Microbial Metabolic Limitations and Their Relationships with Sediment Organic Carbon Across Lake Salinity Gradient in Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
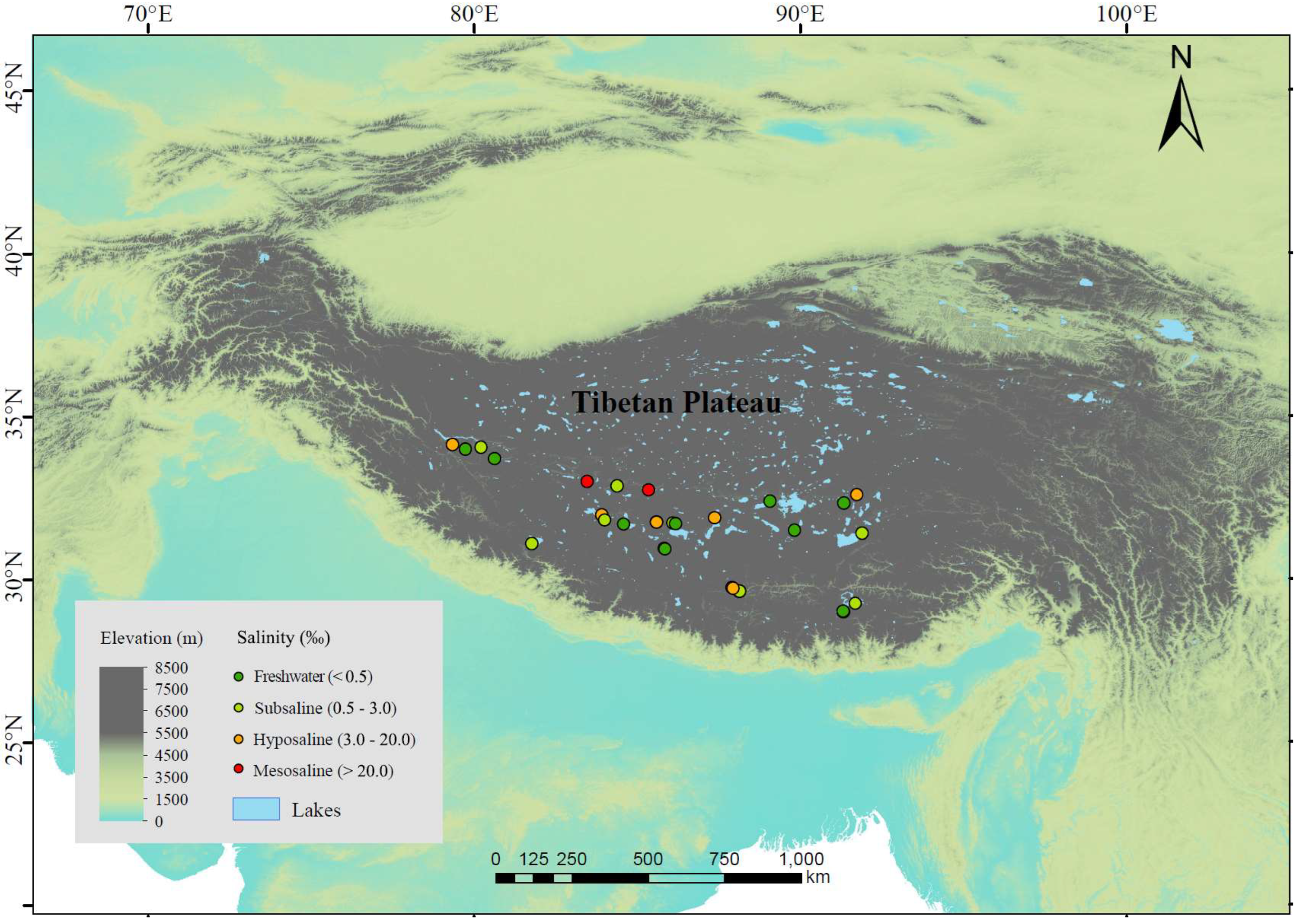
2.1. Study Area and Sampling
2.2. C, N, and P Content Determinations
2.3. Bacterial and Fungal Community Analyses
2.4. EEAs Assays
2.5. MMLs Calculations
2.6. Statistical Analysis
3. Results
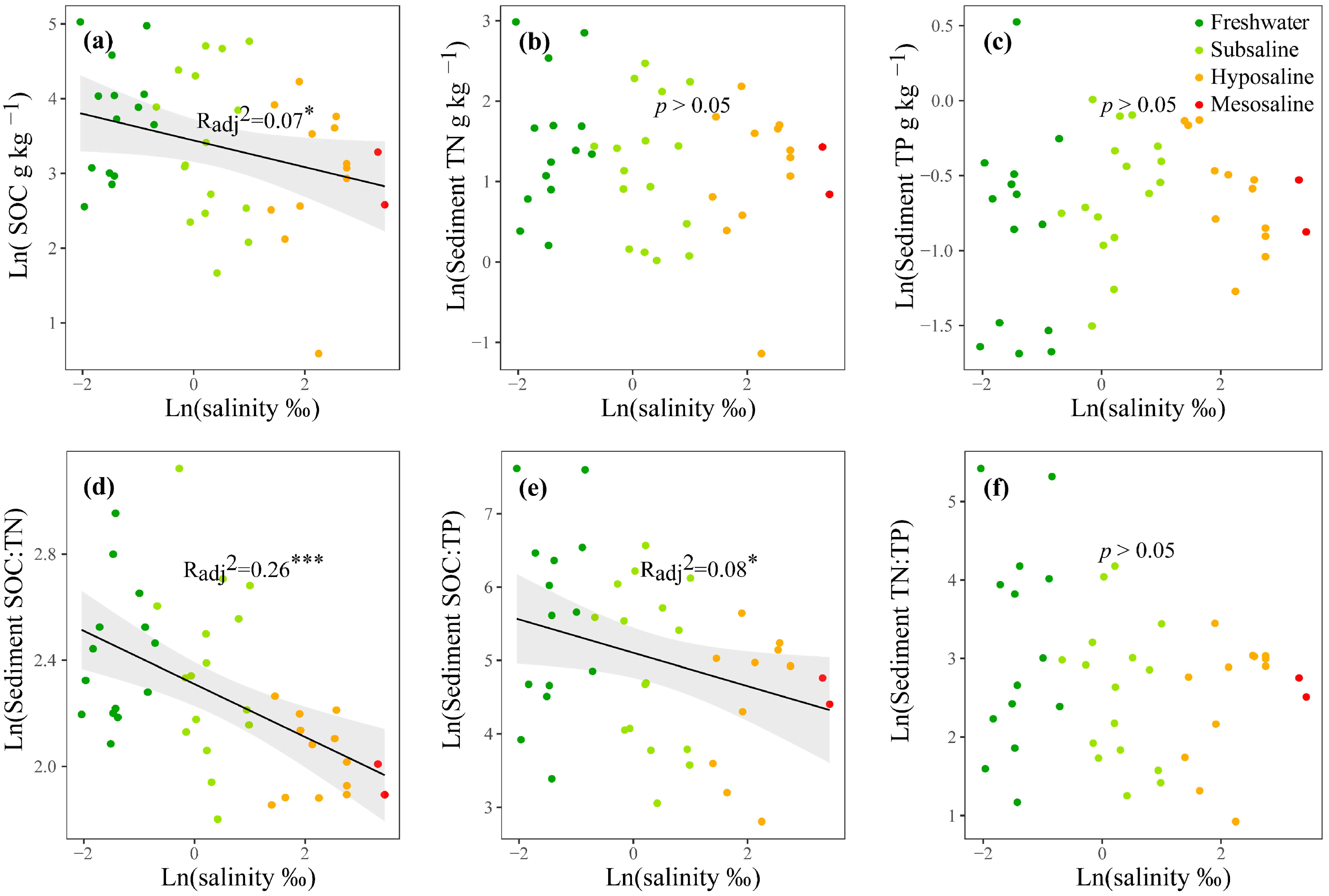
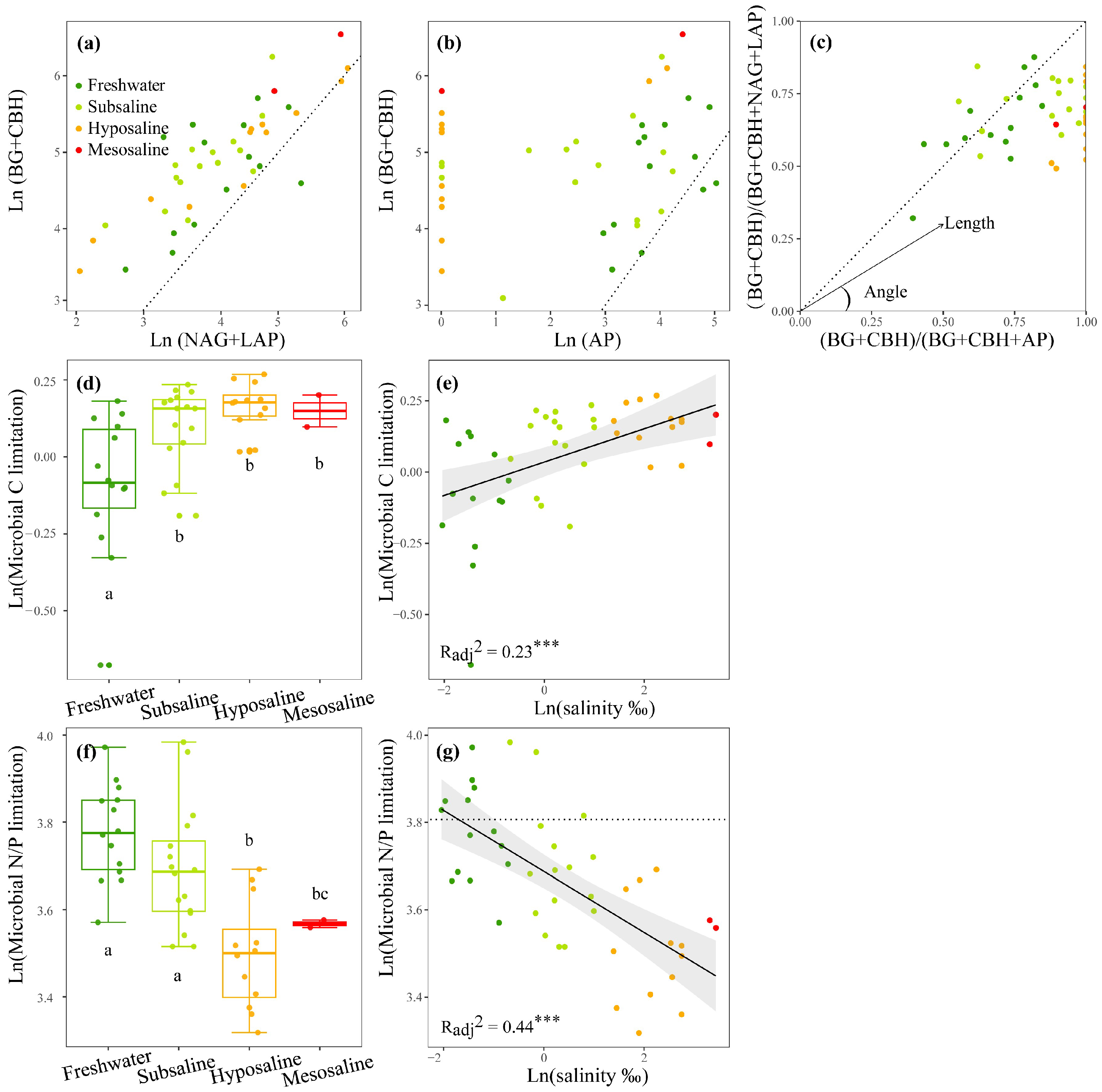
3.1. Nutrients, EEAs, and MMLs
3.2. Bacterial and Fungal Diversities and Their Driving Factors
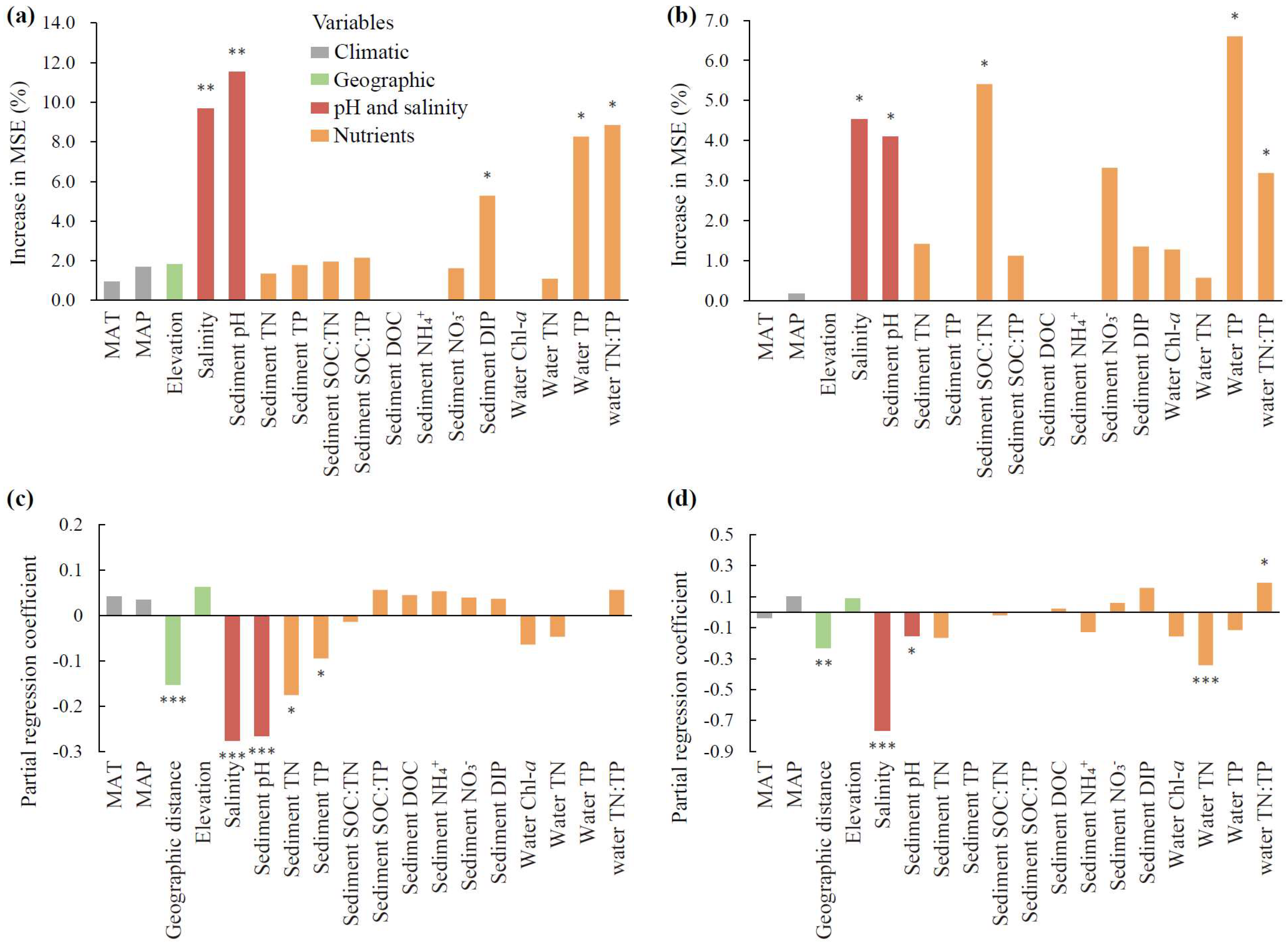
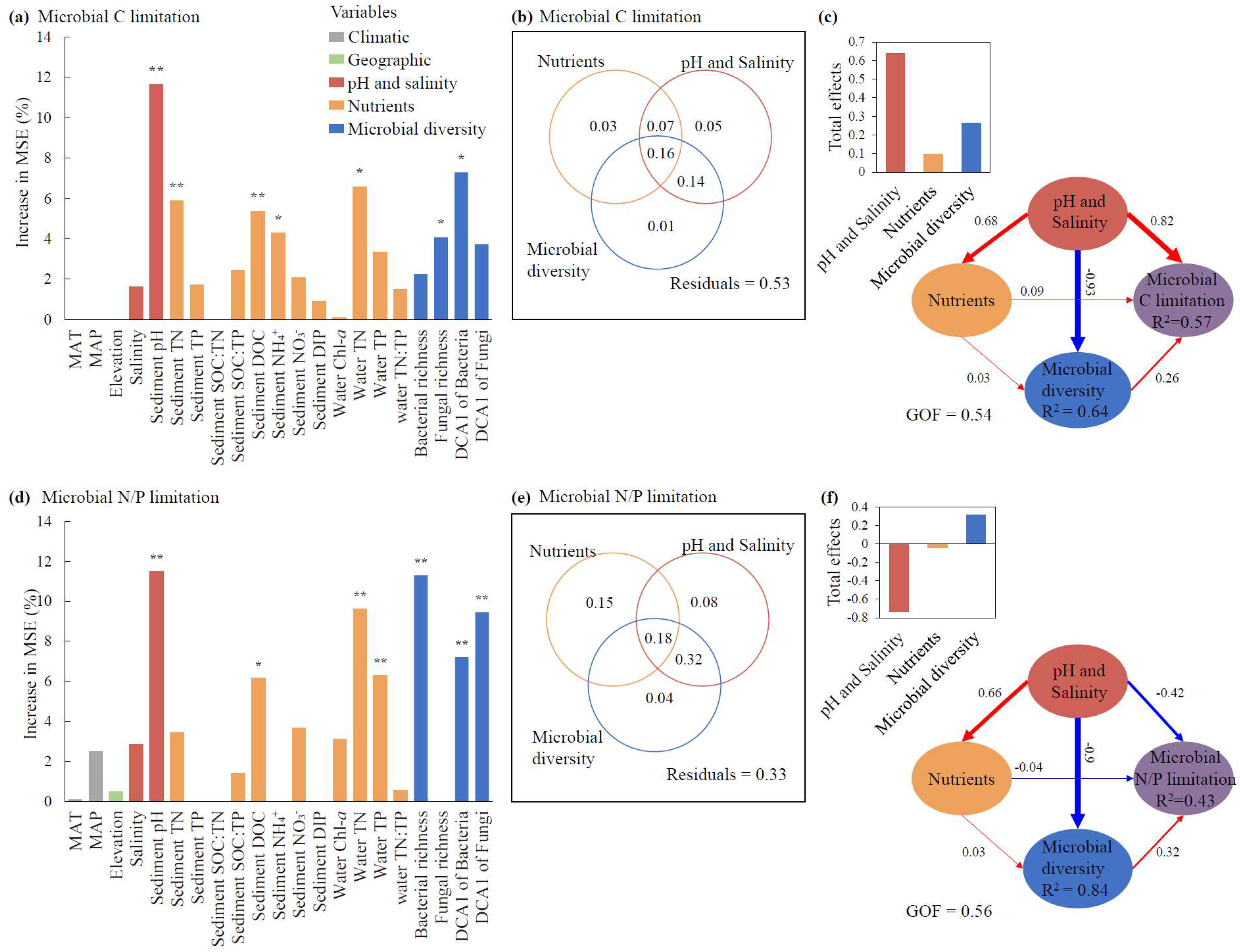
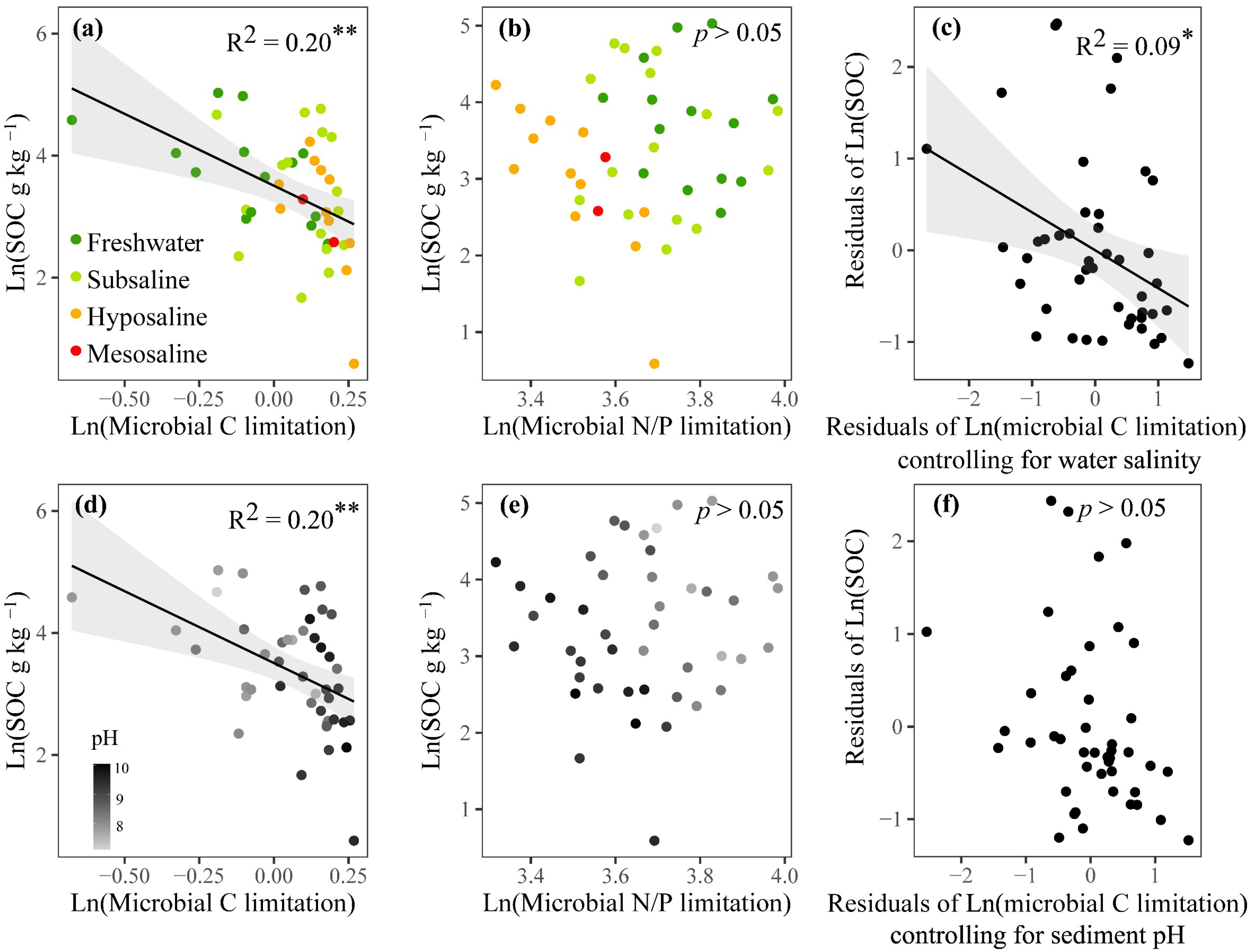
3.3. Drivers of MMLs and Correlations Between MMLs and SOC
4. Discussion
4.1. C and N Limitations Dominated MMLs in Tibetan Lakes
4.2. Predominant Contributions of Water Salinity and Sediment pH to MMLs
4.3. SOC Is Negatively Associated with Microbial C Limitation
4.4. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOC | Sediment organic carbon |
| MML | Microbial metabolic limitation |
| EEA | Extracellular enzymatic activity |
| VPA | Variance partitioning analysis |
| PLS-PM | Partial least squares path modeling |
| DCA | Detrended correspondence analysis |
| MRM | Multiple regression on dissimilarity matrix |
| ANOVA | Analysis of variance |
| TN | Total nitrogen |
| TP | Total phosphorus |
| MAT | Mean annual temperature |
| MAP | Mean annual precipitation |
| ASV | amplicon sequence variant |
| DIP | Dissolved inorganic phosphorus |
| DIN | Dissolved inorganic nitrogen |
| DOC | Dissolved organic carbon |
| Chl-a | Chlorophyll-a |
| OLS | Ordinary Least Square |
| BG | β-1,4-Glucosidase |
| CBH | Cellobiohydrolase |
| NAG | β-1,4-N-acetylglucosaminidase |
