Impact of Tree Species Mixture on Microbial Diversity and Community Structure in Soil Aggregates of Castanopsis hystrix Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Forest Stands
2.3. Plot Establishment and Sample Collection
2.4. Determination of Abiotic Characteristics of Bulk Soil and Aggregates
2.4.1. Aggregate Size Distribution and Stability Parameter
2.4.2. Physicochemical Factors
| Indicator | Stand Type | Soil Aggregate Size | |||
|---|---|---|---|---|---|
| >2 mm | 1–2 mm | 0.25–1 mm | <0.25 mm | ||
| pH | CK | 4.51 ± 0.02 Aa | 4.50 ± 0.00 Aa | 4.50 ± 0.02 Aa | 4.50 ± 0.05 Aa |
| MCA | 4.15 ± 0.02 Aa | 4.18 ± 0.05 Ab | 4.23 ± 0.11 Ab | 4.24 ± 0.01 Ab | |
| MCM | 4.52 ± 0.03 Aa | 4.47 ± 0.07 Aa | 4.47 ± 0.11 Aa | 4.54 ± 0.08 Aa | |
| MCP | 4.50 ± 0.12 Aa | 4.47 ± 0.10 Aa | 4.46 ± 0.13 Aa | 4.48 ± 0.10 Aa | |
| OC (g/kg) | CK | 17.4 ± 2.72 Bb | 21.1 ± 2.11 ABa | 23.9 ± 1.76 Ab | 22.2 ± 0.72 Ab |
| MCA | 28.8 ± 0.88 Ba | 26.9 ± 1.82 Ba | 32.3 ± 1.54 Aa | 33.3 ± 1.86 Aa | |
| MCM | 18.7 ± 2.45 Ab | 20.1 ± 2.59 Aa | 22.9 ± 3.99 Ab | 24.9 ± 1.36 Ab | |
| MCP | 20.6 ± 2.16 Ab | 22.4 ± 4.78 Aa | 23.6 ± 4.02 Ab | 24.8 ± 3.95 Ab | |
| TN (g/kg) | CK | 1.35 ± 0.17 Ca | 1.62 ± 0.12 Ba | 1.85 ± 0.05 Aa | 1.75 ± 0.07 ABa |
| MCA | 1.83 ± 0.30 Aa | 1.78 ± 0.27 Aa | 2.11 ± 0.46 Aa | 2.14 ± 0.51 Aa | |
| MCM | 1.38 ± 0.11 Ba | 1.54 ± 0.15 Ba | 1.73 ± 0.26 ABa | 1.90 ± 0.17 Aa | |
| MCP | 1.51 ± 0.17 Aa | 1.53 ± 0.22 Aa | 1.65 ± 0.23 Aa | 1.70 ± 0.21 Aa | |
| TP (g/kg) | CK | 0.24 ± 0.03 Aa | 0.23 ± 0.01 Aa | 0.23 ± 0.01 Aa | 0.22 ± 0.01 Aa |
| MCA | 0.22 ± 0.02 Aa | 0.21 ± 0.02 Aa | 0.22 ± 0.03 Aa | 0.22 ± 0.04 Aa | |
| MCM | 0.23 ± 0.01 Aa | 0.22 ± 0.02 Aa | 0.24 ± 0.01 Aa | 0.23 ± 0.02 Aa | |
| MCP | 0.22 ± 0.04 Aa | 0.21 ± 0.02 Aa | 0.23 ± 0.05 Aa | 0.23 ± 0.05 Aa | |
| NH4+-N (mg/kg) | CK | 35.4 ± 2.09 Ba | 46.0 ± 3.95 ABa | 53.9 ± 6.38 Aa | 51.8 ± 11.15 Ab |
| MCA | 24.3 ± 6.40 Ab | 25.7 ± 6.61 Ab | 33.3 ± 9.20 Ab | 32.0 ± 7.06 Ac | |
| MCM | 40.7 ± 5.70 Ca | 43.5 ± 0.79 Ca | 55.8 ± 2.44 Ba | 66.5 ± 5.24 Aa | |
| MCP | 37.6 ± 2.93 Ca | 42.3 ± 1.49 BCa | 45.0 ± 3.56 ABa | 48.7 ± 3.09 Ab | |
| NO3−-N (mg/kg) | CK | 1.62 ± 0.14 Aa | 1.27 ± 0.15 Ac | 1.50 ± 0.14 Ac | 1.75 ± 0.46 Ac |
| MCA | 3.96 ± 0.51 Ab | 3.71 ± 0.48 Ab | 3.91 ± 0.70 Ab | 3.55 ± 0.58 Ab | |
| MCM | 3.00 ± 0.58 Bc | 6.44 ± 1.35 Aa | 6.29 ± 1.21 Aa | 5.56 ± 0.54 Aa | |
| MCP | 5.96 ± 0.13 Ba | 6.58 ± 0.25 Aa | 6.48 ± 0.13 ABa | 6.43 ± 0.46 ABa | |
| AP (mg/kg) | CK | 5.65 ± 0.49 Bab | 6.00 ± 0.22 Bb | 7.10 ± 0.40 Aa | 7.82 ± 0.75 Aa |
| MCA | 6.50 ± 0.76 Aa | 6.40 ± 1.08 Ab | 7.53 ± 1.45 Aa | 7.05 ± 1.65 Aa | |
| MCM | 3.58 ± 0.23 Bc | 3.13 ± 0.52 Bc | 4.15 ± 0.87 Bb | 6.13 ± 0.90 Aa | |
