Analysis of the Changes in Diversity of Culturable Bacteria in Different Niches of Mulberry Fields and Assessment of Their Plant Growth-Promoting Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of the Samples
2.2. Isolation of Mulberry Field Bacteria
2.3. Identification of the Bacteria
2.4. Determination of the Antimicrobial Activity of the Bacteria
2.5. Determination of Plant Growth-Promoting (PGP) Traits of Bacteria
2.6. PCR Detection of Genes Related to Antibiotic Biosynthesis
2.7. Effects of Plant Growth-Promoting Bacteria on the Growth of Mulberry Saplings
2.8. Analysis of Bacterial Metabolites Based on the LC-Q/TOF-MS Technique
2.9. Statistical Analysis
3. Results
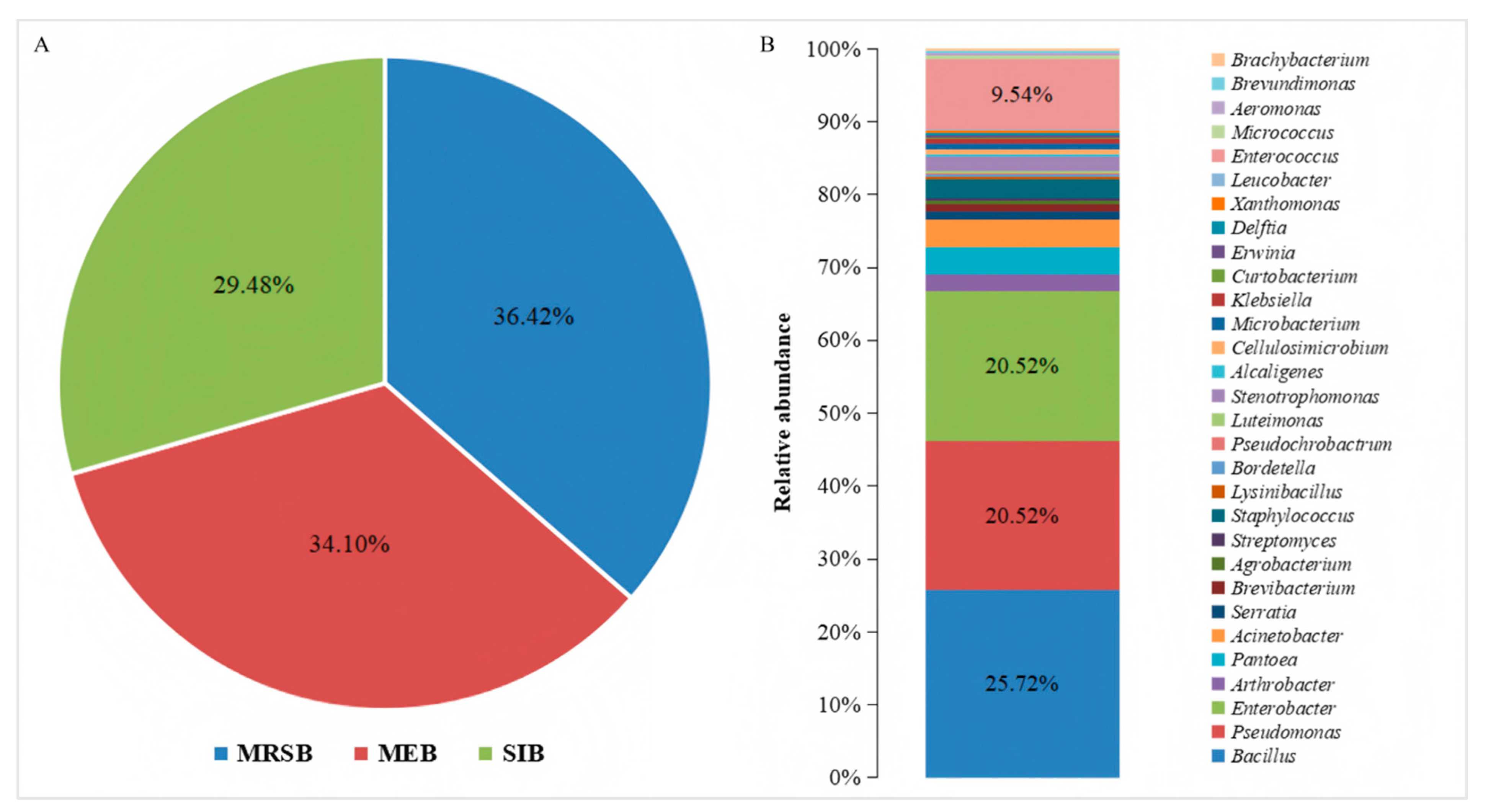
3.1. Isolation and Composition of the Cultured Bacteria in the Mulberry Field
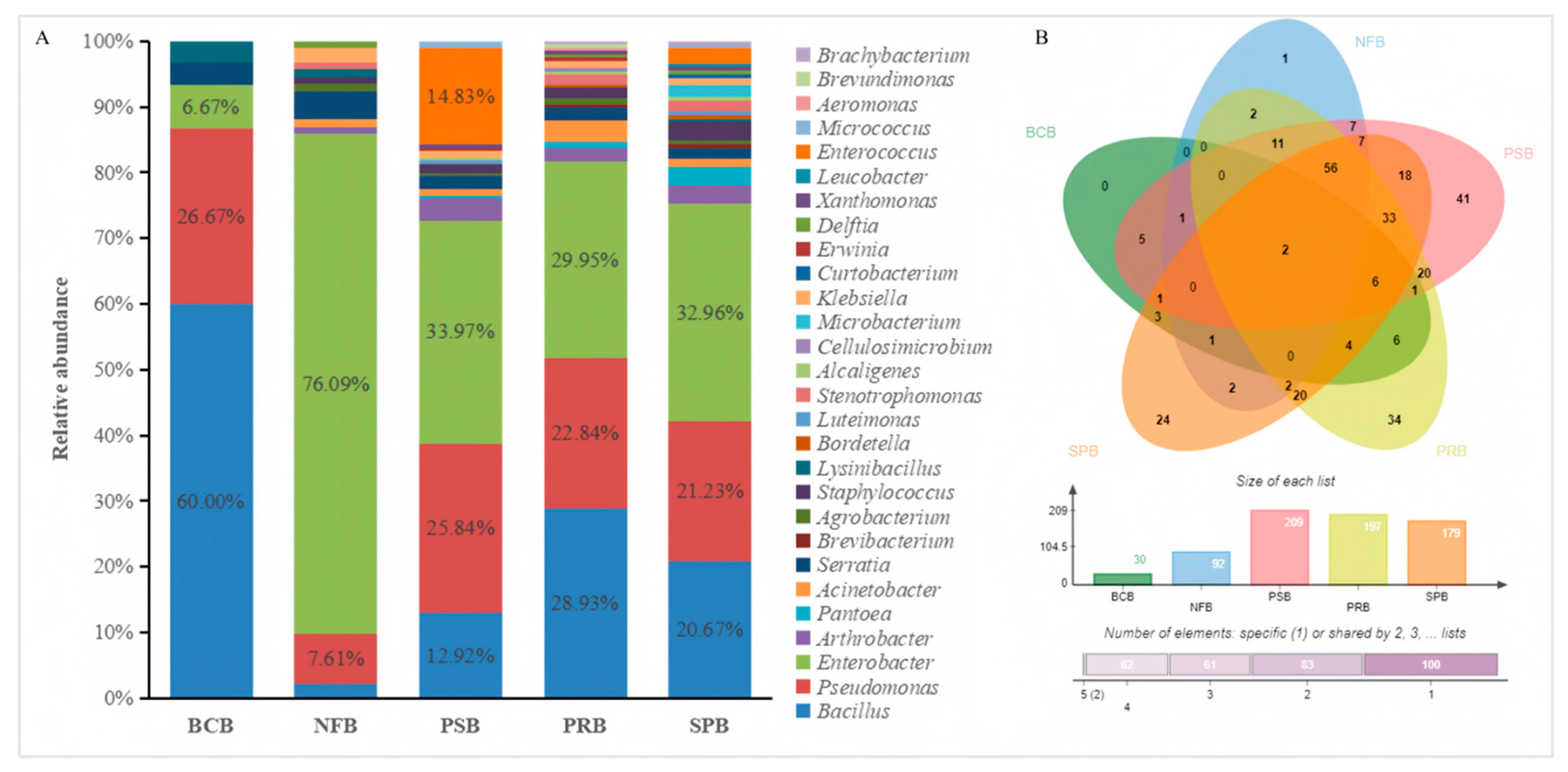
3.2. Diversity of Bacterial Communities in Different Niches in Mulberry Fields
3.3. Plant Growth-Promoting Bacterial Composition and Diversity Analysis
3.4. Screening of Plant Growth-Promoting Bacteria
3.4.1. Determination of Antibacterial Activity and Antibacterial Spectrum of Biological Control Bacteria
3.4.2. Analysis of the Plant Growth-Promoting Properties of Biological Control Bacteria
3.4.3. Analysis of Antibacterial Mechanism of Biological Control Bacteria
3.4.4. Determination and Analysis of Plant Growth-Promoting Properties
3.5. Evaluation of Mulberry Sapling Growth
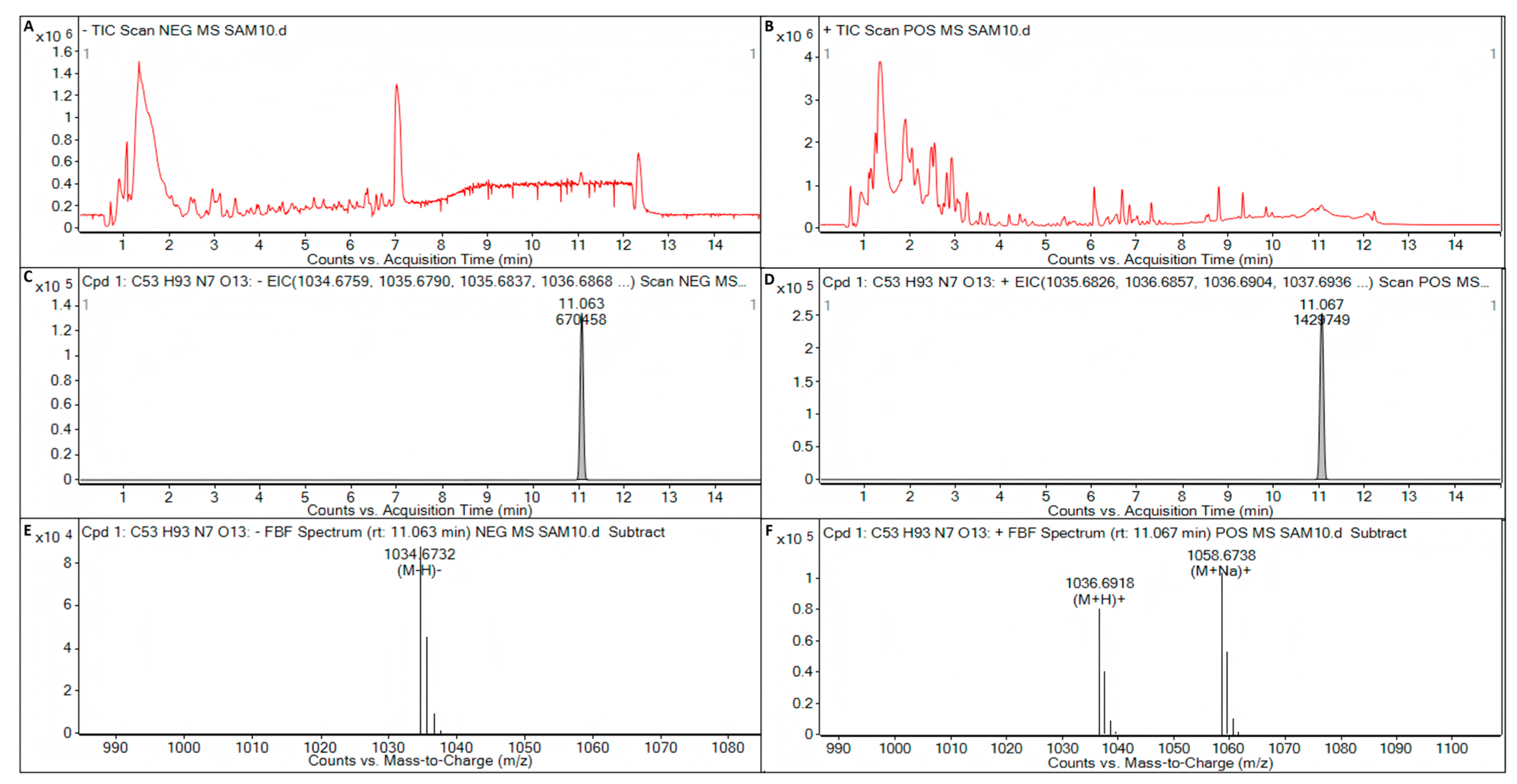
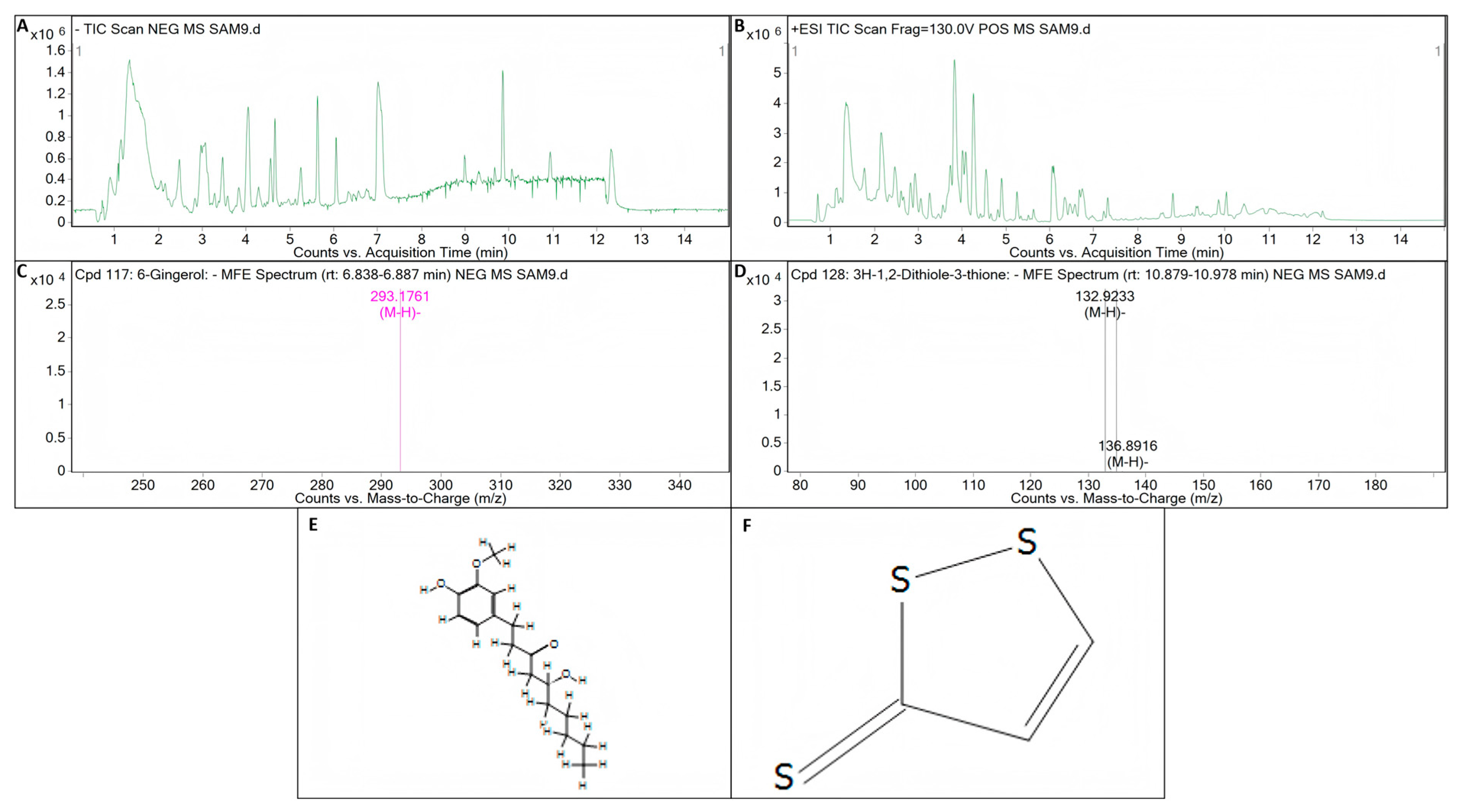
3.6. Putative Bioactive Compounds Detected by LC-Q/TOF-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atieno, M.; Herrmann, L.; Nguyen, H.T.; Phan, H.T.; Nguyen, N.K.; Srean, P.; Than, M.M.; Zhiyong, R.; Tittabutr, P.; Shutsrirung, A.; et al. Assessment of Biofertilizer Use for Sustainable Agriculture in the Great Mekong Region. J. Environ. Manag. 2020, 275, 111300. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Razdan, N.; Saharan, K. Rhizospheric Microbiome: Bio-Based Emerging Strategies for Sustainable Agriculture Development and Future Perspectives. Microbiol. Res. 2022, 254, 126901. [Google Scholar] [CrossRef]