| LAP | Leucine aminopeptidase |
| AP | Alkaline phosphatase |
| AMC | 7-amino-4-methyl coumarin |
| MUB | 4-methylumbelliferone |
References
- Lee, H.; Romero, J. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; 184p. [Google Scholar] [CrossRef]
- Saini, J.; Pandey, S. Environmental threat and change detection in saline lakes from 1960 to 2021: Background, present, and future. Environ. Sci. Pollut. Res. 2023, 30, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Luo, S.; Liu, K.; Chen, T.; Zhang, P.; Fan, C. Widespread declines in water salinity of the endorheic Tibetan Plateau lakes. Environ. Res. Commun. 2022, 4, 091002. [Google Scholar] [CrossRef]
- Jeppesen, E.; Beklioğlu, M.; Zadereev, E. The Effects of Global Climate Change on Water Level and Salinity: Causes and Effects. Water 2023, 15, 2853. [Google Scholar] [CrossRef]
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic carbon burial in global lakes and reservoirs. Nat. Commun. 2017, 8, 1694. [Google Scholar] [CrossRef]
- Chen, X.; Meng, X.; Song, Y.; Zhang, B.; Wan, Z.; Zhou, B.; Zhang, E. Spatial patterns of organic and inorganic carbon in Lake Qinghai surficial sediments and carbon burial estimation. Front. Earth Sci. 2021, 9, 714936. [Google Scholar] [CrossRef]
- Liu, D.; Shi, K.; Chen, P.; Yan, N.; Ran, L.; Kutser, T.; Tyler, A.N.; Spyrakos, E.; Woolway, R.I.; Zhang, Y.; et al. Substantial increase of organic carbon storage in Chinese lakes. Nat. Commun. 2024, 15, 8049. [Google Scholar] [CrossRef]
- Jellison, R.; Anderson, R.F.; Melack, J.M.; Heil, D. Organic matter accumulation in sediments of hypersaline Mono Lake during a period of changing salinity. Limnol. Oceanogr. 2003, 41, 1539–1544. [Google Scholar] [CrossRef]
