Perineal Urethrostomy Enables Susceptibility of Bull Calves as a Natural Host Model for Bovine Trichomonosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites
2.2. Animals and Experimental Infections
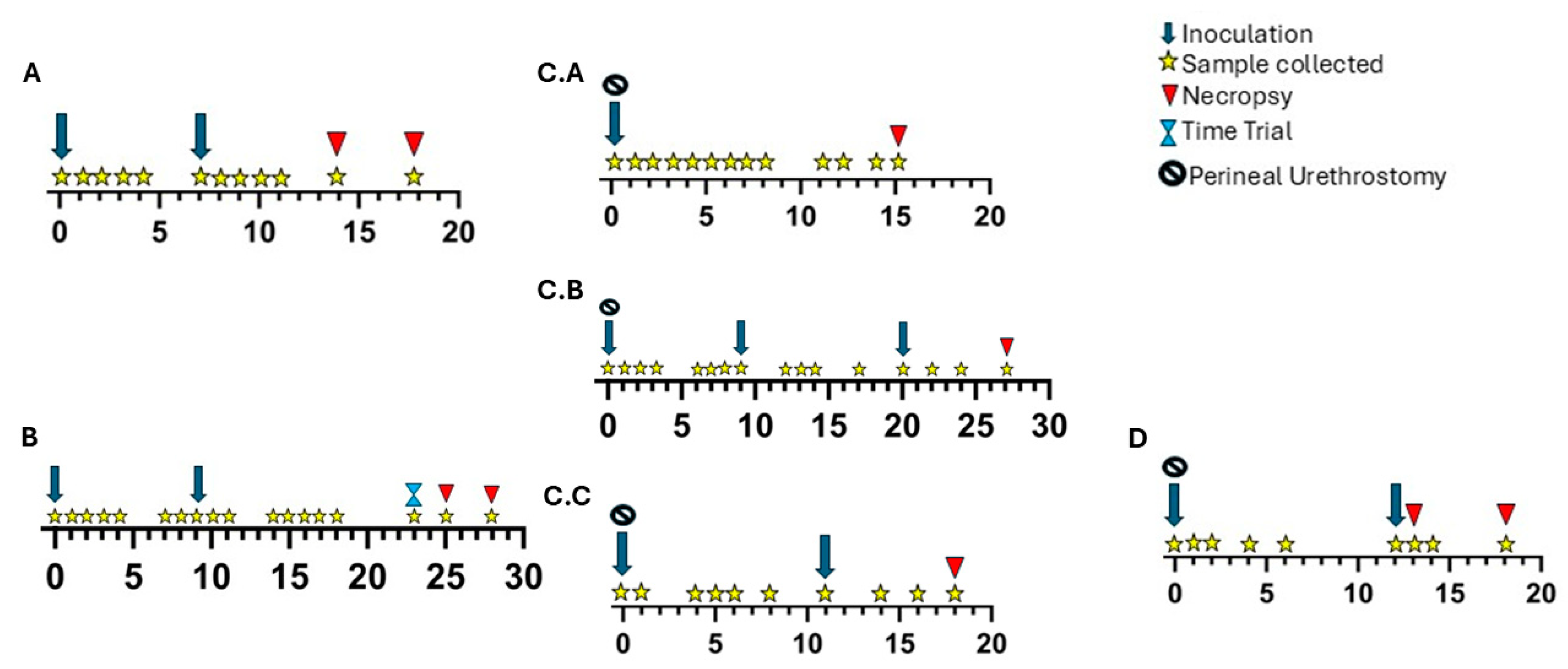
2.2.1. Experiment A
2.2.2. Experiment B
2.2.3. Experiment C
2.2.4. Experiment D
2.3. PCR
2.4. Culture
2.5. Histopathology
2.6. Galectin-1 Immunohistochemistry
2.7. Urine Co-Incubation Studies
3. Results
3.1. Sporadic and Transient PCR Positivity in Non-Urethrostomized Calves
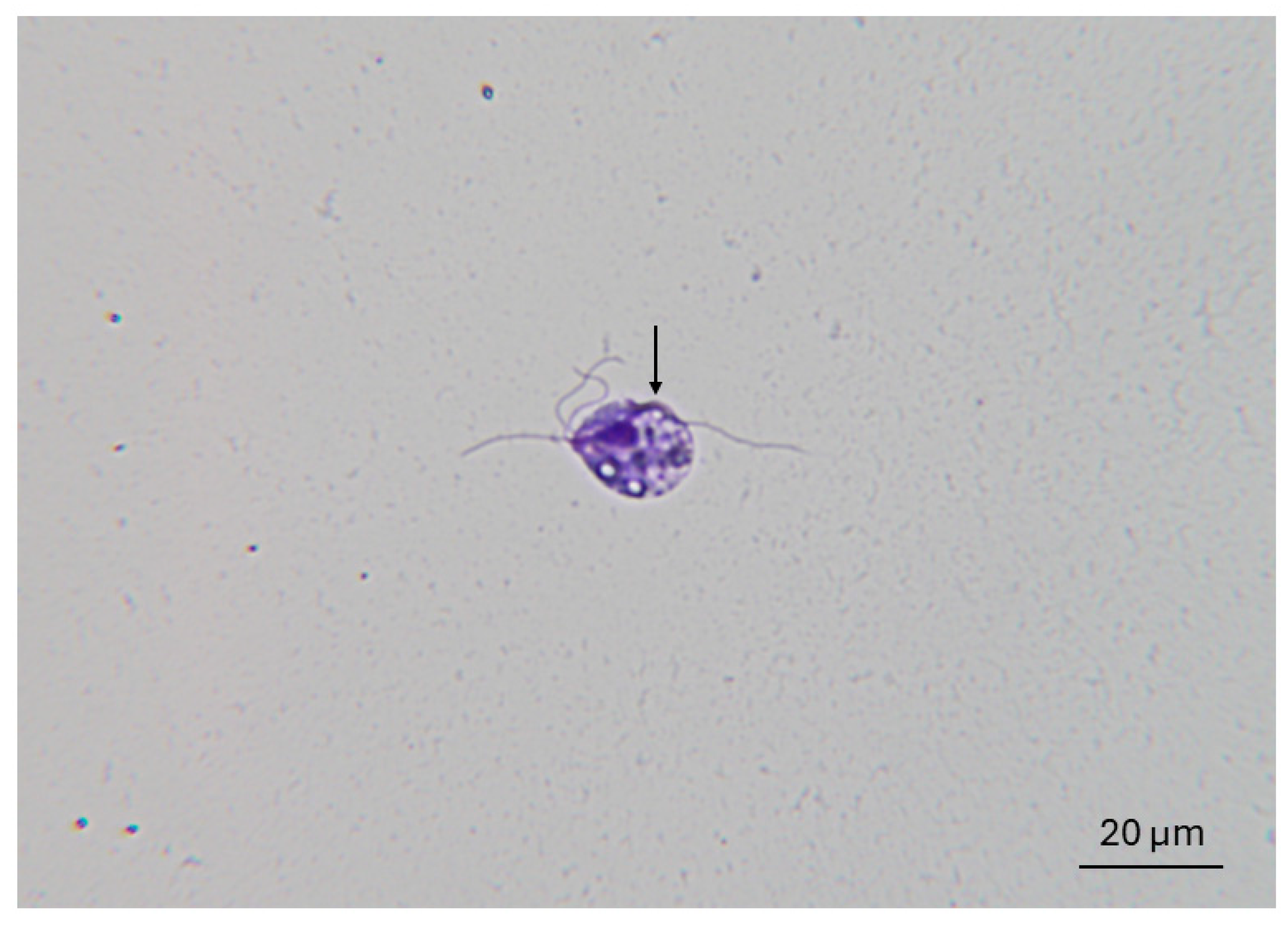
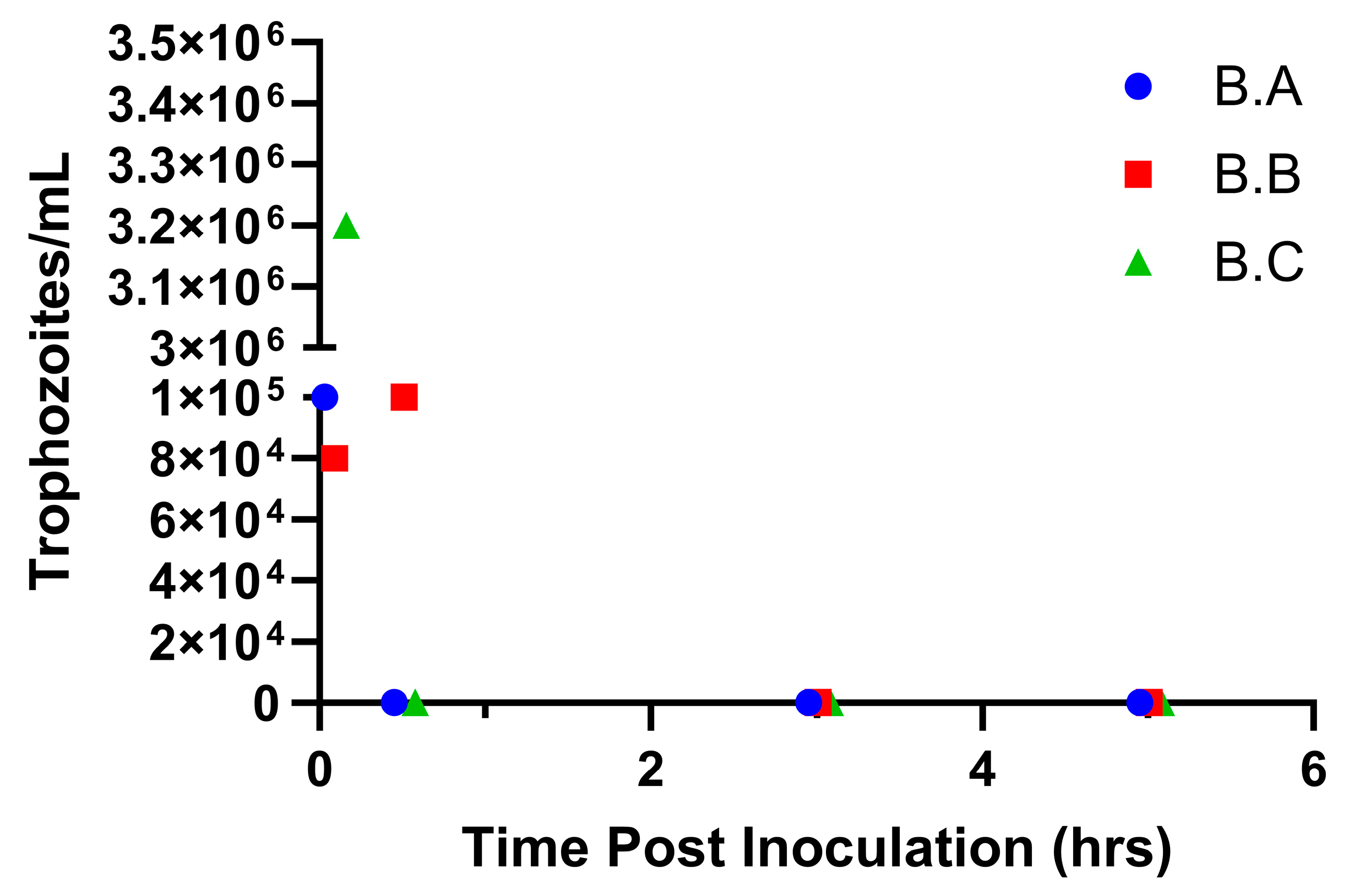
3.2. Trophozoites Do Not Survive More Than 30 min in the Prepuce of Non-Urethrostomized Calves
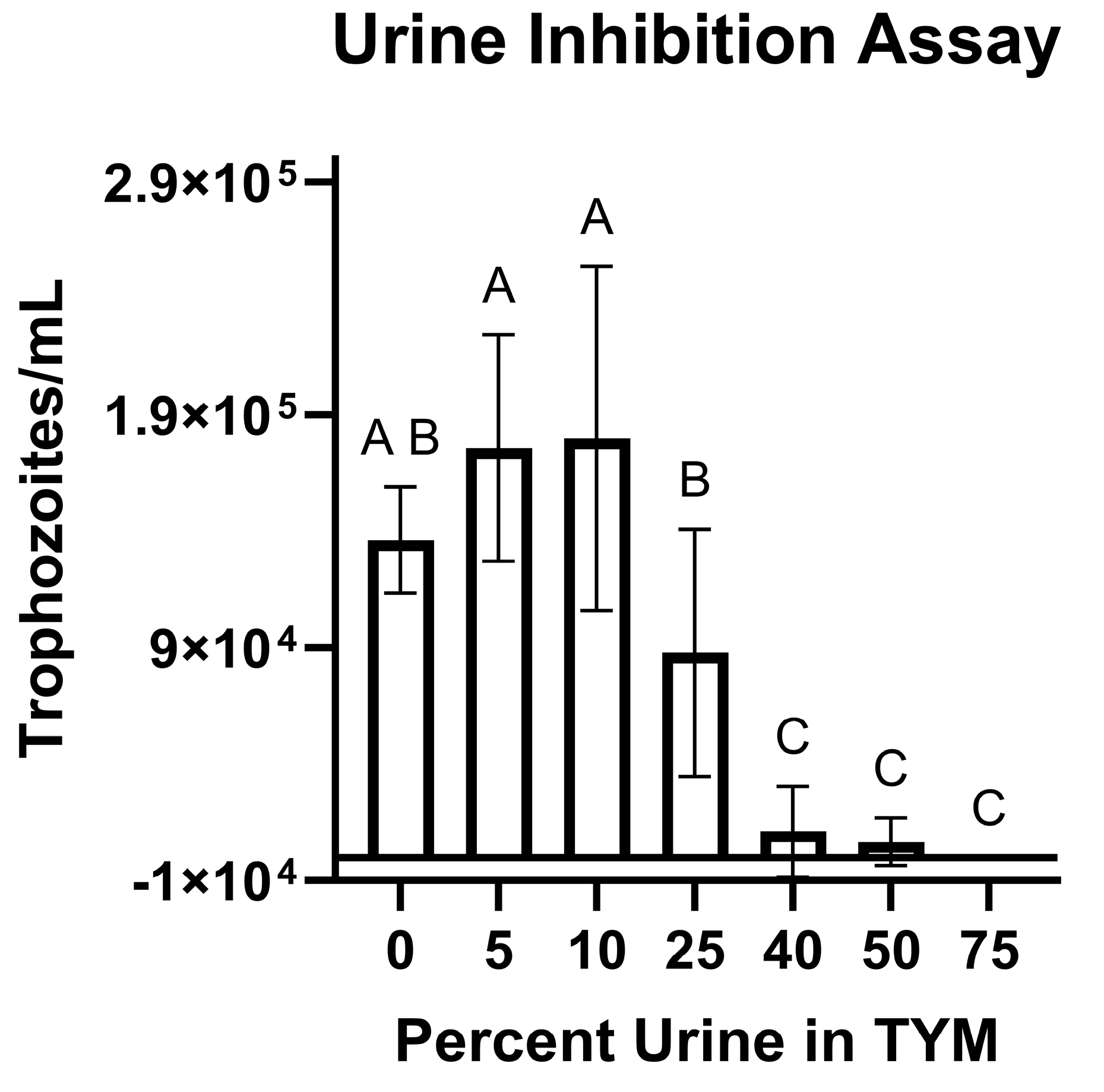
3.3. Trophozoites Are Killed Rapidly in the Presence of Urine
3.4. Putative Host Cell Receptor Galectin-1 Epitopes Are Present in the Reproductive Tract of Prepubescent Bull Calves
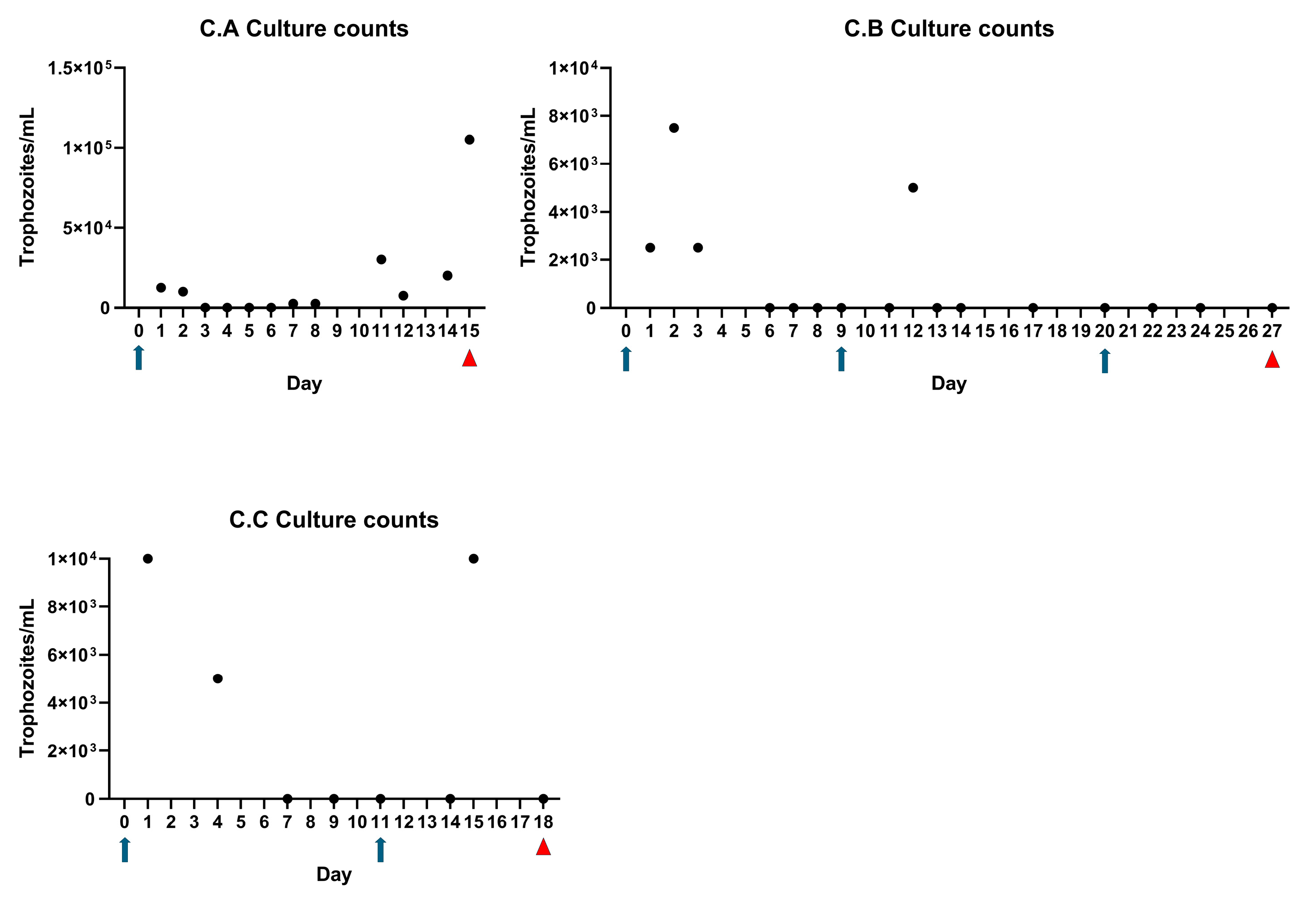
3.5. Perineal Urethrostomy Permits Survival and Growth of T. foetus in Bull Calves
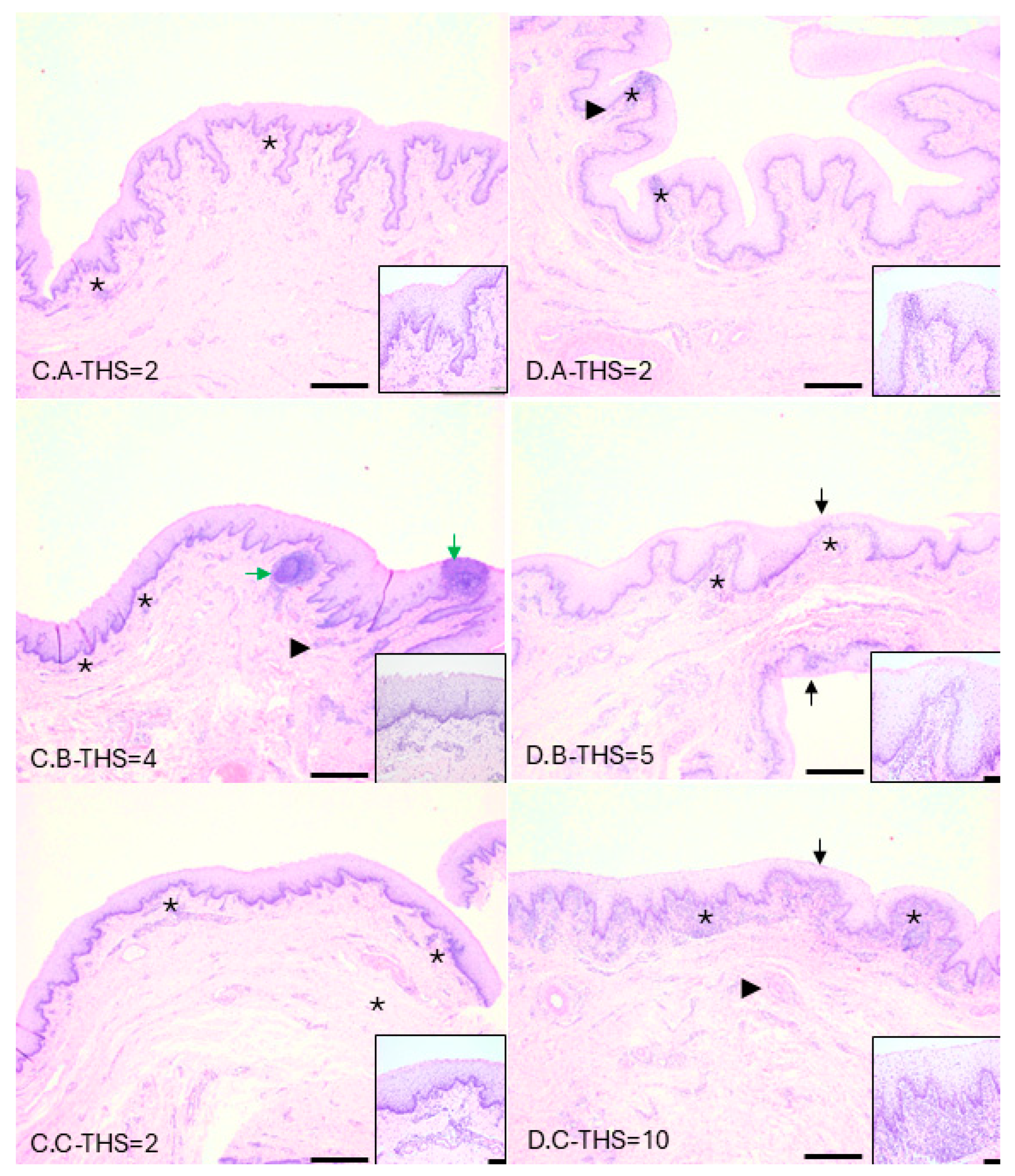
3.6. Inflammatory Cell Infiltrates Consistent with Trichomonosis Present in Infected Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yule, A.; Skirrow, S.Z.; Bonduran, R.H. Bovine trichomoniasis. Parasitol. Today 1989, 5, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Skirrow, S.Z.; BonDurant, R.H. Induced Tritrichomonas foetus infection in beef heifers. J. Am. Vet. Med. Assoc. 1990, 196, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Parsonson, I.M.; Clark, B.L.; Dufty, J.H. Early pathogenesis and pathology of Tritrichomonas foetus infection in virgin heifers. J. Comp. Pathol. 1976, 86, 59–66. [Google Scholar] [CrossRef]
