Virulence Is More than Adhesion and Invasion Ability, an In Vitro Cell Infection Assay of Bovine Mycoplasma spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media, and Culture
2.2. Culture Conditions of MDBK Cells
2.3. Cell Infection and Gentamicin Protection Assay
2.4. Assessment of Mycoplasmas Survival in Cell Lysates by Culture
2.5. DNA Extraction, Target Genes, and Real-Time PCR (qPCR) Conditions
2.6. Calculation of the Ratio of Mycoplasma spp. to MDBK Cells
2.7. Confocal and Scanning Electron Microscopy Analysis
2.8. Impact of the Variation of Infection Conditions
3. Results
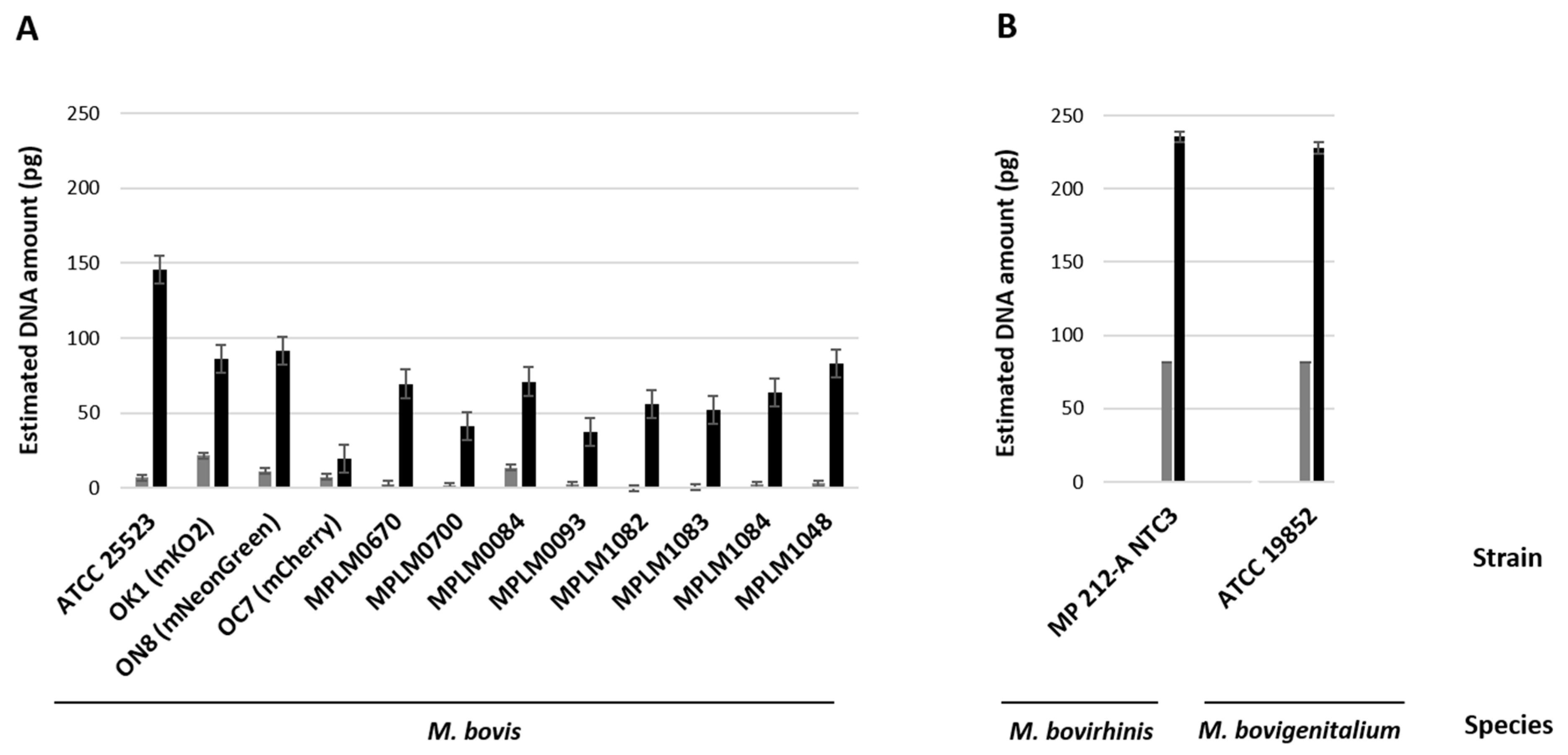
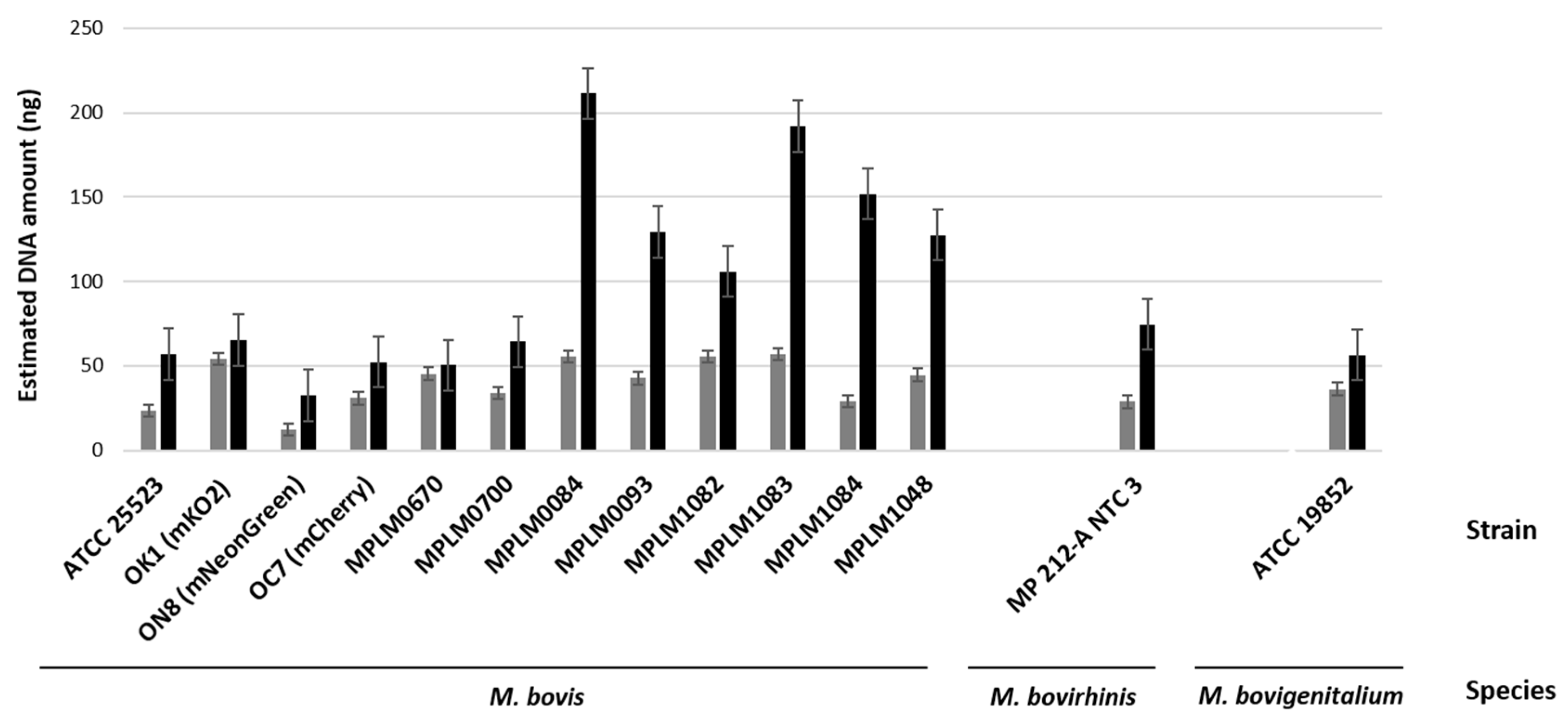
3.1. Biological and Molecular Evidence of Replication of Bovine Mycoplasma spp. Within MDBK Cells
3.2. Myc/Cell Ratios
3.3. Microscopy Studies
3.4. Variation of Myc/Cell Ratios According to Infection Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef] [PubMed]
- Domingue, G.J., Sr.; Woody, H.B. Bacterial persistence and expression of disease. Clin. Microbiol. Rev. 1997, 10, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Caswell, J.L.; Archambault, M. Mycoplasma bovis pneumonia in cattle. Anim. Health Res. Rev. 2007, 8, 161–186. [Google Scholar] [CrossRef]
- González, R.N.; Wilson, D.J. Mycoplasmal mastitis in dairy herds. Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, H.; Sachse, K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev. Sci. Tech. 1996, 15, 1477–1494. [Google Scholar] [CrossRef]
- Walz, P.H.; Mullaney, T.P.; Render, J.A.; Walker, R.D.; Mosser, T.; Baker, J.C. Otitis media in preweaned Holstein dairy calves in Michigan due to Mycoplasma bovis. J. Vet. Diagn. Investig. 1997, 9, 250–254. [Google Scholar] [CrossRef]
- Bürki, S.; Frey, J.; Pilo, P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 2015, 179, 15–22. [Google Scholar] [CrossRef]
- Fox, L.K. Mycoplasma mastitis: Causes, transmission, and control. Vet. Clin. N. Am. Food. Anim. Pract. 2012, 28, 225–237. [Google Scholar] [CrossRef]
- Nicholas, R.A. Bovine mycoplasmosis: Silent and deadly. Vet. Rec. 2011, 168, 459–462. [Google Scholar] [CrossRef]
- ter Laak, E.A.; Noordergraaf, J.H.; Dieltjes, R.P. Prevalence of mycoplasmas in the respiratory tracts of pneumonic calves. Zentralbl. Veterinarmed. B 1992, 39, 553–562. [Google Scholar] [CrossRef]