- Hemingway, J. Fossil organic carbon utilization in marine Arctic fjord sediments by subsurface micro-organisms. Nat. Geosci. 2023, 16, 558–559. [Google Scholar] [CrossRef]
- Sardinha, M.; Müller, T.; Schmeisky, H.; Joergensen, R.G. Microbial performance in soils along a salinity gradient under acidic conditions. Appl. Soil Ecol. 2003, 23, 237–244. [Google Scholar] [CrossRef]
- Oren, A. Thermodynamic limits to microbial life at high salt concentrations. Environ. Microbiol. 2011, 13, 1908–1923. [Google Scholar] [CrossRef]
- Halverson, L.J.; Jones, T.M.; Firestone, M.K. Release of intracellular solutes by four soil bacteria exposed to dilution stress. Soil Sci. Soc. Am. J. 2000, 64, 1630–1637. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Jiang, H.; Wu, G.; Liu, W.; Wang, B.; Xiao, H.; Han, J. Microbial responses to simulated salinization and desalinization in the sediments of the Qinghai-Tibetan lakes. Front. Microbiol. 2020, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, H.; Li, Y.; Zhang, J.; Ma, C.; Weng, N.; Gao, X.; Wu, F.; Huo, S. Strong associations between dissolved organic matter and microbial communities in the sediments of Qinghai-Tibetan Plateau lakes depend on salinity. Sci. Total Environ. 2024, 926, 171857. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, H.; Yang, J.; Wu, G. Gammaproteobacterial diversity and carbon utilization in response to salinity in the lakes on the Qinghai-Tibetan Plateau. Geomicrobiol. J. 2018, 35, 392–403. [Google Scholar] [CrossRef]
- Teixeira, C.; Magalhaes, C.; Joye, S.B.; Bordalo, A.A. The role of salinity in shaping dissolved inorganic nitrogen and N2O dynamics in estuarine sediment-water interface. Mar. Pollut. Bull. 2013, 66, 225–229. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Huang, X.-L. Effect of temperature and salinity on phosphate sorption on marine sediments. Environ. Sci. Technol. 2011, 45, 6831–6837. [Google Scholar] [CrossRef]