- Rhyan, J.C.; Stackhouse, L.L.; Quinn, W.J. Fetal and placental lesions in bovine abortion due to Tritrichomonas foetus. Vet. Pathol. 1988, 25, 350–355. [Google Scholar] [CrossRef]
- Yao, C. Control and eradication of bovine trichomonosis in Wyoming, USA by testing and culling positive bulls. Vet. Res. 2021, 52, 129. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Henderson, J.; Brewer, M. Bovine Trichomonosis Cases in the United States 2015–2019. Front. Vet. Sci. 2021, 8, 692199. [Google Scholar] [CrossRef]
- Yao, C.; Köster, L.S. Tritrichomonas foetus infection, a cause of chronic diarrhea in the domestic cat. Vet. Res. 2015, 46, 35. [Google Scholar] [CrossRef]
- Singh, B.N.; Lucas, J.J.; Beach, D.H.; Shin, S.T.; Gilbert, R.O. Adhesion of Tritrichomonas foetus to Bovine Vaginal Epithelial Cells. Infect. Immun. 1999, 67, 3847–3854. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ratner, D.M.; Ryan, C.M.; Johnson, P.J.; O’ Keefe, B.R.; Secor, W.E.; Anderson, D.J.; Robbins, P.W.; Samuelson, J. Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model. PLoS ONE 2015, 10, e0135340. [Google Scholar] [CrossRef]
- Hirt, R.P.; de Miguel, N.; Nakjang, S.; Dessi, D.; Liu, Y.-C.; Diaz, N.; Rappelli, P.; Acosta-Serrano, A.; Fiori, P.-L.; Mottram, J.C. Chapter 2—Trichomonas vaginalis Pathobiology: New Insights from the Genome Sequence. In Advances in Parasitology; Rollinson, D., Hay, S.I., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 77, pp. 87–140. [Google Scholar]