- Thomas, A.; Ball, H.; Dizier, I.; Trolin, A.; Bell, C.; Mainil, J.; Linden, A. Isolation of Mycoplasma species from the lower respiratory tract of healthy cattle and cattle with respiratory disease in Belgium. Vet. Rec. 2002, 151, 472–476. [Google Scholar] [CrossRef]
- Centeno-Martinez, R.E.; Glidden, N.; Mohan, S.; Davidson, J.L.; Fernández-Juricic, E.; Boerman, J.P.; Schoonmaker, J.; Pillai, D.; Koziol, J.; Ault, A.; et al. Identification of bovine respiratory disease through the nasal microbiome. Anim. Microbiome 2022, 4, 15. [Google Scholar] [CrossRef]
- Chen, S.; Hao, H.; Zhao, P.; Liu, Y.; Chu, Y. Genome-Wide Analysis of Mycoplasma bovirhinis GS01 Reveals Potential Virulence Factors and Phylogenetic Relationships. G3 (Bethesda) 2018, 8, 1417–1424. [Google Scholar] [CrossRef]
- McDaneld, T.G.; Workman, A.M.; Chitko-McKown, C.G.; Kuehn, L.A.; Dickey, A.; Bennett, G.L. Detection of Mycoplasma bovirhinis and bovine coronavirus in an outbreak of bovine respiratory disease in nursing beef calves. Front. Microbiomes Sec. Environ. Microbiomes 2022, 1, 1051241. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Higuchi, H.; Tezuka, E.; Ito, H.; Devkota, B.; Izaike, Y.; Osawa, T. Mycoplasma infection in the uterus of early postpartum dairy cows and its relation to dystocia and endometritis. Theriogenology 2013, 79, 180–185. [Google Scholar] [CrossRef]
- Cornelissen, J.B.W.J.; de Bree, F.M.; van der Wal, F.J.; Kooi, E.A.; Koene, M.G.J.; Bossers, A.; Smid, B.; Antonis, A.F.; Wisselink, H.J. Mycoplasma detection by triplex real-time PCR in bronchoalveolar lavage fluid from bovine respiratory disease complex cases. BMC Vet. Res. 2017, 13, 97. [Google Scholar] [CrossRef]
- McAuliffe, L.; Ellis, R.J.; Miles, K.; Ayling, R.D.; Nicholas, R.A. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 2006, 152, 913–922. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Qi, J.; Zhu, X.; Chenfei, H.; Menghwar, H.; Khan, F.A.; Zhao, G.; Zubair, M.; Hu, C.; Chen, Y.; et al. Comparative Genomics of Mycoplasma bovis Strains Reveals That Decreased Virulence with Increasing Passages Might Correlate with Potential Virulence-Related Factors. Front. Cell. Infect. Microbiol. 2017, 7, 177. [Google Scholar] [CrossRef]