- Saidi, S.; Chebil, S.; Gtari, M.; Mhamdi, R. Characterization of Root-Nodule Bacteria Isolated From Vicia Faba and Selection of Plant Growth Promoting Isolates. World J. Microbiol. Biotechnol. 2013, 29, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Novello, G.; Gamalero, E.; Massa, N.; Cesaro, P.; Lingua, G.; Todeschini, V.; Caramaschi, A.; Favero, F.; Cora, D.; Manfredi, M.; et al. Proteome and Physiological Characterization of Halotolerant Nodule Endophytes: The Case of Rahnella aquatilis and Serratia plymuthica. Microorganisms 2022, 10, 890. [Google Scholar] [CrossRef]
- Wu, L.; Xie, Y.; Li, J.; Han, M.; Yang, X.; Chang, F. The Effect of Two Siderophore-Producing Bacillus Strains On the Growth Promotion of Perennial ryegrass Under Cadmium Stress. Microorganisms 2024, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El, H.H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Riaz, H.M.; Chohan, S.; Yuen, G.Y.; Abid, M. Biological Control of Tomato Early Blight in Pakistan Using Local Rhizobacteria. Pest Manag. Sci. 2024, 80, 1412–1422. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Frances, J.; Rosello, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Bal, H.B.; Das, S.; Dangar, T.K.; Adhya, T.K. Acc Deaminase and IAA Producing Growth Promoting Bacteria From the Rhizosphere Soil of Tropical Rice Plants. J. Basic Microbiol. 2013, 53, 972–984. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-Tolerant Bacillus megaterium Isolated From Semi-Arid Conditions Induces Systemic Tolerance of Wheat Under Drought Conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (Pgpr): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-Garcia, Y.E.; Quintero-Hernandez, V.; Munoz-Rojas, J. Next Generation of Microbial Inoculants for Agriculture and Bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Xie, S.; Vallet, M.; Sun, C.; Kunert, M.; David, A.; Zhang, X.; Chen, B.; Lu, X.; Boland, W.; Shao, Y. Biocontrol Potential of A Novel Endophytic Bacterium From Mulberry (Morus) Tree. Front. Bioeng. Biotechnol. 2019, 7, 488. [Google Scholar] [CrossRef]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, Pharmacology, and Clinical Trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Jiang, W.; Lin, Y.; Qian, L.; Miao, L.; Liu, B.; Ge, X.; Shen, H. Mulberry Leaf Meal: A Potential Feed Supplement for Juvenile Megalobrama amblycephala “Huahai No. 1”. Fish Shellfish. Immunol. 2022, 128, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Morus alba (Mulberry), A Natural Potent Compound in Management of Obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids Extracted From Mulberry (Morus alba L.) Leaf Improve Skeletal Muscle Mitochondrial Function by Activating Ampk in Type 2 Diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef]