- Okumura, C.Y.; Baum, L.G.; Johnson, P.J. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell. Microbiol. 2008, 10, 2078–2090. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Yamamoto, H.S.; Fashemi, T.; Foley, E.; Ryan, S.; Beatty, N.; Dawood, H.; Hayes, G.R.; St-Pierre, G.; Sato, S.; et al. Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and -3 to Modulate Epithelial Immunity. J. Biol. Chem. 2016, 291, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Seyrek, K.; Heck, A.; Sinowatz, F.; Gabius, H.J. Galectin-1 and galectin-3 in fetal development of bovine respiratory and digestive tracts. Comparison of cell type-specific expression profiles and subcellular localization. Cell Tissue Res. 2002, 307, 35–46. [Google Scholar] [CrossRef]
- Irons, P.; McGowan, M.; de Assis, P.; Randhawa, I.; Awawdeh, L.; Mugwabana, J.; Barnes, T.; Boe-Hansen, G.; McCosker, K.; Fordyce, G. Prevalence of Tritrichomonas foetus in beef bulls slaughtered at two abattoirs in northern Australia. Aust. Vet. J. 2022, 100, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Michi, A.N.; Favetto, P.H.; Kastelic, J.; Cobo, E.R. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology 2016, 85, 781–791. [Google Scholar] [CrossRef]
- Clark, B.; Parsonson, I.; Dufty, J. Experimental infection of bulls with Tritrichomonas foetus. Aust. Vet. J. 1974, 50, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.G.; Diamond, L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef]