- Calcutt, M.J.; Lysnyansky, I.; Sachse, K.; Fox, L.K.; Nicholas, R.A.J.; Ayling, R.D. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound. Emerg. Dis. 2018, 65, 91–109. [Google Scholar] [CrossRef]
- Josi, C.; Bürki, S.; Vidal, S.; Dordet-Frisoni, E.; Citti, C.; Falquet, L.; Pilo, P. Large-Scale Analysis of the Mycoplasma bovis Genome Identified Non-essential, Adhesion- and Virulence-Related Genes. Front. Microbiol. 2019, 10, 2085. [Google Scholar] [CrossRef]
- Manso-Silván, L.; Tardy, F.; Baranowski, E.; Barré, A.; Blanchard, A.; Breton, M.; Couture, C.; Citti, C.; Dordet-Frisoni, E.; Dupuy, V.; et al. Draft genome sequences of Mycoplasma alkalescens, Mycoplasma arginini, and Mycoplasma bovigenitalium, three species with equivocal pathogenic status for cattle. Genome Announc. 2013, 1, e00348-13. [Google Scholar] [CrossRef]
- Hata, E.; Nagai, K.; Murakami, K. Complete Genome Sequence of Mycoplasma bovigenitalium Strain HAZ 596 from a Bovine Vagina in Japan. Genome Announc. 2017, 5, e01554-16. [Google Scholar] [CrossRef]
- Bonnefois, T.; Vernerey, M.S.; Rodrigues, V.; Totté, P.; Puech, C.; Ripoll, C.; Thiaucourt, F.; Manso-Silván, L. Development of fluorescence expression tools to study host-mycoplasma interactions and validation in two distant mycoplasma clades. J. Biotechnol. 2016, 236, 35–44. [Google Scholar] [CrossRef]
- Vanden Bush, T.J.; Rosenbusch, R.F. Mycoplasma bovis induces apoptosis of bovine lymphocytes. FEMS Immunol. Med. Microbiol. 2002, 32, 97–103. [Google Scholar] [CrossRef]
- Thomas, A.; Sachse, K.; Farnir, F.; Dizier, I.; Mainil, J.; Linden, A. Adherence of Mycoplasma bovis to bovine bronchial epithelial cells. Microb. Pathog. 2003, 34, 141–148. [Google Scholar] [CrossRef]
- Lu, X.; Rosenbusch, R.F. Endothelial cells from bovine pulmonary microvasculature respond to Mycoplasma bovis preferentially with signals for mononuclear cell transmigration. Microb. Pathog. 2004, 37, 253–261. [Google Scholar] [CrossRef]
- Mulongo, M.; Prysliak, T.; Scruten, E.; Napper, S.; Perez-Casal, J. In vitro infection of bovine monocytes with Mycoplasma bovis delays apoptosis and suppresses production of gamma interferon and tumor necrosis factor alpha but not interleukin-10. Infect. Immun. 2014, 82, 62–71. [Google Scholar] [CrossRef]