- Chen, X.; Luo, M.; Liu, Y.; Tan, J.; Zhang, C.; Tan, F.; Huang, J. Linking carbon-degrading enzyme activity to microbial carbon-use trophic strategy under salinization in a subtropical tidal wetland. Appl. Soil Ecol. 2022, 174, 104421. [Google Scholar] [CrossRef]
- Van Gray, J.B.; Roberto, A.A.; Leff, L.G. Acute salt stress promotes altered assembly dynamics of nascent freshwater microbial biofilms. Hydrobiologia 2020, 847, 2465–2484. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Geng, M.; Chen, R.; Wang, J.; Xue, B.; Xie, P.; Wang, J. Extracellular enzyme stoichiometry reveals carbon and nitrogen limitations closely linked to bacterial communities in China’s largest saline lake. Front. Microbiol. 2022, 13, 1002542. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Moorhead, D. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Penton, C.R.; Newman, S. Enzyme-based resource allocated decomposition and landscape heterogeneity in the Florida Everglades. J. Environ. Qual. 2008, 37, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.C.; Lajtha, K.; Rousk, J. Nutrient limitation may induce microbial mining for resources from persistent soil organic matter. Ecology 2021, 102, e03328. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Moorhead, D.L.; Delgado-Baquerizo, M.; Ye, L.; Yu, J.; Zhang, S.; Wang, X.; Peng, S.; Guo, X.; et al. Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun. Earth Environ. 2022, 3, 184. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Peng, S.; Sinsabaugh, R.L. New insights into the patterns of ecoenzymatic stoichiometry in soil and sediment. Soil Biol. Biochem. 2022, 177, 108910. [Google Scholar] [CrossRef]
