.; Vico, G.; Thorslund, J.; Hugelius, G.; Manzoni, S. Water Availability and Land Management Control Catchment-Scale Agricultural Nitrogen and Phosphorous Use Efficiencies. Glob. Biogeochem. Cycles 2023, 37, e2022GB007487. [Google Scholar] [CrossRef]
- García-Orenes, F.; Morugán-Coronado, A.; Zornoza, R.; Cerdá, A.; Scow, K. Changes in Soil Microbial Community Structure Influenced by Agricultural Management Practices in a Mediterranean Agro-Ecosystem. PLoS ONE 2016, 11, e0152958. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wei, Y.W.; Du, H.T.; Zhu, W.X.; Zhou, Y.B.; Yin, Y. Effects of Intercropping between and Nitrogen Fixing Species on Soil Microbial Community Structure and Diversity. Forests 2022, 13, 1345. [Google Scholar] [CrossRef]
- Luan, H.A.; Gao, W.; Huang, S.W.; Tang, J.W.; Li, M.Y.; Zhang, H.Z.; Chen, X.P.; Masiliunas, D. Substitution of manure for chemical fertilizer affects soil microbial community diversity, structure and function in greenhouse vegetable production systems. PLoS ONE 2020, 15, e0214041. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.Q.; Ou, J.M.; Liu, F.D.; Cai, G.Y.; Tan, K.M.; Wang, X.L. Nitrogen substitution practice improves soil quality of red soil (Ultisols) in South China by affecting soil properties and microbial community composition. Soil Till. Res. 2024, 240, 106089. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden, M.G.A. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhong, Y.J.; Liao, H. Partnering crops with root-associated microbes for soil health and agricultural sustainability. Pedosphere 2024, 34, 26–29. [Google Scholar] [CrossRef]
- Yageta, Y.; Osbahr, H.; Morimoto, Y.; Clark, J. Comparing farmers’ qualitative evaluation of soil fertility with quantitative soil fertility indicators in Kitui County, Kenya. Geoderma 2019, 344, 153–163. [Google Scholar] [CrossRef]
- Duan, T.T.; Zhang, J.; Wang, Z. Responses and Indicators of Composition, Diversity, and Productivity of Plant Communities at Different Levels of Disturbance in a Wetland Ecosystem. Diversity 2021, 13, 252. [Google Scholar] [CrossRef]
- Wolejko, E.; Jablonska-Trypuc, A.; Wydro, U.; Butarewicz, A.; Lozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Anderson, T.H. Microbial eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 2022, 13, 6968. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Ju, W.Y.; Li, J.X.; Yu, W.R.; Zhang, R.C. iGraph: An incremental data processing system for dynamic graph. Front. Comput. Sci. 2016, 10, 462–476. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef]
- López-Carmona, D.A.; Alarcón, A.; Martínez-Romero, E.; Peña-Cabriales, J.J.; Larsen, J. Maize plant growth response to whole rhizosphere microbial communities in different mineral N and P fertilization scenarios. Rhizosphere 2019, 9, 38–46. [Google Scholar] [CrossRef]
- Yu, L.L.; Tang, Y.L.; Wang, Z.G.; Gou, Y.G.; Wang, J.W. Nitrogen-cycling genes and rhizosphere microbial community with reduced nitrogen application in maize/soybean strip intercropping. Nutr. Cycl. Agroecosyst. 2019, 113, 35–49. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.Y.; Lv, F.L.; Yin, Y.N.; Wang, Z.H.; Zhao, Z.Y.; Li, Z.Y.; Zhai, B.N. Fungal alpha diversity influences stochasticity of bacterial and fungal community assemblies in soil aggregates in an apple orchard. Appl. Soil Ecol. 2021, 162, 103878. [Google Scholar] [CrossRef]
- Zhao, X.C.; Tian, P.; Sun, Z.L.; Liu, S.E.; Wang, Q.K.; Zeng, Z.Q. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Cheng, W.X.; Johnson, D.W.; Fu, S.L. Rhizosphere effects on decomposition: Controls of plant species, phenology, and fertilization. Soil. Sci. Soc. Am. J. 2003, 67, 1418–1427. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef]