- Gondaira, S.; Higuchi, H.; Iwano, H.; Nakajima, K.; Kawai, K.; Hashiguchi, S.; Konnai, S.; Nagahata, H. Cytokine mRNA profiling and the proliferative response of bovine peripheral blood mononuclear cells to Mycoplasma bovis. Vet. Immunol. Immunopathol. 2015, 165, 45–53. [Google Scholar] [CrossRef]
- Suleman, M.; Prysliak, T.; Clarke, K.; Burrage, P.; Windeyer, C.; Perez-Casal, J. Mycoplasma bovis isolates recovered from cattle and bison (Bison bison) show differential in vitro effects on PBMC proliferation, alveolar macrophage apoptosis and invasion of epithelial and immune cells. Vet. Microbiol. 2016, 186, 28–36. [Google Scholar] [CrossRef]
- Jimbo, S.; Suleman, M.; Maina, T.; Prysliak, T.; Mulongo, M.; Perez-Casal, J. Effect of Mycoplasma bovis on bovine neutrophils. Vet. Immunol. Immunopathol. 2017, 188, 27–33. [Google Scholar] [CrossRef]
- van der Merwe, J.; Prysliak, T.; Perez-Casal, J. Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect. Immun. 2010, 78, 4570–4578. [Google Scholar] [CrossRef]
- Bürgi, N.; Josi, C.; Burki, S.; Schweizer, M.; Pilo, P. Mycoplasma bovis co-infection with bovine viral diarrhea virus in bovine macrophages. Vet. Res. 2018, 49, 2. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, S.; Chao, J.; Lu, D.; Zhao, G.; Chen, Y.; Chen, H.; Faisal, M.; Yang, L.; Hu, C.; et al. The attenuated Mycoplasma bovis strain promotes apoptosis of bovine macrophages by upregulation of CHOP expression. Front. Microbiol. 2022, 13, 925209. [Google Scholar] [CrossRef]
- Bürki, S.; Gaschen, V.; Stoffel, M.H.; Stojiljkovic, A.; Frey, J.; Kuehni-Boghenbor, K.; Pilo, P. Invasion and persistence of Mycoplasma bovis in embryonic calf turbinate cells. Vet. Res. 2015, 46, 53. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Z.; Xu, S.; Liu, G.; Lin, Y.; Khan, S.; Gao, J.; Qu, W.; Kastelic, J.P.; Han, B. Mycoplasma bovis subverts autophagy to promote intracellular replication in bovine mammary epithelial cells cultured in vitro. Vet. Res. 2021, 52, 130. [Google Scholar] [CrossRef]
- Josi, C.; Bürki, S.; Stojiljkovic, A.; Wellnitz, O.; Stoffel, M.H.; Pilo, P. Bovine Epithelial in vitro Infection Models for Mycoplasma bovis. Front. Cell. Infect. Microbiol. 2018, 8, 329. [Google Scholar] [CrossRef]
- Volokhov, D.V.; Kong, H.; George, J.; Anderson, C.; Chizhikov, V.E. Biological Enrichment of Mycoplasma Agents by Cocultivation with Permissive Cell Cultures. Appl. Environ. Microbiol. 2008, 74, 5383–5391. [Google Scholar] [CrossRef]