- Maqsood, M.; Anam, S.R.; Sahar, A.; Khan, M.I. Mulberry Plant as A Source of Functional Food with Therapeutic and Nutritional Applications: A Review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef]
- Xu, W.; Wang, F.; Zhang, M.; Ou, T.; Wang, R.; Strobel, G.; Xiang, Z.; Zhou, Z.; Xie, J. Diversity of Cultivable Endophytic Bacteria in Mulberry and their Potential for Antimicrobial and Plant Growth-Promoting Activities. Microbiol. Res. 2019, 229, 126328. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.; Gao, H.; Jiang, K.; Yu, J.; Zhao, R.; Liu, X.; Zhou, Z.; Xiang, Z.; Xie, J. Endophytic Klebsiella aerogenes Hgg15 Stimulates Mulberry Growth in Hydro-Fluctuation Belt and the Potential Mechanisms as Revealed by Microbiome and Metabolomics. Front. Microbiol. 2022, 13, 978550. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, X.; Wu, X.; Liu, W.; Liu, J. Identification of Gonatophragmium Mori Causing Mulberry Zonate Leaf Spot Disease and Characterization of their Biological Enemies in Guangxi, China. Plant Dis. 2024, 108, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, W.; Zhang, M.; Qiu, C.; Liu, J.; Wisniewski, M.; Ou, T.; Zhou, Z.; Xiang, Z. The Impact of the Endophytic Bacterial Community On Mulberry Tree Growth in the Three Gorges Reservoir Ecosystem, China. Environ. Microbiol. 2021, 23, 1858–1875. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Qazi, I.H.; Yang, P.; Zhang, X.; Li, J.; Liu, J. Analysis of Endophytic Bacterial Flora of Mulberry Cultivars Susceptible and Resistant to Bacterial Wilt Using Metagenomic Sequencing and Culture-Dependent Approach. World J. Microbiol. Biotechnol. 2023, 39, 163. [Google Scholar] [CrossRef]
- Ou, T.; Xu, W.F.; Wang, F.; Strobel, G.; Zhou, Z.Y.; Xiang, Z.H.; Liu, J.; Xie, J. A Microbiome Study Reveals Seasonal Variation in Endophytic Bacteria Among Different Mulberry Cultivars. Comp. Struct. Biotechnol. J. 2019, 17, 1091–1100. [Google Scholar] [CrossRef]
- Yuan, T.; Qazi, I.H.; Li, J.; Yang, P.; Yang, H.; Zhang, X.; Liu, W.; Liu, J. Analysis of Changes in Bacterial Diversity in Healthy and Bacterial Wilt Mulberry Samples Using Metagenomic Sequencing and Culture-Dependent Approaches. Front. Plant Sci. 2023, 14, 1206691. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, A Widespread Bacterial Plant Pathogen in the Post-Genomic Era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Shu, Q.; Guo, X.; Tian, C.; Wang, Y.; Zhang, X.; Cheng, J.; Li, F.; Li, B. Homeostatic Regulation of the Duox-Ros Defense System: Revelations Based On the Diversity of Gut Bacteria in Silkworms (Bombyx mori). Int. J. Mol. Sci. 2023, 24, 12731. [Google Scholar] [CrossRef]