- Bader, C.; Jesudoss Chelladurai, J.; Thompson, K.; Hall, C.; Carlson, S.A.; Brewer, M.T. Evaluation of high-throughput assays for in vitro drug susceptibility testing of Tritrichomonas foetus trophozoites. Vet. Parasitol. 2016, 223, 34–37. [Google Scholar] [CrossRef]
- Schaut, R.G.; Corbeil, L.B.; Blake, C.N.; Brewer, M.T. Development of a bead-agglutination assay for rapid detection of. Vet. Parasitol. 2017, 243, 188–191. [Google Scholar] [CrossRef]
- Ewoldt, J.M.; Jones, M.L.; Miesner, M.D. Surgery of Obstructive Urolithiasis in Ruminants. Vet. Clin. Food Anim. Pract. 2008, 24, 455–465. [Google Scholar] [CrossRef]
- Loy, D.S.; Spuri Gomes, R.; Dutta, E.; Brodersen, B.W.; Loy, J.D. Time and temperature stability of Tritrichomonas foetus in phosphate-buffered saline as evaluated by a reverse transcription real-time PCR assay and field analysis. Front. Vet. Sci. 2023, 10, 1101502. [Google Scholar] [CrossRef]
- Felleisen, R.S.; Lambelet, N.; Bachmann, P.; Nicolet, J.; Müller, N.; Gottstein, B. Detection of Tritrichomonas foetus by PCR and DNA enzyme immunoassay based on rRNA gene unit sequences. J. Clin. Microbiol. 1998, 36, 513–519. [Google Scholar] [CrossRef]
- Reece, W.O.; Erickson, H.H.; Goff, J.P.; Uemura, E.E. Dukes’ Physiology of Domestic Animals, 13th ed.; Reece, W., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zimmerman, L.C.; Schroeder, T.C.; Dhuyvetter, K.C.; Olson, K.C.; Stokka, G.L.; Seeger, J.T.; Grotelueschen, D.M. The Effect of Value-Added Management on Calf Prices at Superior Livestock Auction Video Markets. J. Agric. Resour. Econ. 2012, 37, 128–143. [Google Scholar]