- Thomas, A.; Sachse, K.; Dizier, I.; Grajetzki, C.; Farnir, F.; Mainil, J.G.; Linden, A. Adherence to various host cell lines of Mycoplasma bovis strains differing in pathogenic and cultural features. Vet. Microbiol. 2003, 91, 101–113. [Google Scholar] [CrossRef]
- Nishi, K.; Gondaira, S.; Fujiki, J.; Katagata, M.; Sawada, C.; Eguchi, A.; Iwasaki, T.; Iwano, H.; Higuchi, H. Invasion of Mycoplasma bovis into bovine synovial cells utilizing the clathrin-dependent endocytosis pathway. Vet. Microbiol. 2021, 253, 108956. [Google Scholar] [CrossRef]
- Thomas, L.H.; Howard, C.J. Effect of Mycoplasma dispar, Mycoplasma bovirhinis, Acholeplasma laidlawii and T-Mycoplasmas on explant cultures of bovine trachea. J. Comp. Pathol. 1974, 84, 193–201. [Google Scholar] [CrossRef]
- Afshar, A. The Growth of Mycoplasma bovigenitalium in Cell Cultures. J. Gen. Microbiol. 1967, 47, 103–110. [Google Scholar] [CrossRef]
- Andres-Lasheras, S.; Zaheer, R.; Ha, R.; Lee, C.; Jelinski, M.; McAllister, T.A. A direct qPCR screening approach to improve the efficiency of Mycoplasma bovis isolation in the frame of a broad surveillance study. J. Microbiol. Methods 2019, 169, 105805. [Google Scholar] [CrossRef]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, j.; Li, Y.; Yang, C.; Zhao, S.; Liu, J.; Liu, A.; Liu, G.; Yin, H.; Guan, G.; et al. Validation of Reference Genes for Quantitative Real-Time PCR in Bovine PBMCs Transformed and Non-transformed by Theileria annulata. Korean J. Parasitol. 2016, 54, 39–46. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA content and genome size of trout and human. Cytometry A 2003, 51, 127–128. [Google Scholar] [CrossRef]
- Ball, H.J. Experimental Mastitis caused by Mycoplasma bovigenitalium and M. canadense in the ewe. Vet. Microbiol. 1990, 22, 383–388. [Google Scholar] [CrossRef]
- Premachandre, C.K.; Vaz, P.K.; Sharma, S.; Condello, A.K.; Browning, G.F.; Wawegama, N.K. Genes required for survival and proliferation of Mycoplasma bovis in association with host cells. Appl. Environ. Microbiol. 2024, 90, e00687-24. [Google Scholar] [CrossRef]
- Soni, J.; Sinha, S.; Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front. Microbiol. 2024, 15, 1370818. [Google Scholar] [CrossRef]