| MCP | 5.33 ± 0.23 Cb | 11.3 ± 1.62 Aa | 6.40 ± 0.45B Ca | 7.28 ± 0.28 Ba | |
| C/N | CK | 12.9 ± 0.46 Aa | 13.1 ± 0.52 Aa | 12.9 ± 0.61 Aa | 12.7 ± 0.20 Aa |
| MCA | 16.0 ± 2.33 Aa | 15.2 ± 1.25 Aa | 15.7 ± 3.18 Aa | 16.1 ± 3.41 Aa | |
| MCM | 13.5 ± 1.31 Aa | 13.0 ± 0.81 Aa | 13.2 ± 0.31 Aa | 13.1 ± 0.46 Aa | |
| MCP | 13.6 ± 0.45 Aa | 14.5 ± 1.17 Aa | 14.3 ± 0.75 Aa | 14.5 ± 0.58 Aa | |
| C/P | CK | 75.1 ± 16.9 Bb | 91.3 ± 11.5 ABa | 106 ± 5.60 Ab | 102 ± 4.80 Ab |
| MCA | 133 ± 5.64 Aa | 130 ± 8.21 Aa | 147 ± 17.1 Aa | 155 ± 19.8 Aa | |
| MCM | 82.0 ± 14.2 Ab | 91.4 ± 17.5 Aa | 97.4 ± 18.4 Ab | 109 ± 1.50 Ab | |
| MCP | 96.0 ± 18.4 Ab | 107 ± 27.6 Aa | 106 ± 24.9 Ab | 109 ± 18.6 Ab | |
| N/P | CK | 5.79 ± 1.21 Ba | 6.98 ± 0.71 ABa | 8.26 ± 0.37 Aa | 8.07 ± 0.27 Ab |
| MCA | 8.47 ± 0.82 Aa | 8.58 ± 0.71 Aa | 9.44 ± 0.78 Aa | 9.72 ± 0.90 Aa | |
| MCM | 6.05 ± 0.83 Aa | 7.00 ± 1.11 Aa | 7.38 ± 1.22 Aa | 8.30 ± 0.22 Aab | |
| MCP | 7.08 ± 1.61 Aa | 7.37 ± 1.47 Aa | 7.45 ± 1.85 Aa | 7.54 ± 1.25 Ab | |
| Composition (%) | CK | 28.0 ± 0.16 b | 27.6 ± 0.02 a | 33.8 ± 0.01 ab | 10.4 ± 0.01 b |
| MCA | 38.8 ± 0.02 a | 26.7 ± 0.06 c | 25.0 ± 0.02 a | 9.46 ± 0.00 b | |
| MCM | 33.1 ± 0.01 ab | 32.6 ± 0.01 b | 27.4 ± 0.01 b | 6.93 ± 0.00 ab | |
| MCP | 34.7 ± 0.06 a | 30.5 ± 0.03 a | 28.1 ± 0.04 b | 6.59 ± 0.01 a | |
| MWD (mm) | CK | 1.62 ± 0.03 b | |||
| MCA | 1.93 ± 0.05 a | ||||
| MCM | 1.83 ± 0.03 a | ||||
| MCP | 1.86 ± 0.14 a | ||||
2.5. High-Throughput Sequencing of Soil Aggregate Microbiota
2.6. Statistical Analysis
3. Results
3.1. Physical and Chemical Environment of Forest Land and Soil
3.2. Microbial Diversity in Soil Aggregates
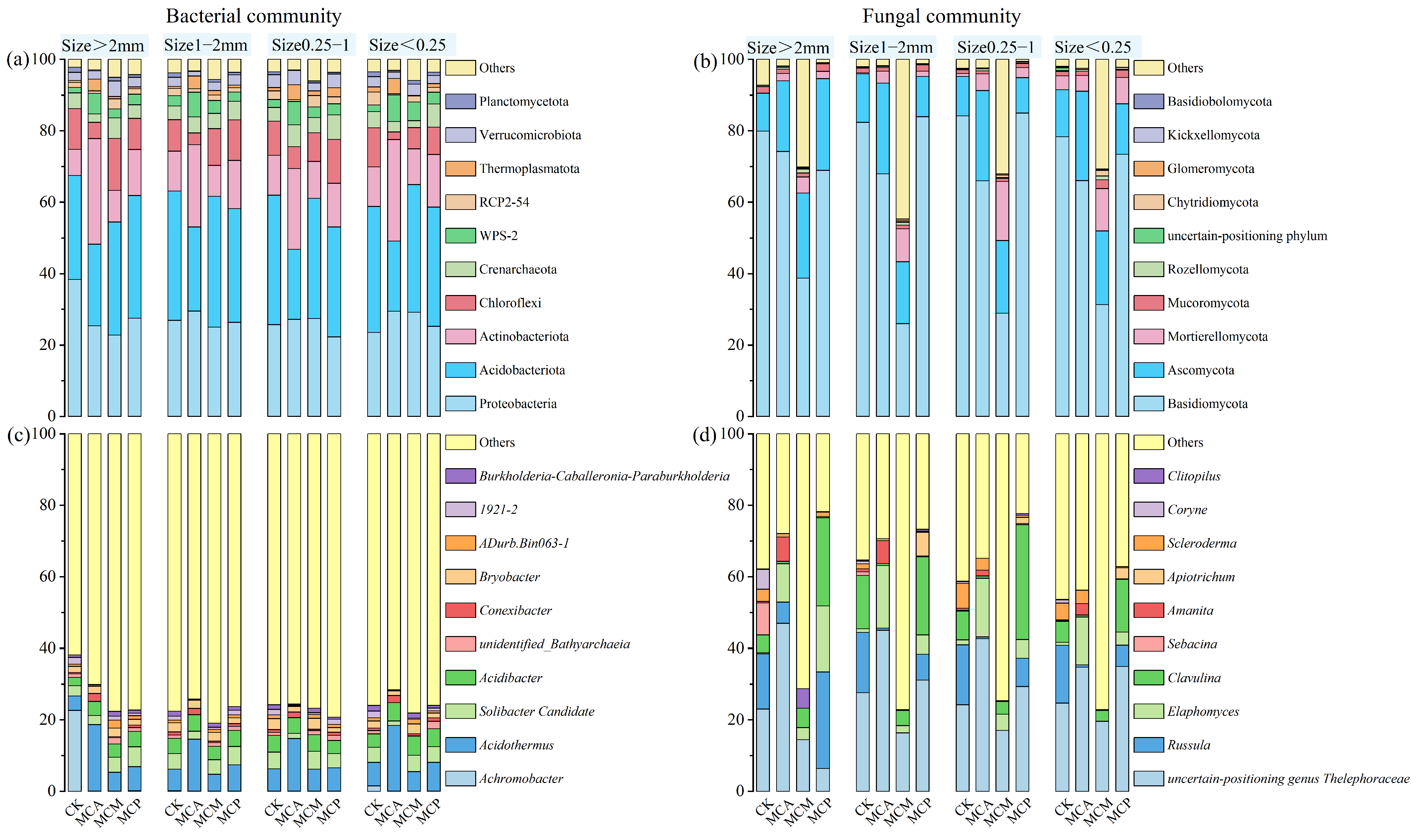
3.3. Analysis of Microbial Community Composition in Soil Aggregates
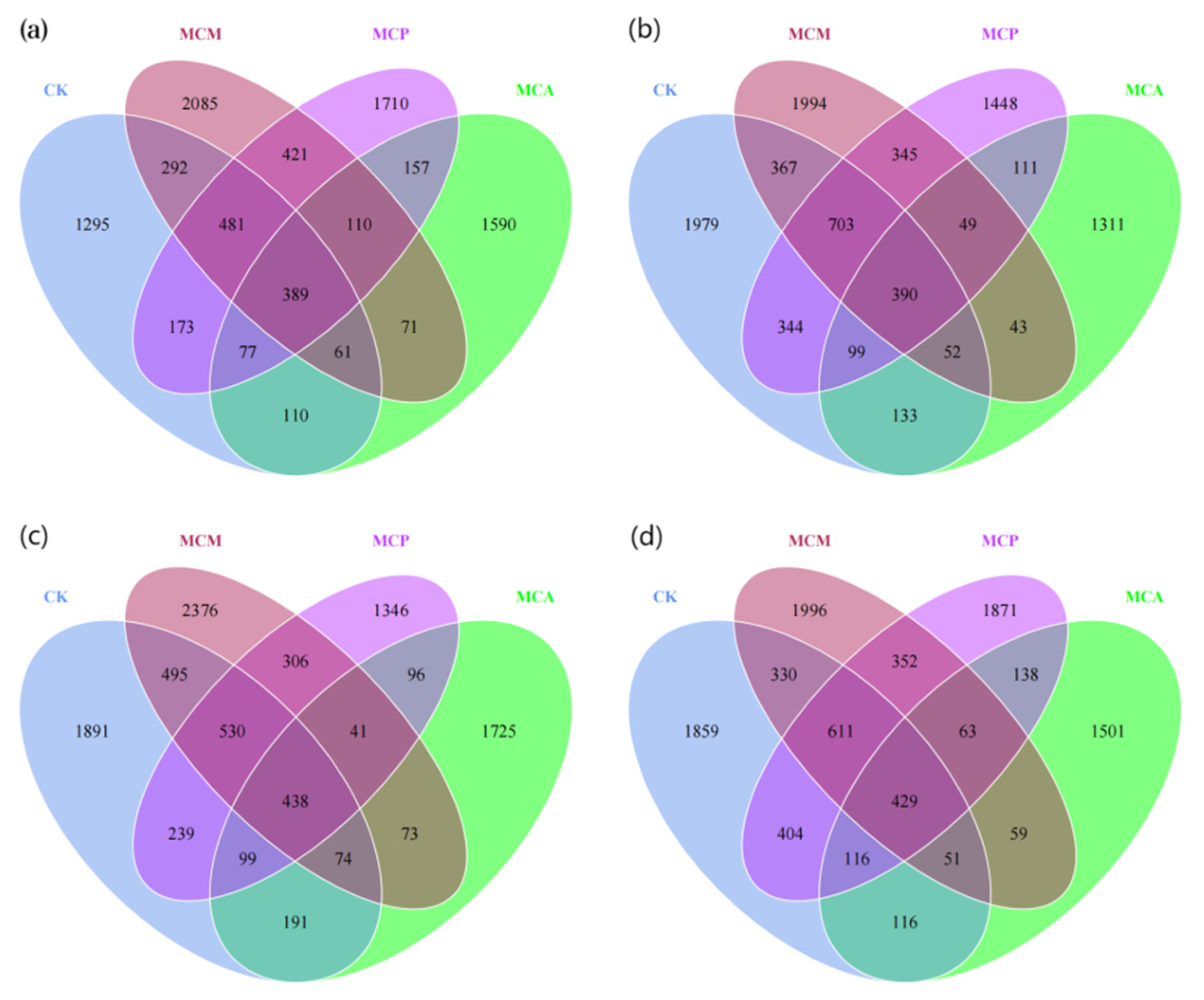
3.4. Distribution of ASV Counts in Soil Aggregates
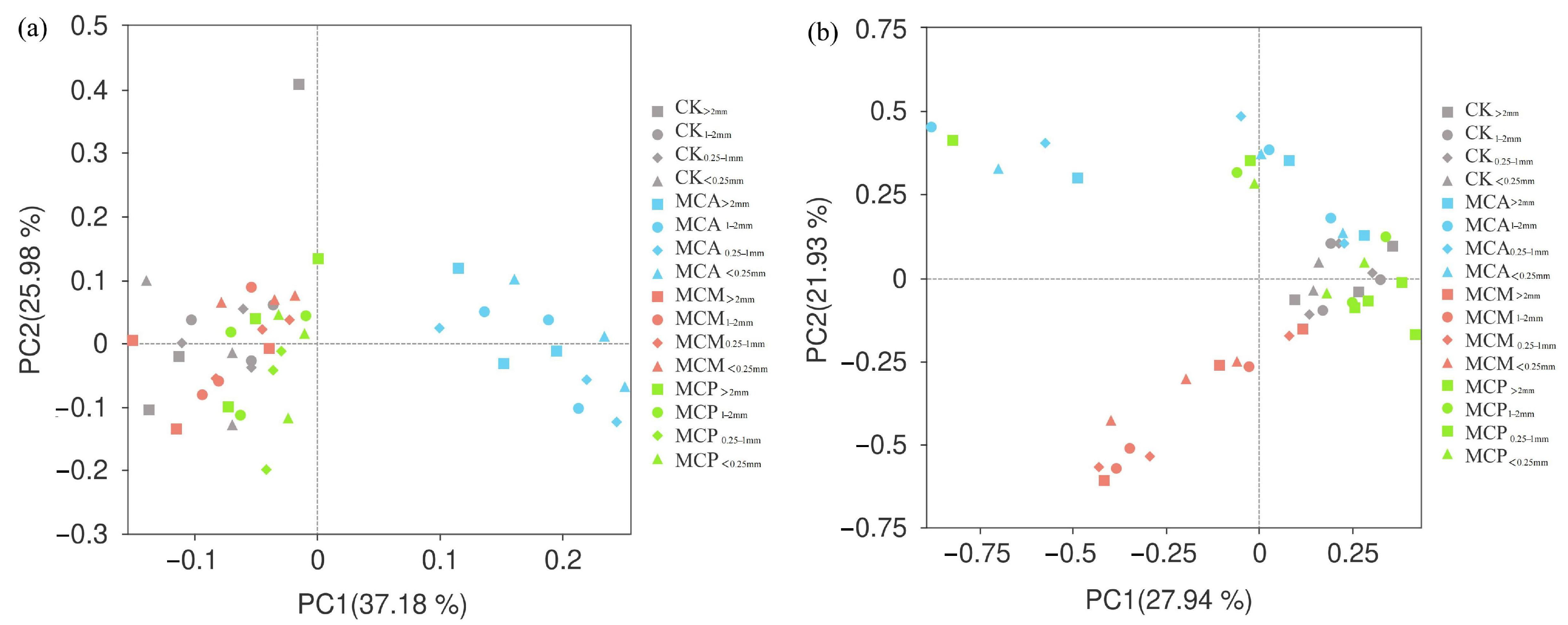
3.5. β-Diversity of Microorganisms in Soil Aggregates
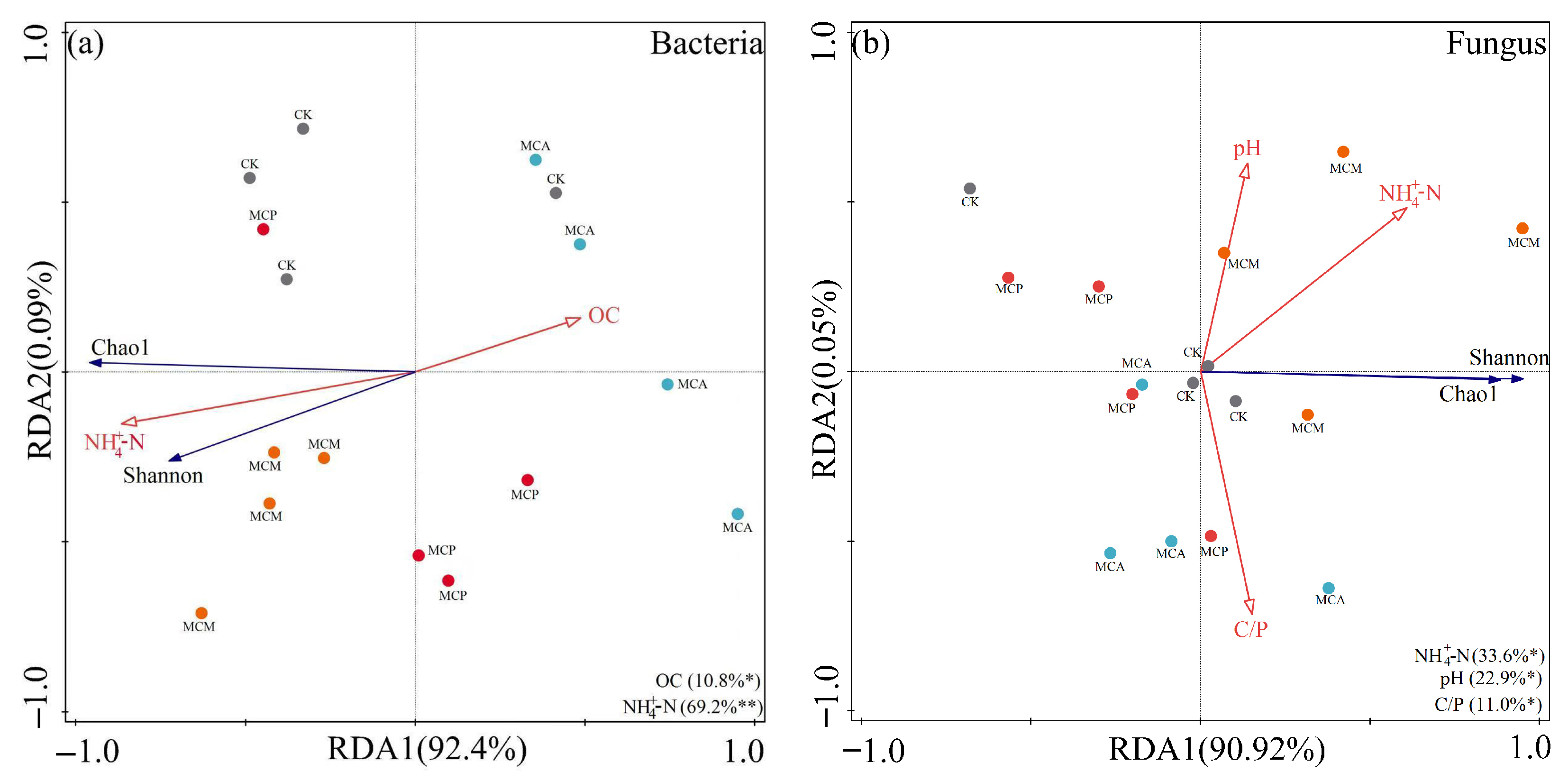
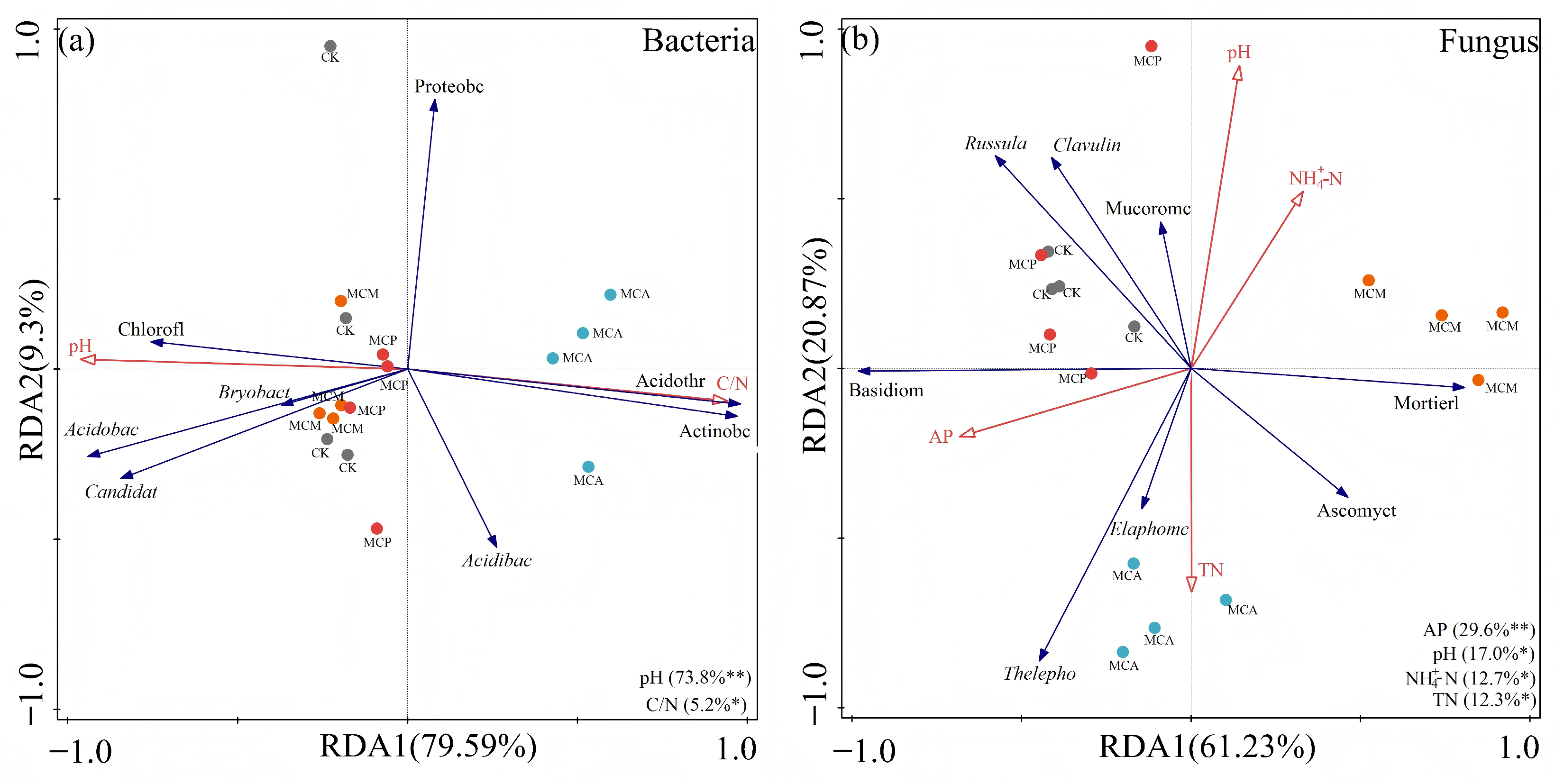
3.6. Relationships Between Soil Aggregate Chemical Factors and Microbial Communities
3.6.1. Microbial Diversity
3.6.2. Distribution of Dominant Microbial Taxa
3.6.3. PLS-PM Analysis
4. Discussion
4.1. Relationships Between Soil Aggregate Factors and Microbial Communities
4.2. Soil Aggregate Microbial Community Structure in Different Forest Types
4.3. Relationship Between Environmental Factors and Soil Aggregate Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, X. Productivity and carbon sink capacity of eucalyptus plantations in China from 1973 to 2018. Sci. Silvae Sin. 2023, 59, 54–64. [Google Scholar]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Lin, L.; Yu, D.; Jin, W.; Gai, Y.; Dai, J.; Ling, W. Effects of plantation on aggregate distribution and stability of lateritic red soil in south subtropical China. Chin. J. Appl. Ecol. 2020, 31, 3647–3656. [Google Scholar]