- Shook, J.N.; Vanoverbeke, D.L.; Scanga, J.A.; Belk, K.E.; Savell, J.W.; Lawrence, T.E.; Morgan, J.B.; Griffin, D.B.; Hale, D.S.; Smith, G.C. The National Beef Quality Audit-2005, Phase I: Views of Producers, Packers, and Merchandisers on Current Quality Characteristics of the Beef Industry1. Prof. Anim. Sci. 2008, 24, 189–197. [Google Scholar] [CrossRef]
- Martin, K.; Jesudoss Chelladurai, J.; Bader, C.; Carreiro, E.; Long, K.; Thompson, K.; Brewer, M. Repurposing the open access malaria box reveals compounds with activity against Tritrichomonas foetus trophozoites. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 89–93. [Google Scholar] [CrossRef]
- Martin, K.A.; Kovach, K.; Moscoso, E.; Carreiro, E.; Jesudoss Chelladurai, J.R.J.; Brewer, M.T. Assessment of in vitro efficacy for common surface disinfectants and antiseptics against Tritrichomonas foetus trophozoites. Front. Vet. Sci. 2023, 10, 1282274. [Google Scholar] [CrossRef]
- Gilbert, R.O. 9—Reproductive Diseases. In Rebhun’s Diseases of Dairy Cattle, 3rd ed.; Peek, S.F., Divers, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 466–507. [Google Scholar] [CrossRef]
- Wolfe, D.F. Review: Abnormalities of the bull—Occurrence, diagnosis and treatment of abnormalities of the bull, including structural soundness. Animal 2018, 12, s148–s157. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, T.J. 24—Specific Infectious Diseases Causing Infertility and Subfertility in Cattle. In Veterinary Reproduction and ObstetricsI, 10th ed.; Noakes, D.E., Parkinson, T.J., England, G.C.W., Eds.; W.B. Saunders: St. Louis, MO, USA, 2019; pp. 434–466. [Google Scholar] [CrossRef]