- Diard, M.; Hardt, W.-D. Evolution of bacterial virulence. FEMS. Microbiol. Rev. 1997, 41, 679–697. [Google Scholar] [CrossRef]
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror ‘real-world’ pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Shi, A.; Feiyu Fan, F.; Broach, J.R. Microbial adaptive evolution. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab076. [Google Scholar] [CrossRef]
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef]
- Sachse, K. Detection and analysis of mycoplasma adhesins. Methods Mol. Biol. 1998, 104, 299–307. [Google Scholar] [CrossRef]
- Sachse, K.; Grajetzki, C.; Rosengarten, R.; Hänel, I.; Heller, M.; Pfützner, H. Mechanisms and factors involved in Mycoplasma bovis adhesion to host cells. Zentralbl. Bakteriol. 1996, 284, 80–92. [Google Scholar] [CrossRef]
- Sachse, K.; Pfützner, H.; Heller, M.; Hänel, I. Inhibition of Mycoplasma bovis cytadherence by a monoclonal antibody and various carbohydrate substances. Vet. Microbiol. 1993, 36, 307–316. [Google Scholar] [CrossRef]
- Hegde, S.; Hegde, S.; Spergser, J.; Brunthaler, R.; Rosengarten, R.; Chopra- Dewasthaly, R. In vitro and in vivo cell invasion and systemic spreading of Mycoplasma agalactiae in the sheep infection model. Int. J. Med. Microbiol. 2014, 304, 1024–1031. [Google Scholar] [CrossRef]
- Groebel, K.; Hoelzle, K.; Wittenbrink, M.M.; Ziegler, U.; Hoelzle, L.E. Mycoplasma suis invades porcine erythrocytes. Infect. Immun. 2009, 77, 576–584. [Google Scholar] [CrossRef]
- Raymond, B.B.A.; Turnbull, L.; Jenkins, C.; Madhkoor, R.; Schleicher, I.; Uphoff, C.C.; Whitchurch, C.B.; Rohde, M.; Djordjevic, S.P. Mycoplasma hyopneumoniae resides intracellularly within porcine epithelial cells. Sci. Rep. 2018, 8, 17697. [Google Scholar] [CrossRef]
- Buim, M.R.; Buzinhani, M.; Yamaguti, M.; Oliveira, R.C.; Mettifogo, E.; Ueno, P.M.; Timenetsky, J.; Santelli, G.M.; Ferreira, A.J. Mycoplasma synoviae cell invasion: Elucidation of the Mycoplasma pathogenesis in chicken. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 41–47. [Google Scholar] [CrossRef]
- Vogl, G.; Plaickner, A.; Szathmary, S.; Stipkovits, L.; Rosengarten, R.; Szostak, M.P. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect. Immun. 2008, 76, 71–77. [Google Scholar] [CrossRef]