- Shi, C.; Urbina-Malo, C.; Tian, Y.; Heinzle, J.; Kengdo, S.K.; Inselsbacher, E.; Borken, W.; Schindlbacher, A.; Wanek, W. Does long-term soil warming affect microbial element limitation? A test by short-term assays of microbial growth responses to labile C, N and P additions. Glob. Change Biol. 2023, 29, 2188–2202. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhang, X.C.; Zheng, B.Y.; Yue, S.C.; Razavi, B.S. Effects of plastic and straw mulching on soil microbial P limitations in maize fields: Dependency on soil organic carbon demonstrated by ecoenzymatic stoichiometry. Geoderma 2021, 388, 114928. [Google Scholar] [CrossRef]
- Luo, L.; Gu, J.-D. Nutrient limitation status in a subtropical mangrove ecosystem revealed by analysis of enzymatic stoichiometry and microbial abundance for sediment carbon cycling. Int. Biodeterior. Biodegrad. 2018, 128, 3–10. [Google Scholar] [CrossRef]
- Velasco Ayuso, S.; Medina-Sánchez, J.M.; Guénon, R.; Carrillo, P. Ecoenzyme activity ratios reveal interactive effects of nutrient inputs and UVR in a Mediterranean high-mountain lake. Biogeochemistry 2017, 132, 71–85. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Zhang, X.; Ju, W.; Duan, C.; Wang, Y.; Fang, L. A novel extracellular enzyme stoichiometry method to evaluate soil heavy metal contamination: Evidence derived from microbial metabolic limitation. Sci. Total Environ. 2020, 738, 139709. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fang, L.; Deng, L.; Guo, X.; Han, F.; Ju, W.; Wang, X.; Chen, H.; Tan, W.; Zhang, X. Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci. Total Environ. 2019, 658, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zheng, M. Influence of climate change on saline lakes of the Tibet Plateau, 1973–2010. Geomorphology 2015, 246, 68–78. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, K.; Wang, B.; Sheng, Y.; Bird, B.W.; Zhang, G.; Tian, L. Response of inland lake dynamics over the Tibetan Plateau to climate change. Clim. Change 2014, 125, 281–290. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Xu, J.; Shen, Z.; Yang, Y.; Chen, A.; Wang, S.; Liang, E.; Piao, S. Enhanced habitat loss of the Himalayan endemic flora driven by warming-forced upslope tree expansion. Nat. Ecol. Evol. 2022, 6, 890–899. [Google Scholar] [CrossRef]
- Meyer-Reil, L.-A. Measurement of hydrolytic activity and incorporation of dissolved organic substrates by microorganisms in marine sediments. Mar. Ecol. Prog. Ser. 1986, 31, 143–149. [Google Scholar] [CrossRef]
- Poremba, K.; Hoppe, H.-G. Spatial variation of benthic microbial production and hydrolytic enzymatic activity down the continental slope of the Celtic Sea. Mar. Ecol. Prog. Ser. 1995, 118, 237–245. [Google Scholar] [CrossRef]
- Boadella, J.; Rodríguez-Baliu, C.; Butturini, A.; Romaní, A.M. Day-night variation of microbial organic matter use in sediments of a saline shallow lake. Freshw. Biol. 2023, 68, 711–725. [Google Scholar] [CrossRef]
- Hewins, D.B.; Chuan, X.; Bork, E.W.; Carlyle, C.N. Measuring the effect of freezing on hydrolytic and oxidative extracellular enzyme activities associated with plant litter decomposition. Pedobiologia 2016, 59, 253–256. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis Part 3—Chemical Methods, 1st ed.; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Environmental Monitoring Technical Specifications for Soil; National Environmental Protection Standards of the People’s Republic of China: Beijing, China, 2018.