- Bredow, C.; Azevedo, J.L.; Pamphile, J.A.; Mangolin, C.A.; Rhoden, S.A. In Silico Analysis of the 16S Rrna Gene of Endophytic Bacteria, Isolated From the Aerial Parts and Seeds of Important Agricultural Crops. Genet. Mol. Res. 2015, 14, 9703–9721. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped Blast and Psi-Blast: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Dowarah, B.; Agarwal, H.; Krishnatreya, D.B.; Sharma, P.L.; Kalita, N.; Agarwala, N. Evaluation of Seed Associated Endophytic Bacteria From Tolerant Chilli Cv. Firingi Jolokia for their Biocontrol Potential Against Bacterial Wilt Disease. Microbiol. Res. 2021, 248, 126751. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Venter, S.N. Pantoea ananatis: An Unconventional Plant Pathogen. Mol. Plant Pathol. 2009, 10, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Huang, Y.; Luo, L.; Wang, J.; Li, J.; Chen, J.; Qin, Y.; Liu, J. Complete Genome Sequence of Pantoea ananatis Strain Lcfj-001 Isolated From Bacterial Wilt Mulberry. Plant Dis. 2023, 107, 2500–2505. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Lv, M.; Liao, L.; Chen, Y.; Gu, Y.; Liu, S.; Jiang, Z.; Xiong, Y.; Zhang, L. The Complete Genome Sequence of Dickeya zeae Ec1 Reveals Substantial Divergence From Other Dickeya Strains and Species. BMC Genom. 2015, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A Critical Examination of the Specificity of the Salkowski Reagent for Indolic Compounds Produced by Phytopathogenic Bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Fu, R.; Yu, F.; Chang, H.; Zhang, H.; Chen, W. Isolation, Identification and Characterization of an Antagonistic Bacterium Against Penicillium expansum. Microbiol. China 2016, 43, 1715–1724. [Google Scholar]
- Ran, J.J.; Xu, J.H.; Hu, X.D.; Shi, J.R. Identification of A Bacillus Strain Producing Lipopeptide and Cloning of Genes Related to Lipopeptide. Food Sci. 2016, 37, 127–132. [Google Scholar]
- Xiang, Y.; Zhou, H.; Liu, Y.; Chen, Z. Isolation and Identification of Lipopeptide Antibiotics Produced by Bacillus Amyloliquefaciens B1619 and the Inhibition of the Lipopeptide Antibiotics to Fusarium oxysporum F. Sp. Lycopersici. Sci. Agric. Sin. 2016, 49, 2935–2944. [Google Scholar]