- Ueno, P.M.; Timenetsky, J.; Centonze, V.E.; Wewer, J.J.; Cagle, M.; Stein, M.A.; Krishnan, M.; Baseman, J.B. Interaction of Mycoplasma genitalium with host cells: Evidence for nuclear localization. Microbiology 2008, 154, 3033–3041. [Google Scholar] [CrossRef]
- Yavlovich, A.; Rottem, S. Binding of host extracellular matrix proteins to Mycoplasma fermentans and its effect on adherence to, and invasion of HeLa cells. FEMS Microbiol. Lett. 2007, 266, 158–162. [Google Scholar] [CrossRef]
- Yavlovich, A.; Tarshis, M.; Rottem, S. Internalization and intracellular survival of Mycoplasma pneumoniae by non-phagocytic cells. FEMS. Microbiol. Lett. 2004, 233, 241–246. [Google Scholar] [CrossRef]
- Díaz-García, F.J.; Herrera-Mendoza, A.P.; Giono-Cerezo, S.; Guerra-Infante, F.M. Mycoplasma hominis attaches to and locates intracellularly in human spermatozoa. Hum. Reprod. 2006, 21, 1591–1598. [Google Scholar] [CrossRef]
- Yamada, E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445–458. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Hybiske, K.; Stephens, R.S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Nat. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef]
- Hybiske, K.; Stephens, R.S. Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 2008, 6, 99–110. [Google Scholar] [CrossRef]
- Friedrich, N.; Hagedorn, M.; Soldati-Favre, D.; Soldati, T. Prison break: Pathogens’ strategies to egress from host cells. Microbiol. Mol. Biol. Rev. 2012, 76, 707–720. [Google Scholar] [CrossRef]
- Lamberti, Y.; Gorgojo, J.; Massillo, C.; Rodriguez, M.E. Bordetella pertussis entry into respiratory epithelial cells and intracellular survival. Pathog. Dis. 2013, 69, 194–204. [Google Scholar] [CrossRef]
- Razin, S.; Jacobs, E. Mycoplasma adhesion. J. Gen. Microbiol. 1992, 138, 407–422. [Google Scholar] [CrossRef] [PubMed]


| Country of Origin | Host Species | Health Status | Anatomical Isolation Site | Isolation Year | |||
|---|---|---|---|---|---|---|---|
| M. bovis | Type strain | PG45 (ATCC 25523) | USA | Cattle | Mastitis | Milk | 1962 |
| Fluorescent strains | OK1 (mKO2) | France * | Cattle | Diseased | Lung | 1975/2016 ** | |
| ON8 (mNeonGreen) | Diseased | Lung | |||||
| OC7 (mCherry) | Diseased | Lung | |||||
| Field strains | MPLM0670 | Canada | Cattle | Asymptomatic | Nasopharynx | 2007 | |