- Ding, Z.J.; Tang, M.; Chen, X.; Yin, L.M.; Gui, H.C.; Zhu, B. Measuring rhizosphere effects of two tree species in a temperate forest: A comprehensive method comparison. Rhizosphere 2019, 10, 100153. [Google Scholar] [CrossRef]
- Ma, Y.D.; Yue, K.; Hedenec, P.; Li, C.H.; Li, Y.; Wu, Q.Q. Global patterns of rhizosphere effects on soil carbon and nitrogen biogeochemical processes. Catena 2023, 220, 106661. [Google Scholar] [CrossRef]
- Ao, G.K.L.; Qin, W.K.; Wang, X.D.; Yu, M.; Feng, J.G.; Han, M.G.; Zhu, B. Linking the rhizosphere effects of 12 woody species on soil microbial activities with soil and root nitrogen status. Rhizosphere 2023, 28, 100809. [Google Scholar] [CrossRef]
- Maurer, D.; Malique, F.; Alfarraj, S.; Albasher, G.; Horn, M.A.; Butterbach-Bahl, K.; Dannenmann, M.; Rennenberg, H. Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 2021, 467, 107–127. [Google Scholar] [CrossRef]
- Lopes, L.D.; Fernandes, M.F. Changes in microbial community structure and physiological profile in a kaolinitic tropical soil under different conservation agricultural practices. Appl. Soil Ecol. 2020, 152, 103545. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tang, Z.H.; Wang, J.D.; Zhang, Y.C. Long-term organic fertilization reshapes the communities of bacteria and fungi and enhances the activities of C- and P-cycling enzymes in calcareous alluvial soil. Appl. Soil Ecol. 2024, 194, 105204. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Guan, Y.P.; Xu, B.; Zhang, X.M.; Yang, W. Tillage Practices and Residue Management Manipulate Soil Bacterial and Fungal Communities and Networks in Maize Agroecosystems. Microorganisms 2022, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, Q.C.; Xu, D.L.; Li, Z.S.; Chao, L.M.; Li, X.Y.; Liu, H.J.; Wang, P.F.; Zheng, Y.X.; Liu, X.Y.; et al. Soil microbial community composition and co-occurrence network responses to mild and severe disturbances in volcanic areas. Sci. Total Environ. 2023, 901, 165889. [Google Scholar] [CrossRef] [PubMed]
- Maurice, K.; Bourceret, A.; Youssef, S.; Boivin, S.; Laurent-Webb, L.; Damasio, C.; Boukcim, H.; Selosse, M.A.; Ducousso, M. Anthropic disturbances impact the soil microbial network structure and stability to a greater extent than natural disturbances in an arid ecosystem. Sci. Total Environ. 2024, 907, 167969. [Google Scholar] [CrossRef]
- Gottshall, C.B.; Cooper, M.; Emery, S.M. Activity, diversity and function of arbuscular mycorrhizae vary with changes in agricultural management intensity. Agric. Ecosyst. Environ. 2017, 241, 142–149. [Google Scholar] [CrossRef]
- Gonzalez-Chávez, M.D.A.; Aitkenhead-Peterson, J.A.; Gentry, T.J.; Zuberer, D.; Hons, F.; Loeppert, R. Soil microbial community, C, N, and P responses to long-term tillage and crop rotation. Soil Till. Res. 2010, 106, 285–293. [Google Scholar] [CrossRef]
- Aravena, C.; Valle, S.R.; Vergara, R.; Chang, M.G.; Martínez, O.; Clunes, J.; Caurapan, B.; Asenjo, J. Effect of Agricultural Management Intensity on the Organic Carbon Fractions and Biological Properties of a Volcanic-Ash-Derived Soil. Sustainability 2025, 17, 2704. [Google Scholar] [CrossRef]
- Wan, P.X.; Zhou, Z.K.; Yuan, Z.Q.; Wei, H.H.; Huang, F.Q.; Li, Z.S.; Li, F.M.; Zhang, F. Fungal community composition changes and reduced bacterial diversity drive improvements in the soil quality index during arable land restoration. Environ. Res. 2024, 244, 117931. [Google Scholar] [CrossRef]
- Su, H.Y.; Ma, L.; Chang, T.; Qin, R.M.; Zhang, Z.H.; She, Y.D.; Wei, J.J.; Zhou, C.Y.; Hu, X.; Shi, Z.C.; et al. Effects of Main Land-Use Types on Plant and Microbial Diversity and Ecosystem Multifunctionality in Degraded Alpine Grasslands. Land 2023, 12, 638. [Google Scholar] [CrossRef]