- Horňák, K.; Kasalický, V.; Šimek, K.; Grossart, H.P. Strain-specific consumption and transformation of alga-derived dissolved organic matter by members of the Limnohabitans-C and Polynucleobacter-B clusters of Betaproteobacteria. Environ. Microbiol. 2017, 19, 4519–4535. [Google Scholar] [CrossRef] [PubMed]
- Shao, L. Comparison and improvement on the extraction method for chlorophyll a in phytoplankton. Ecol. Sci. 2005, 24, 9–11. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Michael, S.R., II; Devon, R.O.R.; Benjamin, D.K.; Michal, Z.; Matthew, R.D.; Jeffrey, T.F.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management for the masses. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Freeman, C.; Liska, G.; Ostle, N.J.; Jones, S.E.; Lock, M.A. The use of fluorogenic substrates for measuring enzyme activity in peatlands. Plant Soil 1995, 175, 147–152. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by RandomForest. Forest 2001, 23, 18–22. [Google Scholar]
- Lichstein, J.W. Multiple regression on distance matrices: A multivariate spatial analysis tool. Plant Ecol. 2007, 188, 117–131. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Data from: Climatologies at high resolution for the earth’s land surface areas. EnviDat 2021. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Modell. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Vinzi, V.E.; Chatelin, Y.M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Healy, K. An R and S-PLUS companion to applied regression. Sociol. Method Res. 2005, 34, 137–140. [Google Scholar] [CrossRef]
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Anderson, L.E.; Lehrter, J.C. Microbial respiration and ecoenzyme activity in sediments from the Gulf of Mexico hypoxic zone. Aquat. Microb. Ecol. 2014, 72, 107–118. [Google Scholar] [CrossRef]
- Kamble, P.N.; Gaikwad, V.B.; Kuchekar, S.R.; Bååth, E. Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India). Eur. J. Soil Biol. 2014, 65, 87–95. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Yang, W.; Xia, F.; Guo, H.; Tan, Q. Coupling and decoupling of soil carbon and nutrients cycles at different salinity levels in a mangrove wetland: Insights from CUE and enzymatic stoichiometry. Sci. Total Environ. 2024, 922, 171039. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial enzyme stoichiometry and nutrient limitation in US streams and rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Herlihy, A.T.; Jicha, T.M.; Serenbetz, G. Microbial ecoenzyme stoichiometry, nutrient limitation, and organic matter decomposition in wetlands of the conterminous United States. Wetl. Ecol. Manag. 2018, 26, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, R.; Hall, J.W. Global patterns and key drivers of stream nitrogen concentration: A machine learning approach. Sci. Total Environ. 2023, 868, 161623. [Google Scholar] [CrossRef]
- Watanabe, M.; Miura, S.; Hasegawa, S.; Koshikawa, M.K.; Takamatsu, T.; Kohzu, A.; Imai, A.; Hayashi, S. Coniferous coverage as well as catchment steepness influences local stream nitrate concentrations within a nitrogen-saturated forest in central Japan. Sci. Total Environ. 2018, 636, 539–546. [Google Scholar] [CrossRef]
- Craig, L.S. Nitrogen Saturation in Streams and Forests of the Maryland Piedmont. Ph.D. Thesis, University of Maryland, College Park, MA, USA, 2009. [Google Scholar]
- Chen, J.; Seven, J.; Zilla, T.; Dippold, M.A.; Blagodatskaya, E.; Kuzyakov, Y. Microbial C:N:P stoichiometry and turnover depend on nutrients availability in soil: A 14C, 15N and 33P triple labelling study. Soil Biol. Biochem. 2019, 131, 206–216. [Google Scholar] [CrossRef]
- Sundareshwar, P.V.; Morris, J.T. Phosphorus sorption characteristics of intertidal marsh sediments along an estuarine salinity gradient. Limnol. Oceanogr. 1999, 44, 1693–1701. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Johanson, J.B. Effect of pH on enzyme stability in soils. Soil Biol. Biochem. 1982, 14, 433–437. [Google Scholar] [CrossRef]
- Jin, X.C.; Wang, S.R.; Pang, Y.; Wu, F.C. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ. Pollut. 2006, 139, 288–295. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S. Nitrogen addition shapes soil enzyme activity patterns by changing pH rather than the composition of the plant and microbial communities in an alpine meadow soil. Plant Soil 2019, 440, 11–24. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef] [PubMed]
- Stark, S.; Mannisto, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Min, K.; Lehmeier, C.A.; Ballantyne, F.; Tatarko, A.; Billings, S.A. Differential effects of pH on temperature sensitivity of organic carbon and nitrogen decay. Soil Biol. Biochem. 2014, 76, 193–200. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Jiang, H.; Wu, G.; Dong, H. Salinity shapes microbial diversity and community structure in surface sediments of the Qinghai-Tibetan Lakes. Sci. Rep. 2016, 6, 25078. [Google Scholar] [CrossRef]
