Microbial Contamination on High-Touch Surfaces in Outpatient Clinics: Identification of Bacterial Strains from Areas of Patient and Medical Staff Occupancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Study Design
2.3. Microbiological Analysis
2.4. Ethical Considerations
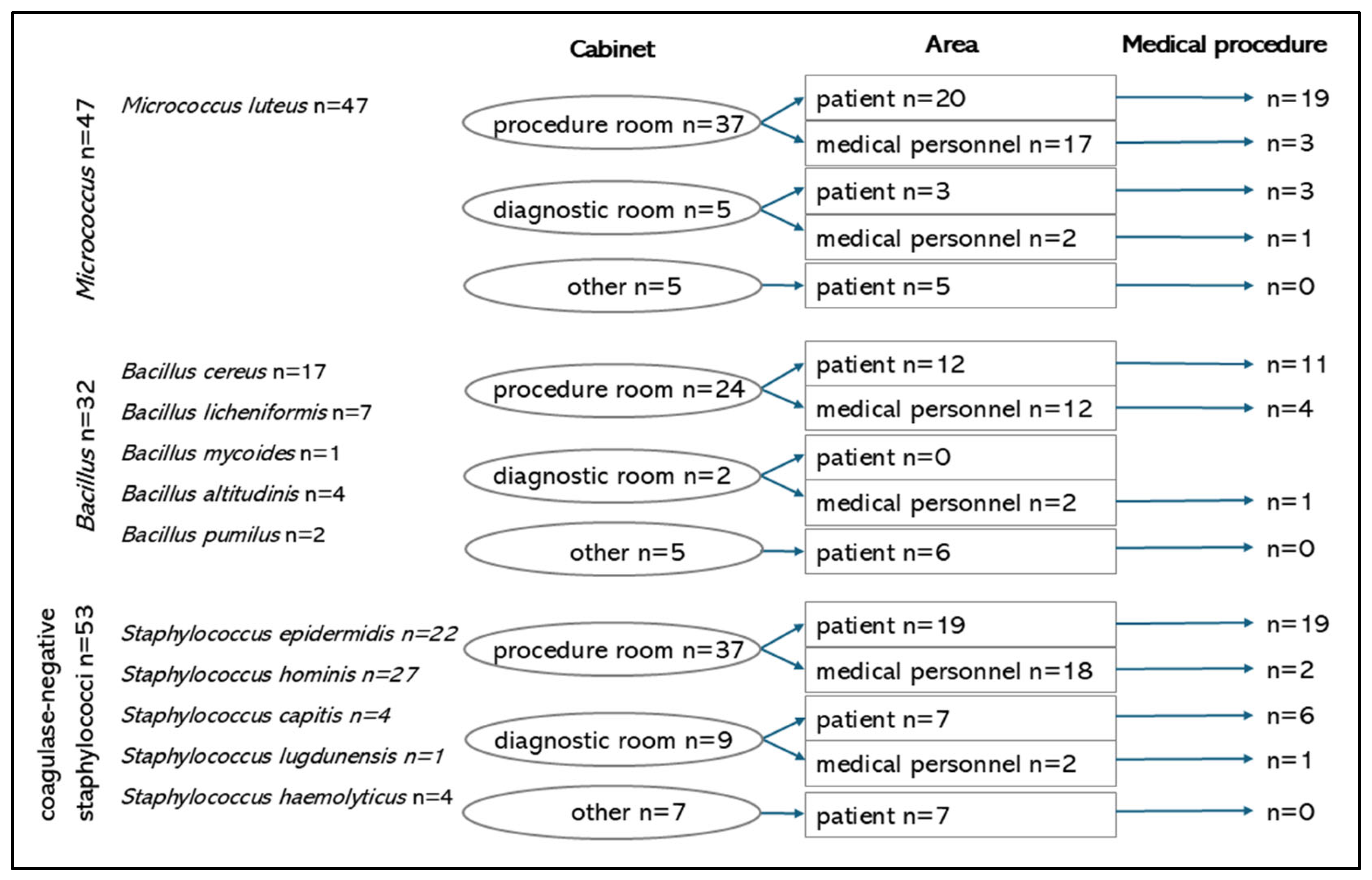
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAIs I | Hospital-acquired infections |
References
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Heredia, E.J.; Chemaly, R.F. Update on infection control practices in cancer hospitals. CA Cancer J. Clin. 2018, 68, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Wolfensberger, A.; Clack, L.; Kuster, S.P.; Passerini, S.; Mody, L.; Chopra, V.; Mann, J.; Sax, H. Transfer of pathogens to and from patients, healthcare providers, and medical devices during care activity-a systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Różańska, A.; Romaniszyn, D.; Chmielarczyk, A.; Bulanda, M. Bacteria contamination of touch surfaces in Polish hospital wards. Med. Pr. 2017, 68, 459–467. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Healthcare Infection Control Practices Advisory Committee (HICPAC). In Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Gauvry, E.; Mathot, A.-G.; Leguérinel, I.; Couvert, O.; Postollec, F.; Broussolle, V.; Coroller, L. Knowledge of the physiology of spore-forming bacteria can explain the origin of spores in the food environment. Res. Microbiol. 2017, 168, 369–378. [Google Scholar] [CrossRef]
- Wałaszek, M.; Kołpa, M.; Wolak, Z.; Różańska, A.; Wójkowska-Mach, J. Poor Hand Hygiene Procedure Compliance among Polish Medical Students and Physicians—The Result of an Ineffective Education Basis or the Impact of Organizational Culture? Int. J. Environ. Res. Public Health 2017, 14, 1026. [Google Scholar] [CrossRef]
- Robakowska, M.; Bronk, M.; Tyrańska-Fobke, A.; Ślęzak, D.; Kraszewski, J.; Balwicki, Ł. Patient Safety Related to Microbiological Contamination of the Environment of a Multi-Profile Clinical Hospital. Int. J. Environ. Res. Public Health 2021, 18, 3844. [Google Scholar] [CrossRef]
- Różańska, A.; Walkowicz, M.; Bulanda, M.; Kasperski, T.; Synowiec, E.; Osuch, P.; Chmielarczyk, A. Evaluation of the Efficacy of UV-C Radiation in Eliminating Microorganisms of Special Epidemiological Importance from Touch Surfaces under Laboratory Conditions and in the Hospital Environment. Healthcare 2023, 11, 3096. [Google Scholar] [CrossRef]
- Gruszecka, J.; Gutkowska, D.; Filip, R. Microbiological assessment of cleanliness of surfaces and equipment in a children’s operating theatre on the example of a selected hospital. Ann. Agric. Environ. Med. 2019, 26, 249–251. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/ESAC-Net_report-2023.pdf (accessed on 12 January 2025).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Clostridioides (Clostridium) Difficile Infections. Annual Epidemiological Report for 2016–2017; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/clostridioides-clostridium-difficile-infections.pdf (accessed on 12 January 2025).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-annual-epidemiological-report-EARS-Net-2023.pdf (accessed on 12 January 2025).