- Xu, H.; Yuan, H.; Yu, M.; Xiang, C. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- You, Z.; Miao, M.; Jin, Z.; Jie, M.; Sheng, L. Study on the composition and stability of soil aggregates of the main forest stands in Fengyang Mountain, Zhejiang Province. J. Nanjing For. Univ. 2018, 61, 84. [Google Scholar]
- Ya, Y.; Xiao, H.; Xue, D.; Lu, L. Distribution of aggregate-associated organic carbon and aggregate stability in rhizosphere of different plants. Chin. J. Soil. Sci. 2012, 43, 320–324. [Google Scholar]
- Peng, Y.; Schmidt, I.K.; Zheng, H.; Hedenec, P.; Luciana, B.; Yue, K.; Fu, W.; Vesterdal, L. Tree species effects on topsoil carbon stock and concentration are mediated by tree species type, mycorrhizal association, and N-fixing ability at the global scale. Forest Ecol. Manag. 2020, 478, 118510. [Google Scholar] [CrossRef]
- Demenois, J.; Carriconde, F.; Bonaventure, P.; Maeght, J.; Stokes, A.; Rey, F. Impact of plant root functional traits and associated mycorrhizas on the aggregate stability of a tropical Ferralsol. Geoderma 2018, 312, 6–16. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Ye, S. Distribution of organic carbon and nutrients in soil aggregates under different stand types of Cunninghamia lanceolata in southern Guangxi of China. Soil. Sci. Plant Nutr. 2021, 67, 427–438. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Lv, F.; Yan, Y.; Zhao, W.; Zheng, Z.; Zi, L.; Bing, Z. Fungal alpha diversity influences stochasticity of bacterial and fungal community assemblies in soil aggregates in an apple orchard. Appl. Soil. Ecol. 2021, 162, 103878. [Google Scholar] [CrossRef]
- Xiang, G.; Chun, L.; Jing, L.; Luo, Y.; Qing, Y.; Wei, Z.; Pu, Y.; Bai, F. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil. Till. Res. 2019, 195, 10435. [Google Scholar]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. Fems Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Rochester, I.J.; Trivedi, C.; Van, J.D.; Zhou, J.Z.; Karunaratne, S.; Anderson, I.; Singh, B.K. Soil aggregate sizemediates the impacts of cropping regimes on soil carbon andmicrobial communities. Soil. Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Wang, H.; Guan, D.; Zhang, R.; Chen, Y.; Hu, Y.; Xiao, L. Soil aggregates and organic carbon affected by the land use change fromrice paddy to vegetable field. Ecol. Eng. 2014, 70, 206–211. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Wei, H.; Deng, Y.; Lin, L.; Huang, J.; Wang, J.; Huang, H.; Huang, Z. Comparative studly on the stability of soil ag grezates in typical microhabitats inkarst ecologically fragile areas. Acta Ecol. Sin. 2022, 42, 2751–2762. [Google Scholar]