- Timms, B.V. Drivers restricting biodiversity in Australian saline lakes: A review. Mar. Freshw. Res. 2021, 72, 462–468. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef]
- Erez, A.; Lopez, J.G.; Weiner, B.G.; Meir, Y.; Wingreen, N.S. Nutrient levels and trade-offs control diversity in a serial dilution ecosystem. eLife 2020, 9, e57790. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2009, 8, 15–25. [Google Scholar] [CrossRef]
- Aranda-Díaz, A.; Willis, L.; Nguyen, T.H.; Ho, P.Y.; Vila, J.; Thomsen, T.; Chavez, T.; Yan, R.; Yu, F.B.; Neff, N.; et al. Assembly of gut-derived bacterial communities follows “early-bird” resource utilization dynamics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, R.; Graham, E.B.; Yu, B.; Bao, Y.; Li, X.; You, X.; Feng, Y. Eco-evolutionary strategies for relieving carbon limitation under salt stress differ across microbial clades. Nat. Commun. 2024, 15, 6013. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.; Adams, H. Dissolved organic matter quantity and quality from freshwater and saltwater lakes in east-central Alberta. Biogeochemistry 1995, 30, 59–76. [Google Scholar] [CrossRef]
- Schimel, J.P.; Scott, W.J.; Killham, K. Changes in cytoplasmic carbon and nitrogen pools in a soil bacterium and a fungus in response to salt stress. Appl. Environ. Microbiol. 1989, 55, 1635–1637. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; She, W.; Xiao, H.; Wang, Z.; Wang, H.; Liu, W.; Jiang, H. Deciphering linkages between microbial communities and priming effects in lake sediments with different salinity. J. Geophys. Res.-Biogeo. 2020, 125, e2019JG005611. [Google Scholar] [CrossRef]
- Yang, J.; Han, M.; Wang, B.; Yao, B.; Wu, Z.; Li, X.; Liu, L.; Dong, H.; Jiang, H. Predominance of positive priming effects induced by algal and terrestrial organic matter input in saline lake sediments. Geochim. Cosmochim. Acta 2023, 349, 126–134. [Google Scholar] [CrossRef]
- Hassani, A.; Smith, P.; Shokri, N. Negative correlation between soil salinity and soil organic carbon variability. Proc. Natl. Acad. Sci. USA 2024, 121, e2317332121. [Google Scholar] [CrossRef]
- Setia, R.; Gottschalk, P.; Smith, P.; Marschner, P.; Baldock, J.; Setia, D.; Smith, J. Soil salinity decreases global soil organic carbon stocks. Sci. Total Environ. 2013, 465, 267–272. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, N.; Zhao, C.M.; Li, J.H. Responses of carbon cycling and soil organic carbon content to nitrogen addition in grasslands globally. Soil Biol. Biochem. 2023, 186, 109164. [Google Scholar] [CrossRef]
- Zhou, S.; Long, H.; Chen, W.; Qiu, C.; Zhang, C.; Xing, H.; Zhang, J.; Cheng, L.; Zhao, C.; Cheng, J.; et al. Temperature seasonality regulates organic carbon burial in lake. Nat. Commun. 2025, 16, 1049. [Google Scholar] [CrossRef]
- Quanliang, J.; Xiaohua, M.; Zhichun, L.; Shuaidong, L.; Changchun, H.; Tao, H.; Bin, X.; Hao, Y. New perspectives on organic carbon storage in lake sediments based on classified mineralization. Catena 2024, 237, 107811. [Google Scholar] [CrossRef]
- McCullough, I.M.; Dugan, H.A.; Farrell, K.J.; Morales-Williams, A.M.; Ouyang, Z.; Roberts, D.; Scordo, F.; Bartlett, S.L.; Burke, S.M.; Doubek, J.P.; et al. Dynamic modeling of organic carbon fates in lake ecosystems. Ecol. Modell. 2018, 386, 71–82. [Google Scholar] [CrossRef]
- Valinia, S.; Futter, M.N.; Cosby, B.J.; Rosen, P.; Folster, J. Simple Models to Estimate Historical and Recent Changes of Total Organic Carbon Concentrations in Lakes. Environ. Sci. Technol. 2015, 49, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, R.; Meng, F.; Yuan, H.; Geng, M.; Cheng , L.; Yin, H.; Xue, B.; Wang, J. Ecosystem functioning is linked to microbial evenness and community composition along depth gradient in a semiarid lake. Ecol. Indic. 2021, 132, 108314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, J.; Li, Y.; Song, C.; Zhou, Y.; Meng, X.; Chen, R. Microbial Metabolic Limitations and Their Relationships with Sediment Organic Carbon Across Lake Salinity Gradient in Tibetan Plateau. Microorganisms 2025, 13, 629. https://doi.org/10.3390/microorganisms13030629
Zhang W, Wang J, Li Y, Song C, Zhou Y, Meng X, Chen R. Microbial Metabolic Limitations and Their Relationships with Sediment Organic Carbon Across Lake Salinity Gradient in Tibetan Plateau. Microorganisms. 2025; 13(3):629. https://doi.org/10.3390/microorganisms13030629
Chicago/Turabian StyleZhang, Weizhen, Jianjun Wang, Yun Li, Chao Song, Yongqiang Zhou, Xianqiang Meng, and Ruirui Chen. 2025. "Microbial Metabolic Limitations and Their Relationships with Sediment Organic Carbon Across Lake Salinity Gradient in Tibetan Plateau" Microorganisms 13, no. 3: 629. https://doi.org/10.3390/microorganisms13030629
APA StyleZhang, W., Wang, J., Li, Y., Song, C., Zhou, Y., Meng, X., & Chen, R. (2025). Microbial Metabolic Limitations and Their Relationships with Sediment Organic Carbon Across Lake Salinity Gradient in Tibetan Plateau. Microorganisms, 13(3), 629. https://doi.org/10.3390/microorganisms13030629