- Facciolà, A.; Pellicanò, G.F.; Visalli, G.; Paolucci, I.A.; Venanzi Rullo, E.; Ceccarelli, M.; D’Aleo, F.; Di Pietro, A.; Squeri, R.; Nunnari, G.; et al. The role of the hospital environment in the healthcare-associated infections: A general review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1266–1278. [Google Scholar] [CrossRef]
- WHO. Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2009; Available online: https://www.who.int/publications/i/item/9789241597906 (accessed on 11 November 2024).
- Reynolds, K.A.; Sexton, J.D.; Pivo, T.; Humphrey, K.; Leslie, R.A.; Gerba, C.P. Microbial transmission in an outpatient clinic and impact of an intervention with an ethanol-based disinfectant. Am. J. Infect. Control 2019, 47, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, S.Y.; Neilson, R.; An, X.L.; Su, J.Q. Skin microbiota interact with microbes on office surfaces. Environ. Int. 2022, 168, 107493. [Google Scholar] [CrossRef]
- Peters, A.; Otter, J.; Moldovan, A.; Parneix, P.; Voss, A.; Pittet, D. Keeping hospitals clean and safe without breaking the bank; summary of the Healthcare Cleaning Forum 2018. Antimicrob. Resist. Infect. Control. 2018, 7, 132. [Google Scholar] [CrossRef]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins—The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Veysseyre, F.; Fourcade, C.; Lavigne, J.P.; Sotto, A. Bacillus cereus infection: 57 case patients and a literature review. Med. Mal. Infect. 2015, 45, 436–440. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Assadian, O.; Harbarth, S.; Vos, M.; Knobloch, J.K.; Asensio, A.; Widmer, A.F. Practical recommendations for routine cleaning and disinfection procedures in healthcare institutions: A narrative review. J. Hosp. Infect. 2021, 113, 104–114. [Google Scholar] [CrossRef]
- Rozman, U.; Pušnik, M.; Kmetec, S.; Duh, D.; Šostar Turk, S. Reduced Susceptibility and Increased Resistance of Bacteria against Disinfectants: A Systematic Review. Microorganisms 2021, 9, 2550. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, N.; Peters, A.; Pittet, D. Hand hygiene and the novel coronavirus pandemic: The role of healthcare workers. J. Hosp. Infect. 2020, 105, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Robbins, G.; Quinn, J.; Arbogast, J.W. The impact of COVID-19 pandemic on hand hygiene performance in hospitals. Am. J. Infect. Control 2021, 49, 30–33. [Google Scholar] [CrossRef] [PubMed]
| Category | Place | Number (%) |
|---|---|---|
| City | Cracow | 53 (62.4%) |
| Warsaw | 32 (37.6%) | |
| Disinfection | Cleaning company | 23 (27.1%) |
| Medical personnel | 62 (72.9%) | |
| Room | Procedure room | 60 (70.6%) |
| Diagnostic room | 11 (12.9%) | |
| other | 14 (16.5%) | |
| Area | Patient | 53 (62.4%) |
| Medical personnel | 32 (37.6%) | |
| Procedures involving the breach of skin integrity | Yes | 12 (14.1%) |
| No | 73 (85.9%) | |
| Type of procedure | Medical | 45 (52.9%) |
| Non-medical | 40 (47.1%) | |
| Microbiological environmental cleanliness | None + scarce | 55 (64.7%) |
| Abundant | 30 (35.3%) | |
| Growth | None | 5 (5.9%) |