- Yang, R.; Liu, P.; Wang, Z.; Ruan, B.; Wang, Z. Analysis of Antimicrobial Active Metabolites From Antagonistic Strains Against Fusarium solani. Biotechnol. Bull. 2022, 38, 57–66. [Google Scholar]
- Chen, Z.; Liu, B.; Shi, H.; Chen, M.; Chen, Q.; Wang, J.; Deng, W. Anaysis of the Functional Components of Fermentation Products of Bacillus Tequilensis by Lcq/Tof-Ms. Fujian Agric. Sci. Technol. 2021, 52, 19–25. [Google Scholar]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Nagrale, D.T.; Chaurasia, A.; Kumar, S.; Gawande, S.P.; Hiremani, N.S.; Shankar, R.; Gokte-Narkhedkar, N.; Renu; Prasad, Y.G. Pgpr: The Treasure of Multifarious Beneficial Microorganisms for Nutrient Mobilization, Pest Biocontrol and Plant Growth Promotion in Field Crops. World J. Microbiol. Biotechnol. 2023, 39, 100. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer-Shaanan, Y.; Jakoby, G.; Starr, M.L.; Karliner, R.; Eilon, G.; Itkin, M.; Malitsky, S.; Klein, T. A Dynamic Rhizosphere Interplay Between Tree Roots and Soil Bacteria Under Drought Stress. Elife 2022, 11, e79679. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of Flower Senescence by Bacterial Endophytes Expressing 1-Aminocyclopropane-1-Carboxylate Deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef]
- Coutinho, B.G.; Licastro, D.; Mendonca-Previato, L.; Camara, M.; Venturi, V. Plant-Influenced Gene Expression in the Rice Endophyte Burkholderia kururiensis M130. Mol. Plant Microbe Interact. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Shen, S.Y.; Fulthorpe, R. Seasonal Variation of Bacterial Endophytes in Urban Trees. Front. Microbiol. 2015, 6, 427. [Google Scholar] [CrossRef]
- Jardak, M.; Lami, R.; Saadaoui, O.; Jlidi, H.; Stien, D.; Aifa, S.; Mnif, S. Control of Staphylococcus epidermidis Biofilm by Surfactins of an Endophytic Bacterium Bacillus Sp. 15 F. Enzyme Microb. Technol. 2024, 180, 110477. [Google Scholar] [CrossRef] [PubMed]
- Sabino, Y.; de Araujo, D.K.; O’Connor, P.M.; Marques, P.H.; Santos, E.H.; Totola, M.R.; Abreu, L.M.; de Queiroz, M.V.; Cotter, P.D.; Mantovani, H.C. Bacillus velezensis Iturins Inhibit the Hemolytic Activity of Staphylococcus aureus. Sci. Rep. 2024, 14, 9469. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Kumari, A.; Singh, M.; Kumar, S.; Kumar, S.; Dabla, A.; Chaturvedi, S.; Yadav, V.; Chattopadhyay, D.; Prakash Dwivedi, V. [6]-Gingerol Exhibits Potent Anti-Mycobacterial and Immunomodulatory Activity Against Tuberculosis. Int. Immunopharmacol. 2020, 87, 106809. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, N.A.; Sagathevan, K.; Anwar, A.; Siddiqui, R. Biologically Active Metabolite(S) From Haemolymph of Red-Headed Centipede Scolopendra subspinipes Possess Broad Spectrum Antibacterial Activity. AMB Express 2019, 9, 95. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Antimicrobial Potential of A Lipopeptide Biosurfactant Derived From A Marine Bacillus circulans. J. Appl. Microbiol. 2008, 104, 1675–1684. [Google Scholar] [CrossRef]
- Sen, R. Surfactin: Biosynthesis, Genetics and Potential Applications. Adv. Exp. Med. Biol. 2010, 672, 316–323. [Google Scholar]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in Agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Zhen, C.; Ge, X.F.; Lu, Y.T.; Liu, W.Z. Chemical Structure, Properties and Potential Applications of Surfactin, as Well as Advanced Strategies for Improving its Microbial Production. AIMS Microbiol. 2023, 9, 195–217. [Google Scholar] [CrossRef]
- Moyne, A.L.; Shelby, R.; Cleveland, T.E.; Tuzun, S. Bacillomycin D: An Iturin with Antifungal Activity Against Aspergillus flavus. J. Appl. Microbiol. 2001, 90, 622–629. [Google Scholar] [CrossRef]
- Ines, M.; Dhouha, G. Lipopeptide Surfactants: Production, Recovery and Pore Forming Capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Fira, D.; Dimkic, I.; Beric, T.; Lozo, J.; Stankovic, S. Biological Control of Plant Pathogens by Bacillus Species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]

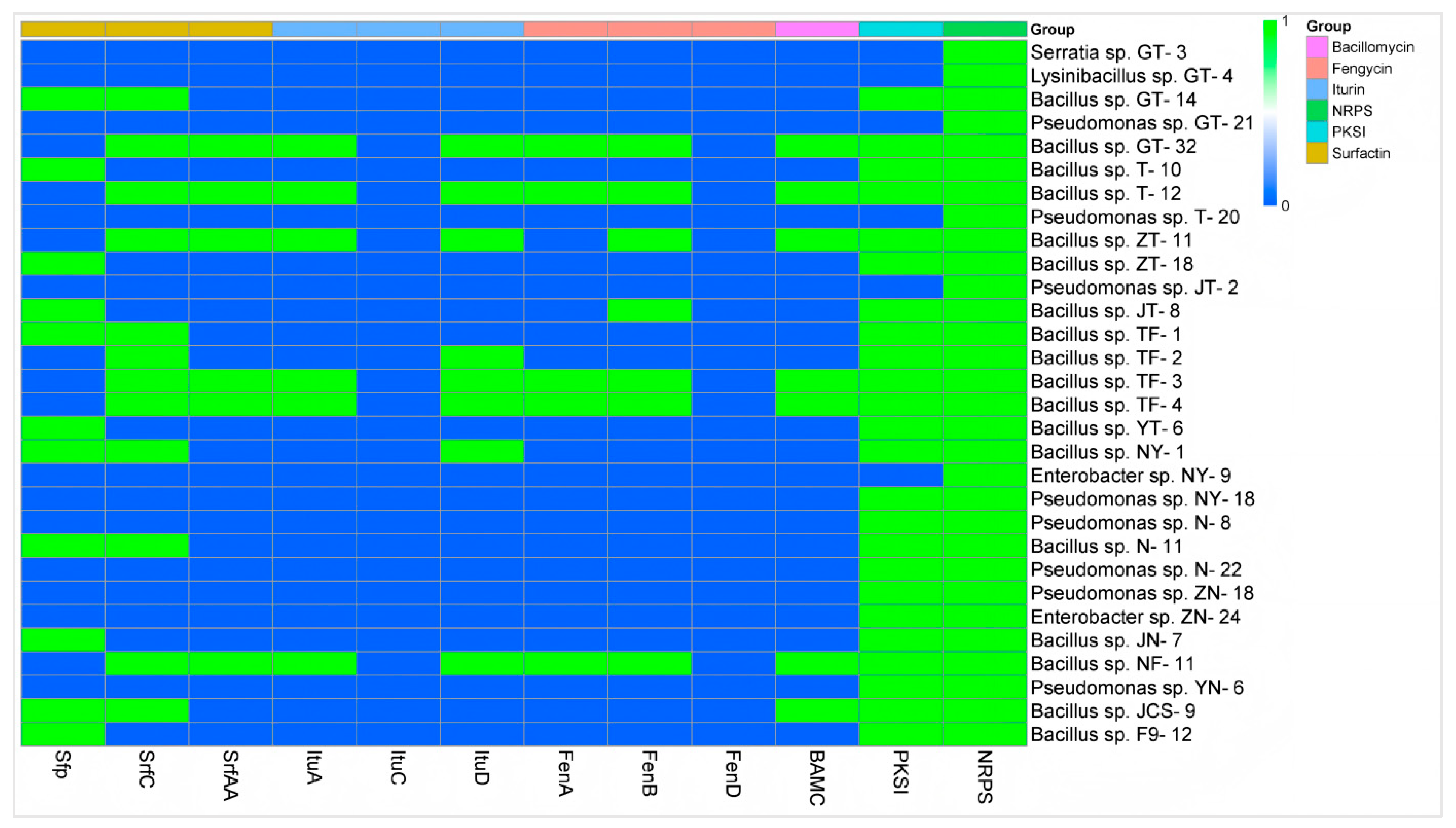
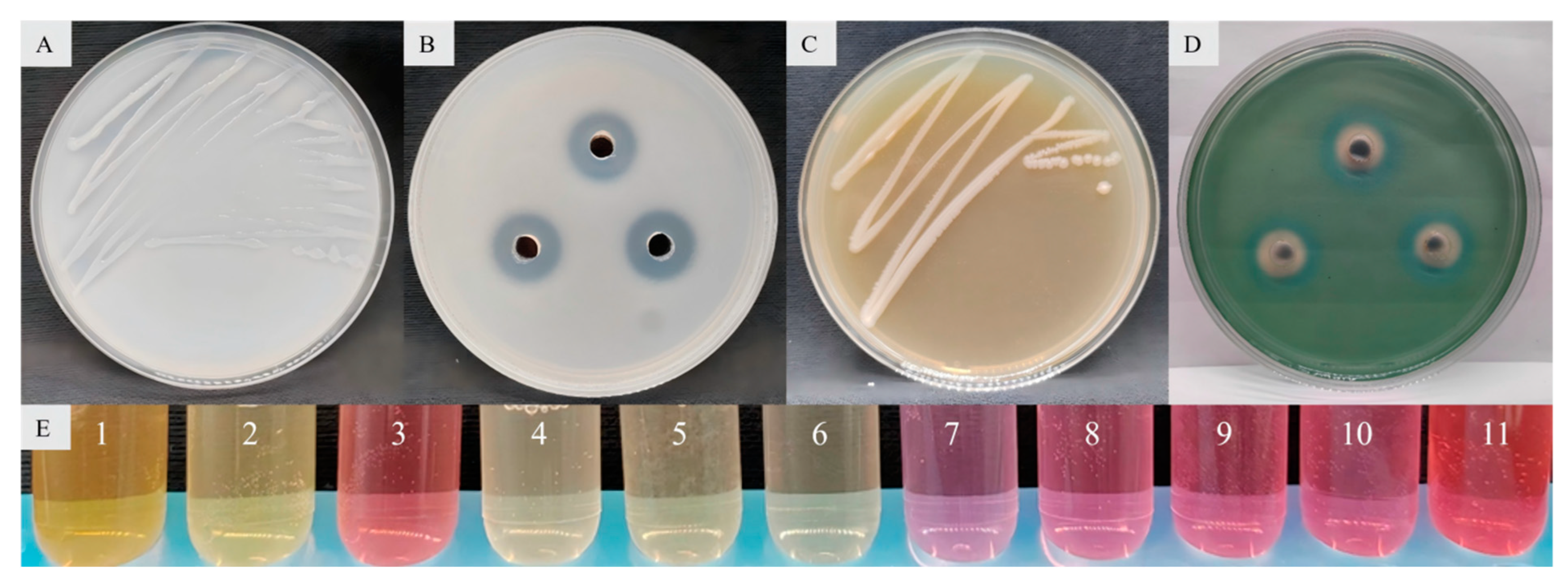

| No. | Strain | Inhibition Zone | ||
|---|---|---|---|---|
| R. solanacearum | P. ananatis | D. zeae | ||
| 1 | Serratia sp. GT-3 | 9.33 ± 0.58 | - | - |
| 2 | Lysinibacillus sp. GT-4 | 6.33 ± 0.58 | - | - |
| 3 | Bacillus sp. GT-14 | 13.00 ± 0.00 | 8.67 ± 0.58 | 11.00 ± 0.00 |
| 4 | Pseudomonas sp. GT-21 | 8.67 ± 0.58 | - | - |
| 5 | Bacillus sp. GT-32 | 14.00 ± 1.00 | 10.67 ± 0.58 | 11.33 ± 0.58 |
| 6 | Bacillus sp. T-10 | 10.00 ± 1.00 | 8.33 ± 0.58 | 9.67 ± 0.58 |
| 7 | Bacillus sp. T-12 | 13.33 ± 0.58 | 10.00 ± 1.00 | 11.00 ± 0.00 |
| 8 | Pseudomonas sp. T-20 | 10.67 ± 0.58 | 8.00 ± 0.00 | 9.00 ± 0.00 |
| 9 | Bacillus sp. ZT-11 | 14.67 ± 0.58 | 12.00 ± 1.00 | 12.67 ± 0.58 |