- Hai, D.; Hai, Y.; Jing, X.; Mu, Y.; Xiang, C. Effects of uneven-aged Cunninghamia lanceolata and evergreen broadleaved mixed plantations on soil aggregate stability and soil organic carbon and nutrients stocks. For. Res. 2020, 33, 107–115. [Google Scholar]
- Ye, Y.; Wang, H.; Luan, J.; Ma, J.; Ming, A.; Liu, S. Early effects of diffrent tree species mixing on aggregate organic carbon fractions. Chin. J. Ecol. 2024, 43, 3350–3356. [Google Scholar]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz, L.A.; Xin, Y.; Hu, D.; Kelin, W.; De, L. Tree species diversity increases soil microbial carbon use efficiency in a subtropical forest. Global Change Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Yin, Z.; Li, X.; Lu, J. Effects of organic carbon and iron oxides on soil aggregate stability under different tillage systems in a rice-rape cropping system. Catena 2019, 177, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Fang, L.; Qiu, T.; Bing, H.; Cui, Y.; Sardans, J. Disconnection between plant–microbial nutrient limitation across forest biomes. Funct. Ecol. 2023, 37, 2271–2281. [Google Scholar] [CrossRef]
- Bertini, S.C.B.; Stromberger, M.; Azevedo, L.C.B.; Cardoso, E.J.B.N. Soil physicochemical and biological profiles as indicators for Araucaria forest disturbance levels. Appl. Soil Ecol. 2021, 158, 103794. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Chen, J.; Xu, H.; Ma, S.; Dippold, M.A.; Kuzyakov, Y. Long-term nitrogen and phosphorus fertilization reveals that phosphorus limitation shapes the microbial community composition and functions in tropical montane forest soil. Sci. Total Environ. 2023, 854, 158709. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhang, X.; Mao, Q.; Li, X.; You, Y.; Wang, J.; Zheng, M.; Zhang, W.; Lu, X.; et al. Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Lu, Y.; Li, K.; Liang, Q.; Li, C.; Zhang, C. Effects of leaf litter decomposition on bacterial community structure in the leaf litter of four dominant tree species in Mount Tai. Acta Ecol. Sin. 2019, 39, 3175–3186. [Google Scholar]
- Wang, P.; Dong, J.; Xia, L.; He, J.; Kuang, S.; Xu, Y.; Cong, P. Effects of straw carbon types on fungal community characteristics of soil aggregates. Acta Pedol. Sin. 2024, 61, 1714–1728. [Google Scholar]
- Ji, Y.; Yong, L.; Chun, L.; Mei, L.; Ji, Z. Soybean-corn intercropping increases fungal community structure and diversity in red soil aggregates. J. Plant Nutr. Fert. 2023, 29, 889–899. [Google Scholar]