- Rhyan, J.C.; Wilson, K.L.; Wagner, B.; Anderson, M.L.; Bondurant, R.H.; Burgess, D.E.; Mutwiri, G.K.; Corbeil, L.B. Demonstration of Tritrichomonas foetus in the External Genitalia and of Specific Antibodies in Preputial Secretions of Naturally Infected Bulls. Vet. Pathol. 1999, 36, 406–411. [Google Scholar] [CrossRef]
- Parsonson, I.M.; Clark, B.L.; Dufty, J. The pathogenesis of Tritrichomonas foetus infection in the bull. Aust. Vet. J. 1974, 50, 421–423. [Google Scholar] [CrossRef]
- Cowley, J.; Hopper, R. Management of urolithiasis in breeding bulls. Clin. Theriogenol. 2023, 15, 5–10. [Google Scholar] [CrossRef]
- Oman, R.E.; Reppert, E.J.; Streeter, R.N.; Jones, M. Outcome and complications in goats treated by perineal urethrostomy for obstructive urolithiasis: 25 cases (2010–2017). J. Vet. Intern. Med. 2019, 33, 292–296. [Google Scholar] [CrossRef]
- Foster, R.A. Chapter 19—Male Reproductive System 1. In Pathologic Basis of Veterinary, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 1194–1222. [Google Scholar]
- Van Der Pol, B. Trichomonas vaginalis Infection: The Most Prevalent Nonviral Sexually Transmitted Infection Receives the Least Public Health Attention. Clin. Infect. Dis. 2007, 44, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Molgora, B.M.; Mukherjee, S.K.; Baumel-Alterzon, S.; Santiago, F.M.; Muratore, K.A.; Sisk, A.E., Jr.; Mercer, F.; Johnson, P.J. Trichomonas vaginalis adherence phenotypes and extracellular vesicles impact parasite survival in a novel in vivo model of pathogenesis. PLoS Negl. Trop. Dis. 2023, 17, e0011693. [Google Scholar] [CrossRef] [PubMed]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and Microbiological Aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Ackers, J. Immunologic Aspects of Human Trichomoniasis. In Trichomonads Parasitic in Humans; Honiberg, B., Ed.; Springer: New York, NY, USA, 1989. [Google Scholar]