- Durán-Viseras, A.; Lindner, B.G.; Hatt, J.K.; Lai, A.M.D.; Wallace, R.; Ginn, O.; Brown, J.; Konstantinidis, K.T. Metagenomic insights into the impact of litter from poultry Concentrated Animal Feeding Operations (CAFOs) to adjacent soil and water microbial communities. Sci. Total Environ. 2024, 920, 170772. [Google Scholar] [CrossRef]
- Kurm, V.; Schilder, M.T.; Haagsma, W.K.; Bloem, J.; Scholten, O.E.; Postma, J. Reduced tillage increases soil biological properties but not suppressiveness against Rhizoctonia solani and Streptomyces scabies. Appl. Soil Ecol. 2023, 181, 104646. [Google Scholar] [CrossRef]
- Liu, Z.X.; Gu, H.D.; Liang, A.Z.; Li, L.J.; Yao, Q.; Xu, Y.X.; Liu, J.J.; Jin, J.; Liu, X.B.; Wang, G.H. Conservation tillage regulates the assembly, network structure and ecological function of the soil bacterial community in black soils. Plant Soil 2022, 472, 207–223. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yan, L.; Gao, Y.H.; Liang, A.Z.; Sui, B.; Zhao, L.P.; Liu, S.X. Long-Term Effects of Tillage Practices on Soil Bacterial Community Abundance and Metabolic Diversity of Black Soil from Northeast China. Int. J. Agric. Biol. 2018, 20, 2753–2762. [Google Scholar] [CrossRef]
- Fan, K.K.; Weisenhorn, P.; Gilbert, J.A.; Shi, Y.; Bai, Y.; Chu, H.Y. Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil. Biol. Biochem. 2018, 121, 185–192. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.D.; Qiu, X.Y.; Zhang, J.; Zhang, J.Y.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Swift, J.F.; Kolp, M.R.; Carmichael, A.; Ford, N.E.; Hansen, P.M.; Sikes, B.A.; Kleiner, M.; Wagner, M.R. Drought stress homogenizes maize growth responses to diverse natural soil microbiomes. Plant Soil 2025, 509, 181–199. [Google Scholar] [CrossRef]


| Maize Height (cm) | Aboveground Biomass (g Plant−1) | |
|---|---|---|
| Con_Mono | 136.15 ± 4.78 a | 99.50 ± 4.01 a |
| Con_Inter | 185.25 ± 4.07 b | 150.35 ± 8.26 b |
| Fer_Mono | 211.05 ± 5.95 c | 146.80 ± 7.52 b |
| Fer_Inter | 235.95 ± 4.68 d | 208.95 ± 15.78 c |
| Alpha Diversity | Con_Mono | Con_Inter | Fer_Mono | Fer_Inter | |
|---|---|---|---|---|---|
| Richness | Intra-Row | 860.50 ± 46.04 a | 904.75 ± 70.81 a | 748.00 ± 53.99 a | 818.25 ± 73.49 a |
| Inter-Row | 1262.13 ± 147.10 a | 1436.13 ± 160.21 a | 988.88 ± 84.89 a | 1407.63 ± 129.98 a | |
| Shannon | Intra-Row | 6.27 ± 0.04 a | 6.32 ± 0.05 a | 6.16 ± 0.09 a | 6.19 ± 0.11 a |
| Inter-Row | 6.59 ± 0.19 ab | 6.60 ± 0.13 ab | 6.31 ± 0.08 a | 6.78 ± 0.09 b | |
| PD | Intra-Row | 89.86 ± 3.16 a | 96.96 ± 3.43 a | 88.26 ± 4.09 a | 93.17 ± 4.98 a |
| Inter-Row | 126.31 ± 7.70 ab | 136.14 ± 8.13 b | 99.83 ± 5.43 a | 127.96 ± 7.70 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, X.; Jin, T.; Jiang, M.; Shi, P.; Wei, G. Spatial Heterogeneity in Soil Microbial Communities Impacts Their Suitability as Bioindicators for Evaluating Productivity in Agricultural Practices. Microorganisms 2025, 13, 1160. https://doi.org/10.3390/microorganisms13051160
Li G, Li X, Jin T, Jiang M, Shi P, Wei G. Spatial Heterogeneity in Soil Microbial Communities Impacts Their Suitability as Bioindicators for Evaluating Productivity in Agricultural Practices. Microorganisms. 2025; 13(5):1160. https://doi.org/10.3390/microorganisms13051160
Chicago/Turabian StyleLi, Guoqiang, Xuanjing Li, Ting Jin, Muyilan Jiang, Peng Shi, and Gehong Wei. 2025. "Spatial Heterogeneity in Soil Microbial Communities Impacts Their Suitability as Bioindicators for Evaluating Productivity in Agricultural Practices" Microorganisms 13, no. 5: 1160. https://doi.org/10.3390/microorganisms13051160
APA StyleLi, G., Li, X., Jin, T., Jiang, M., Shi, P., & Wei, G. (2025). Spatial Heterogeneity in Soil Microbial Communities Impacts Their Suitability as Bioindicators for Evaluating Productivity in Agricultural Practices. Microorganisms, 13(5), 1160. https://doi.org/10.3390/microorganisms13051160