- Chen, X.; Li, Z.; Liu, M.; Chun, J.; Yu, C. Microbial community and functional diversity associated with different aggregate fractions of a paddy soil fertilized with organic manure and/or NPK fertilizer for 20 years. J. Soils Sediments 2015, 15, 292–301. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Wu, Y.; Yu, J.; Shen, G.; Jiang, M.; Wang, X.; Zhang, X. Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- An, C.; Zhang, B.; Fan, J.; Shi, W. Unveiling the mechanism for the rapid development of hydrostatically oxygenic photogranules. China Environ. Sci. 2025, 1, 11. [Google Scholar]
- Ni, X.; Liao, S.; Tan, S.; Wang, D.; Yan, P.; Kai, Y.; Wu, F.; Yang, Y. A quantitative assessment of amino sugars in soil profiles. Soil Biol. Biochem. 2020, 143, 107762. [Google Scholar] [CrossRef]
- Cai, Y.F.; Barber, P.; Dell, B.; Brien, P.; Williams, N.; Bowen, B.; Hardy, G. Soil bacterial functional diversity is associated with the decline of Eucalyptus gomphocephala. Forest Ecol. Manag. 2010, 260, 1047–1057. [Google Scholar] [CrossRef]
- Hu, L.; Xiang, Z.; Wang, G.; Rafique, R.; Liu, W. Changes in soil physicochemical and microbial properties along elevation gradients in two forest soils. Scand. J. Forest Res. 2016, 31, 242–253. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Wu, H.; Ou, S.; Zhou, B.; Zeng, Y.; Chen, Y.; Kuzyakov, Y. Tree species identity surpasses richness in affecting soil microbial richness and community composition in subtropical forests. Soil Biol. Biochem. 2019, 130, 113–121. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Jia, H.; Sun, W.; Ming, A.; Pang, S.; An, N.; Zhang, J.; Tang, C.; Dong, S. Influence of slope direction on the soil seed bank and seedling regeneration of Castanopsis hystrix seed rain. Forests 2021, 12, 500. [Google Scholar] [CrossRef]
- Zhang, D.; Man, X.; Liu, S.; Xu, Z. Litter decomposition and nutrient release of typical forestcommunities in non-growing season in cold temperate zone. J. Beijing For. Univ. 2022, 44, 65–74. [Google Scholar]
- Gao, W.; Ye, G.; Yue, X.; Liu, H.; Huang, Z. Soil microbial communities in different protection forest types in coastal sand dunes of Fujian Province. Res. Soil Water Conserv. 2024, 31, 204–212. [Google Scholar]
- Rahman, M.M.; Tsukamoto, J.; Rahman, M.M.; Yoneyama, A.; Mostafa, K.M. Lignin and its effects on litter decomposition in forest ecosystems. Chem. Ecol. 2013, 29, 540–553. [Google Scholar] [CrossRef]
- Wu, L.; Lin, X.; Lin, W. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. [Google Scholar]
- Plante, A.F.; McGill, W.B. Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil Till. Res. 2002, 66, 79–92. [Google Scholar] [CrossRef]
- Yong, G.; Wang, C.; Zhang, H.; Chao, H.; Xie, J.; Hong, J.; Li, Y.; Meng, H. Responses of cementing materials and stability of reclaimed soil aggregates to different fertilization regimes. Chin. J. Appl. Environ. Biol. 2022, 28, 1042–1050. [Google Scholar]