- Hayward-McClelland, S.F.; Delgaty, K.; Garber, G. Chapter 100—Animal Models of Trichomonas vaginalis Infection with Special Emphasis on the Intravaginal Mouse Model. In Handbook of Animal Models of Infection: Experimental Models in Antimicrobial Chemotherapy; Zak, O., Sande, M., Eds.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]


| Day | A.A (ATCC) | A.B (ATCC) | A.C (ATCC) | A.D (IA-1) | A.E (IA-1) | A.F (IA-1) | Notes |
|---|---|---|---|---|---|---|---|
| Negative | Negative | Negative | Negative | Negative | Negative | Pre-infection | |
| 0 | Positive | Positive | Positive | Positive | Positive | Positive | Day of infection |
| 1 | Negative | Negative | Negative | Suspect | Positive | Negative | |
| 2 | Negative | Negative | Negative | Negative | Negative | Negative | |
| 3 | Negative | Negative | Negative | Negative | Negative | Negative | |
| 4 | Negative | Negative | Negative | Negative | Negative | Negative | |
| 7 | Positive | Negative | Negative | Positive | Negative | Negative | Prior to re-inoculation |
| 8 | Positive | Suspect | Positive | Positive | Suspect | Positive | |
| 9 | Suspect | Negative | Negative | Positive | Negative | Positive | |
| 10 | Positive | Negative | Negative | Suspect | Negative | Negative | |
| 11 | Negative | Negative | Negative | Negative | Negative | Negative | |
| 14 | Negative | Positive | Negative | Negative | Negative | Negative | Necropsy:A.A,A.D |