| 10 | Bacillus sp. ZT-18 | 9.00 ± 0.00 | - | 8.00 ± 0.00 |
| 11 | Pseudomonas sp. JT-2 | 9.00 ± 1.00 | - | - |
| 12 | Bacillus sp. JT-8 | 10.67 ± 0.58 | 7.33 ± 0.58 | 10.00 ± 0.00 |
| 13 | Bacillus sp. TF-1 | 14.33 ± 0.58 | 11.67 ± 0.58 | 12.33 ± 1.15 |
| 14 | Bacillus sp. TF-2 | 15.67 ± 0.58 | - | - |
| 15 | Bacillus sp. TF-3 | 11.00 ± 0.00 | 8.33 ± 0.58 | 10.67 ± 0.58 |
| 16 | Bacillus sp. TF-4 | 14.33 ± 1.15 | 11.33 ± 0.58 | 12.00 ± 1.00 |
| 17 | Bacillus sp. YT-6 | 11.00 ± 0.00 | 9.00 ± 0.00 | 10.00 ± 0.00 |
| 18 | Bacillus sp. NY-1 | 15.00 ± 0.00 | 13.67 ± 1.15 | 15.33 ± 0.58 |
| 19 | Enterobacter sp. NY-9 | 8.67 ± 0.58 | - | - |
| 20 | Pseudomonas sp. NY-18 | 13.00 ± 0.00 | 10.67 ± 0.58 | 12.00 ± 1.00 |
| 21 | Pseudomonas sp. N-8 | 9.33 ± 0.58 | - | 9.00 ± 0.00 |
| 22 | Bacillus sp. N-11 | 13.33 ± 1.15 | 10.33 ± 0.58 | 12.33 ± 0.58 |
| 23 | Pseudomonas sp. N-22 | 13.33 ± 0.58 | 10.00 ± 1.00 | 12.67 ± 0.58 |
| 24 | Pseudomonas sp. ZN-18 | 12.67 ± 1.15 | 9.00 ± 0.00 | 12.00 ± 0.00 |
| 25 | Enterobacter sp. ZN-24 | 7.33 ± 0.58 | - | - |
| 26 | Bacillus sp. JN-7 | 12.00 ± 1.00 | 9.67 ± 0.58 | 11.67 ± 0.58 |
| 27 | Bacillus sp. NF-11 | 13.33 ± 0.58 | 10.67 ± 0.58 | 12.33 ± 1.15 |
| 28 | Pseudomonas sp. YN-6 | 11.33 ± 0.58 | 8.67 ± 0.58 | 11.00 ± 0.00 |
| 29 | Bacillus sp. JCS-9 | 10.00 ± 0.00 | 7.33 ± 0.58 | 8.67 ± 0.58 |
| 30 | Bacillus sp. F9-12 | 8.33 ± 1.15 | - | - |
| No. | Strain | PGP Properties | ||||
|---|---|---|---|---|---|---|
| NF | PB | PR | SP | IAA | ||
| 1 | Serratia sp. GT-3 | + | 9.67 ± 1.15 | + | 12.33 ± 0.58 | + |
| 2 | Lysinibacillus sp. GT-4 | + | − | − | 7.00 ± 0.00 | + |
| 3 | Bacillus sp. GT-14 | − | − | − | 8.67 ± 0.58 | − |
| 4 | Pseudomonas sp. GT-21 | − | − | − | 14.33 ± 1.15 | − |
| 5 | Bacillus sp. GT-32 | − | − | + | − | − |
| 6 | Bacillus sp. T-10 | − | 7.67 ± 0.58 | − | − | − |
| 7 | Bacillus sp. T-12 | − | − | + | 8.00 ± 0.00 | − |
| 8 | Pseudomonas sp. T-20 | − | 8.33 ± 1.15 | + | 11.00 ± 0.00 | + |
| 9 | Bacillus sp. ZT-11 | − | 7.33 ± 0.58 | + | − | − |
| 10 | Bacillus sp. ZT-18 | − | 7.00 ± 0.00 | − | − | − |
| 11 | Pseudomonas sp. JT-2 | − | 8.00 ± 1.00 | + | 12.00 ± 0.00 | + |
| 12 | Bacillus sp. JT-8 | − | 6.67 ± 0.58 | + | 8.33 ± 0.58 | − |
| 13 | Bacillus sp. TF-1 | − | − | + | − | − |
| 14 | Bacillus sp. TF-2 | − | 7.33 ± 0.58 | + | 7.33 ± 0.58 | − |
| 15 | Bacillus sp. TF-3 | − | − | + | − | − |
| 16 | Bacillus sp. TF-4 | − | − | + | − | + |