- Luo, G.; Rensing, C.; Chen, H.; Liu, M.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 2018, 32, 1103–1116. [Google Scholar] [CrossRef]
- Liu, F.; He, W.; Sun, Q.; Xi, L.; Liao, A.; Han, L. Effect of alfalfa green manure on diversity and function of soil bacteria in apple orchards in Tarim Basin. J. Agric. Sci. Technol. 2024, 26, 223–233. [Google Scholar]
- Zhang, X.; Liu, S.; Huang, Y.; Fu, S.; Wang, J.; Ming, A.; Li, X.; Yao, M.; Li, H. Tree species mixture inhibits soil organic carbon mineralization accompanied by decreased r-selected bacteria. Plant Soil 2018, 431, 203–216. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Yin, Y.; Wei, Y.; Qin, S.; Zhu, W. Soil bacterial community structure characteristics in coniferous forests of montane regions ofeastern Liaoning Province, China. Acta Ecol. Sin. 2019, 39, 997–1008. [Google Scholar]
- Feng, X.; Chao, X.; Ju, W.; Liu, K. A study on community characteristics of forest soil bacteria in Lushan national nature reserve. For. Resour. Manag. 2019, 6, 101–107. [Google Scholar]
- Li, S.; Huang, X.; Lang, X.; Shen, J.; Xu, F.; Su, J. Cumulative effects of multiple biodiversity attributes and abiotic factors on ecosystem multifunctionality in the Jinsha River valley of southwestern China. For. Ecol. Manag. 2020, 472, 118281. [Google Scholar] [CrossRef]
- Taibi, Z.; Saoudi, B.; Boudelaa, M.; Trigui, H.; Belghith, H.; Gargouri, A.; Ladjama, A. Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp. strain Cpt20 isolated from poultry compost. Appl. Biochem. Biotech. 2012, 166, 663–679. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Xie, J.; Deng, Y.; Wu, L.; Cheng, X.; Fei, S.; Deng, S.; He, Z.; Nostrand, J.; et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2012, 2, 106–110. [Google Scholar] [CrossRef]
- Barbi, F.; Prudent, E.; Vallon, L.; Buee, M.; Dubost, A.; Legout, A.; Marmeisse, R.; Fraissinet, L.; Luis, P. Tree species select diverse soil fungal communities expressing different sets of lignocellulolytic enzyme-encoding genes. Soil Biol. Biochem. 2016, 100, 149–159. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Liu, S.; Hu, J.; Ming, A.; Chen, H.; Wu, J.; Shi, Z.; Lu, L.; Cai, D. Relationship between tree species richness andsil miembial diversity and commumity composition in a mixed planted south subtropical forest. Acta Ecol. Sin. 2020, 40, 8265–8273. [Google Scholar]
- Egidi, E.; Delgado, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales-one thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sanchez, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef]