| 18 | NA | Negative | Negative | NA | Negative | Negative | Necropsy: A.B,A.C, A.E,A.F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, K.A.; Bayne, J.E.; Chinchilla-Vargas, K.; Reece, S.L.; Jesudoss Chelladurai, J.R.J.; Harm, T.A.; Smith, J.D.; Jones, D.E.; Blake, C.N.; Brewer, M.T. Perineal Urethrostomy Enables Susceptibility of Bull Calves as a Natural Host Model for Bovine Trichomonosis. Microorganisms 2025, 13, 1070. https://doi.org/10.3390/microorganisms13051070
Martin KA, Bayne JE, Chinchilla-Vargas K, Reece SL, Jesudoss Chelladurai JRJ, Harm TA, Smith JD, Jones DE, Blake CN, Brewer MT. Perineal Urethrostomy Enables Susceptibility of Bull Calves as a Natural Host Model for Bovine Trichomonosis. Microorganisms. 2025; 13(5):1070. https://doi.org/10.3390/microorganisms13051070
Chicago/Turabian StyleMartin, Katy A., Jenna E. Bayne, Krystal Chinchilla-Vargas, Sara L. Reece, Jeba R. J. Jesudoss Chelladurai, Tyler A. Harm, Jodi D. Smith, Douglas E. Jones, Courtney N. Blake, and Matthew T. Brewer. 2025. "Perineal Urethrostomy Enables Susceptibility of Bull Calves as a Natural Host Model for Bovine Trichomonosis" Microorganisms 13, no. 5: 1070. https://doi.org/10.3390/microorganisms13051070
APA StyleMartin, K. A., Bayne, J. E., Chinchilla-Vargas, K., Reece, S. L., Jesudoss Chelladurai, J. R. J., Harm, T. A., Smith, J. D., Jones, D. E., Blake, C. N., & Brewer, M. T. (2025). Perineal Urethrostomy Enables Susceptibility of Bull Calves as a Natural Host Model for Bovine Trichomonosis. Microorganisms, 13(5), 1070. https://doi.org/10.3390/microorganisms13051070