| 17 | Bacillus sp. YT-6 | − | − | + | − | + |
| 18 | Bacillus sp. NY-1 | − | − | + | − | − |
| 19 | Enterobacter sp. NY-9 | + | 11.67 ± 1.15 | + | 12.00 ± 1.00 | + |
| 20 | Pseudomonas sp. NY-18 | − | 11.33 ± 0.58 | − | − | − |
| 21 | Pseudomonas sp. N-8 | − | 9.67 ± 1.15 | − | − | − |
| 22 | Bacillus sp. N-11 | − | 7.33 ± 0.58 | − | − | − |
| 23 | Pseudomonas sp. N-22 | − | 8.33 ± 0.58 | + | 9.33 ± 1.15 | − |
| 24 | Pseudomonas sp. ZN-18 | − | 8.00 ± 1.00 | − | 12.00 ± 1.00 | − |
| 25 | Enterobacter sp. ZN-24 | + | 10.67 ± 0.58 | − | − | + |
| 26 | Bacillus sp. JN-7 | − | − | + | 7.00 ± 0.00 | − |
| 27 | Bacillus sp. NF-11 | − | − | + | 8.33 ± 0.58 | − |
| 28 | Pseudomonas sp. YN-6 | − | 8.33 ± 0.58 | + | 9.67 ± 1.15 | − |
| 29 | Bacillus sp. JCS-9 | − | − | + | 8.67 ± 0.58 | − |
| 30 | Bacillus sp. F9-12 | − | − | − | 10.33 ± 0.58 | + |
| Isolate | Best NCBI Database Match with Accession Number | % Identity | GenBank Accession Number of Submitted Sequence |
|---|---|---|---|
| TF-2 | Bacillus licheniformis strain CO-12 (OL354425.1) | 99.54% | PP596549.1 |
| NY-1 | Bacillus subtilis strain K21 (JN587510.1) | 99.74% | MT669326 |
| JCS-3 | Enterobacter hormaechei strain C44 (CP042566.1) | 99.67% | PP440228.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yuan, T.; Wang, M.; Liu, J. Analysis of the Changes in Diversity of Culturable Bacteria in Different Niches of Mulberry Fields and Assessment of Their Plant Growth-Promoting Potential. Microorganisms 2025, 13, 1012. https://doi.org/10.3390/microorganisms13051012
Liu W, Yuan T, Wang M, Liu J. Analysis of the Changes in Diversity of Culturable Bacteria in Different Niches of Mulberry Fields and Assessment of Their Plant Growth-Promoting Potential. Microorganisms. 2025; 13(5):1012. https://doi.org/10.3390/microorganisms13051012
Chicago/Turabian StyleLiu, Weifu, Ting Yuan, Mengya Wang, and Jiping Liu. 2025. "Analysis of the Changes in Diversity of Culturable Bacteria in Different Niches of Mulberry Fields and Assessment of Their Plant Growth-Promoting Potential" Microorganisms 13, no. 5: 1012. https://doi.org/10.3390/microorganisms13051012
APA StyleLiu, W., Yuan, T., Wang, M., & Liu, J. (2025). Analysis of the Changes in Diversity of Culturable Bacteria in Different Niches of Mulberry Fields and Assessment of Their Plant Growth-Promoting Potential. Microorganisms, 13(5), 1012. https://doi.org/10.3390/microorganisms13051012