| Scarce | 50 (58.8%) | |
| Moderate | 13 (15.3%) | |
| Abundant | 17 (20.0%) | |
| Origin * | Procedure room | 60 (70.6%) |
| Diagnostic room | 11 (12.9%) | |
| Other | 14 (16.5%) |
| Category | Place | Microbiota (n = 44) | Others (n = 36) | p | Spore- Forming (n = 29) | Non-Spore- Forming (n = 56) | p |
|---|---|---|---|---|---|---|---|
| City | Cracow | 30 (68.2%) | 18 (50%) | 0.099 | 18 (62.1%) | 35 (62.5%) | 0.969 |
| Warsaw | 14 (31.8%) | 18 (50%) | 11 (37.9%) | 21 (37.5%) | |||
| Disinfection | Cleaning company | 31 (70.5%) | 28 (77.8%) | 0.459 | 11 (37.9%) | 12 (21.4%) | 0.104 |
| Medical personnel | 13 (29.5%) | 8 (22.2%) | 18 (62.1%) | 44 (78.6%) | |||
| Room | Procedure room | 33 (75%) | 24 (69.7%) | 0.409 | 21 (72.4%) | 39 (69.6%) | 0.419 |
| Diagnostic room | 4 (9.1%) | 7 (19.4%) | 2 (6.9%) | 9 (16.1%) | |||
| Other | 7 (15.9%) | 5 (13.9%) | 6 (20.7%) | 8 (14.3%) | |||
| Area | Patient | 28 (63.6%) | 23 (63.9%) | 0.981 | 17 (58.6%) | 36 (64.3%) | 0.610 |
| Medical personnel | 16 (36.4%) | 13 (36.1%) | 12 (41.4%) | 20 (35.7%) | |||
| Procedures involving the breach of skin integrity | Yes | 7 (15.9%) | 3 (8.3%) | 0.497 | 4 (13.8%) | 8 (14.3%) | 0.790 |
| No | 37 (84.1%) | 33 (91.7%) | 25 (86.2%) | 48 (85.7%) | |||
| Type of procedure | Medical | 25 (56.8%) | 18 (50%) | 0.702 | 14 (48.3%) | 31 (55.4%) | 0.535 |
| Non-medical | 19 (43.2%) | 18 (50%) | 15 (51.7%) | 25 (44.6%) | |||
| Growth | No + scarce | 21 (47.7%) | 29 (80.6%) | 0.003 * | 12 (41.4%) | 43 (76.8%) | 0.001 * |
| Moderate + abundant | 23 (52.3%) | 7 (19.4%) | 17 (58.6%) | 13 (23.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasek, K.; Kiersnowska, I.; Wójkowska-Mach, J.; Różańska, A.; Romaniszyn, D.; Foryciarz, E.; Kwiećkowska, L.B.; Krzych-Fałta, E. Microbial Contamination on High-Touch Surfaces in Outpatient Clinics: Identification of Bacterial Strains from Areas of Patient and Medical Staff Occupancy. Microorganisms 2025, 13, 698. https://doi.org/10.3390/microorganisms13030698
Prasek K, Kiersnowska I, Wójkowska-Mach J, Różańska A, Romaniszyn D, Foryciarz E, Kwiećkowska LB, Krzych-Fałta E. Microbial Contamination on High-Touch Surfaces in Outpatient Clinics: Identification of Bacterial Strains from Areas of Patient and Medical Staff Occupancy. Microorganisms. 2025; 13(3):698. https://doi.org/10.3390/microorganisms13030698
Chicago/Turabian StylePrasek, Karolina, Iwona Kiersnowska, Jadwiga Wójkowska-Mach, Anna Różańska, Dorota Romaniszyn, Ewelina Foryciarz, Lucyna Barbara Kwiećkowska, and Edyta Krzych-Fałta. 2025. "Microbial Contamination on High-Touch Surfaces in Outpatient Clinics: Identification of Bacterial Strains from Areas of Patient and Medical Staff Occupancy" Microorganisms 13, no. 3: 698. https://doi.org/10.3390/microorganisms13030698
APA StylePrasek, K., Kiersnowska, I., Wójkowska-Mach, J., Różańska, A., Romaniszyn, D., Foryciarz, E., Kwiećkowska, L. B., & Krzych-Fałta, E. (2025). Microbial Contamination on High-Touch Surfaces in Outpatient Clinics: Identification of Bacterial Strains from Areas of Patient and Medical Staff Occupancy. Microorganisms, 13(3), 698. https://doi.org/10.3390/microorganisms13030698