| MPLM0700 | Nasopharynx | 2007 | |||||
| MPLM0084 | Dead | Lung | 2017 | ||||
| MPLM0093 | Lung | 2017 | |||||
| MPLM1082 | Bison | Asymptomatic | Nasopharynx | 2014 | |||
| MPLM1083 | Nasopharynx | 2014 | |||||
| MPLM1084 | Dead | Lung | 2015 | ||||
| MPLM1048 | Joint | 2011 | |||||
| M. bovirhinis | Field strain | MP 212-A NTC 3 | Canada | Cattle | Dead | Lung | 2014 |
| M. bovigenitalium | Type strain | NCTC 10122 (ATCC 19852) | Cattle | Unknown | Bovine genital tract | 1955 | |
| Species | Group | Mycoplasma Strain | Myc/Cell Ratio (6 h) | Myc/Cell Ratio (54 h) |
|---|---|---|---|---|
| M. bovis | Laboratory strains | PG45 (ATCC 25523) | 1.7 | 13.9 |
| OK1 (mKO2) | 2.2 | 7.1 | ||
| ON8 (mNeonGreen) | 5.0 | 15.2 | ||
| OC7 (mCherry | 1.3 | 2.1 | ||
| Mean value of Myc/Cell ratios | 2.5 | 9.6 | ||
| Field strains | MPLM0670 | 0.3 | 7.4 | |
| MPLM0700 | 0.3 | 3.5 | ||
| MPLM0084 | 1.3 | 1.8 | ||
| MPLM0093 | 0.3 | 1.6 | ||
| MPLM1082 | 0.02 | 2.8 | ||
| MPLM1083 | 0.04 | 1.5 | ||
| MPLM1084 | 0.4 | 2.3 | ||
| MPLM1048 | 0.4 | 3.5 | ||
| Mean value of Myc/Cell ratios | 0.4 | 3.0 | ||
| Mean value of Myc/Cell ratios | 1.1 | 5.2 | ||
| M. bovirhinis | MP 212-A NTC 3 | |||
| Myc/Cell ratio | 5.6 | 6.2 | ||
| M. bovigenitalium | NCTC 10122 (ATCC 19852) | |||
| Myc/Cell ratio | 7.3 | 13.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yacoub, E.; Kos, D.; Jelinski, M. Virulence Is More than Adhesion and Invasion Ability, an In Vitro Cell Infection Assay of Bovine Mycoplasma spp. Microorganisms 2025, 13, 632. https://doi.org/10.3390/microorganisms13030632
Yacoub E, Kos D, Jelinski M. Virulence Is More than Adhesion and Invasion Ability, an In Vitro Cell Infection Assay of Bovine Mycoplasma spp. Microorganisms. 2025; 13(3):632. https://doi.org/10.3390/microorganisms13030632
Chicago/Turabian StyleYacoub, Elhem, Daniel Kos, and Murray Jelinski. 2025. "Virulence Is More than Adhesion and Invasion Ability, an In Vitro Cell Infection Assay of Bovine Mycoplasma spp." Microorganisms 13, no. 3: 632. https://doi.org/10.3390/microorganisms13030632
APA StyleYacoub, E., Kos, D., & Jelinski, M. (2025). Virulence Is More than Adhesion and Invasion Ability, an In Vitro Cell Infection Assay of Bovine Mycoplasma spp. Microorganisms, 13(3), 632. https://doi.org/10.3390/microorganisms13030632