- Li, W. Effects of Mixing with Broad-Leaved Trees on Dynamics of Carbon, Nitrogen, and Phosphorus and Microbial Characteristics in Rhizosphere Soil in Subtropical Coniferous Forests. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2021. [Google Scholar]
- Yuan, J.; Wen, T.; Zhang, H.; Zhao, M.; Penton, C.R.; Thomashow, L.S.; Shen, Q. Predicting disease occurrence with high accuracy based on soil macroecological patterns of fusarium wilt. ISME J. 2020, 14, 2936–2950. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Zhou, B.; Yang, J.; Xing, S. Effect of biochar-based fertilizer on soil bacteria and fungi quantity and community structure in acidified tea garden. J. Fujian Agric. For. Univ. 2023, 52, 247–257. [Google Scholar]
- Mackay, J.E.; Macdonald, L.M.; Smernik, R.J.; Cavagnaro, T.R. Organic amendments as phosphorus fertilisers: Chemical analyses, biological processes and plant P uptake. Soil Biol. Biochem. 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Liu, N.; Yang, Y.; Tong, C.; Luo, M.; Huang, J. Soil microbial metabolism limitation and carbon use efficiency in four different wetlandhabitats of Jiulong River estuary. Acta Sci. Circumstantiae 2023, 43, 471–482. [Google Scholar]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Marschner, P.; Yang, C.H.; Lieberei, R.; Crowley, D.E. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol. Biochem. 2001, 33, 1437–1445. [Google Scholar] [CrossRef]
- Jiang, J.; Song, M. Review of the roles of plants and soil microorganisms in regulating ecosystem nutrient cycling. J. Plant Ecol. 2010, 34, 979–988. [Google Scholar]
- Zhang, S.; Li, Q.; Lu, Y.; Zhang, X.; Liang, W. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Lu, D.; Mao, Z.; Tang, Y.; Feng, B.; Xu, L. Driving factors influencing soil microbial community succession of coal mining subsidence areas during natural recovery in inner mongolia grasslands. Microorganisms 2023, 12, 87. [Google Scholar] [CrossRef]
- Fang, W.; Huang, B.; Sun, Y.; Yan, D.; Li, Y.; Bruno, T.; Roncada, P.; Wang, Q.; Cao, A. Soil amendments promoting nitrifying bacteria recovery faster than the denitrifying bacteria at post soil fumigation. Sci. Total Environ. 2024, 908, 168041. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, X.; Zhao, Y.; Wang, X.; Zhang, Y.; Liang, Y.; Weng, Y.; Chen, Y. Effects of mine remediation mode on microbial community structure and function in the rhizosphere soil of Robinia pseudoacacia. Chin. J. Environ. Sci. 2024, 1–17. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Ingwersen, J.; Poll, C.; Streck, T.; Kandeler, E. Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 2008, 40, 864–878. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.; Zuo, Q.; Du, B.; Chen, W.; Wei, D.; Huang, Q. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of northeastern China. Sci. Total Environ. 2018, 635, 784–792. [Google Scholar] [CrossRef]



| Stand Types | Altitudes (Masl) | Slope (°) | CD | DBH (cm) | Tm (m) | ||
|---|---|---|---|---|---|---|---|
| Main Species | Mixed-Breed Tree Species | Main Species | Mixed-Breed Tree Species | ||||
| CK | 226 | 27 | 0.7 | 22.7 | – | 18.7 | – |
| MCA | 165 | 25 | 0.8 | 20.0 | 36.0 | 18.1 | 25.4 |
| MCM | 173 | 26 | 0.8 | 19.8 | 24.3 | 21.7 | 23.5 |
| MCP | 182 | 28 | 0.7 | 21.6 | 23.1 | 17.1 | 19.5 |
| Indicator | Stand Type | |||
|---|---|---|---|---|
| CK | MCA | MCM | MCP | |
| BD (g/cm3) | 1.20 ± 0.04 ab | 1.13 ± 0.09 b | 1.27 ± 0.03 a | 1.16 ± 0.06 ab |
| SP (%) | 49.7 ± 2.65 a | 47.5 ± 4.20 a | 49.6 ± 3.04 a | 50.2 ± 2.49 a |
| WC (%) | 30.1 ± 3.06 a | 29.2 ± 4.74 a | 32.5 ± 1.76 a | 29.3 ± 1.76 a |
| pH | 4.51 ± 0.04 a | 4.06 ± 0.06 c | 4.48 ± 0.03 ab | 4.43 ± 0.04 b |
| OC (g/kg) | 30.6 ± 2.12 ab | 40.1 ± 1.49 a | 27.7 ± 4.50 ab | 21.5 ± 1.50 b |
| TN (g/kg) | 2.16 ± 0.22 b | 2.58 ± 0.10 a | 1.59 ± 0.14 c | 1.94 ± 0.19 b |
| TP (g/kg) | 0.26 ± 0.01 a | 0.23 ± 0.02 ab | 0.25 ± 0.02 ab | 0.22 ± 0.03 b |
| AP (mg/kg) | 11.3 ± 0.15 a | 8.42 ± 1.10 b | 8.03 ± 1.17 b | 8.10 ± 0.84 b |
| NH4+-N (mg/kg) | 75.0 ± 8.01 a | 34.9 ± 10.6 b | 70.4 ± 11.4 a | 47.7 ± 7.08 b |
| NO3−-N (mg/kg) | 3.22 ± 0.14 a | 3.60 ± 0.86 a | 4.29 ± 0.83 a | 3.88 ± 0.16 a |
| C/N | 14.2 ± 0.53 ab | 15.6 ± 0.01 a | 17.6 ± 4.05 a | 11.1 ± 1.38 b |
| N/P | 8.17 ± 0.38 bc | 11.1 ± 1.19 a | 6.30 ± 0.73 c | 8.98 ± 1.54 b |
| C/P | 116 ± 2.97 b | 174 ± 18.5 a | 109 ± 13.3 b | 98.6 ± 5.49 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Liu, Y.; Cheng, F. Impact of Tree Species Mixture on Microbial Diversity and Community Structure in Soil Aggregates of Castanopsis hystrix Plantations. Microorganisms 2025, 13, 578. https://doi.org/10.3390/microorganisms13030578
Zhang R, Liu Y, Cheng F. Impact of Tree Species Mixture on Microbial Diversity and Community Structure in Soil Aggregates of Castanopsis hystrix Plantations. Microorganisms. 2025; 13(3):578. https://doi.org/10.3390/microorganisms13030578
Chicago/Turabian StyleZhang, Rongao, Yijun Liu, and Fei Cheng. 2025. "Impact of Tree Species Mixture on Microbial Diversity and Community Structure in Soil Aggregates of Castanopsis hystrix Plantations" Microorganisms 13, no. 3: 578. https://doi.org/10.3390/microorganisms13030578
APA StyleZhang, R., Liu, Y., & Cheng, F. (2025). Impact of Tree Species Mixture on Microbial Diversity and Community Structure in Soil Aggregates of Castanopsis hystrix Plantations. Microorganisms, 13(3), 578. https://doi.org/10.3390/microorganisms13030578






