Abstract
Lactococcosis caused by Lactococcus garvieae is a bacterial infection affecting fish with a considerable economic impact. Recently, L. garvieae has established itself as an opportunistic pathogen in humans. The aim of the current study was to test classical and molecular-biological methods for the identification of L. garvieae and examine antimicrobial susceptibility and capsule production, an important virulence factor. Additionally, tests for differentiation from closely related species, as well as epidemiological typing, were performed. In a period of 18 years (2002–2019), 24 isolates presumptively identified as L. garvieae were collected from Oncorhynchus mykiss and Salmo salar fish obtained either from retail stores or fish farms. In order to confirm the species, optimized PCR-based protocols were used. As a result, 21 of the tested strains were proved to be L. garvieae (n = 21). The remaining three isolates were Lactococcus lactis, Streptococcus iniae, and Enterococcus faecalis. Epidemiological typing by randomly amplified polymorphic DNA was performed. Except for a single KG+ isolate, all other strains belonged to the European capsular serotype KG−. All L. garvieae isolates showed susceptibility to all tested antibiotics with the exception of clindamycin, which was a diagnostic sign. A thorough optimization of diagnostic methods is essential to determining the etiology of specific infections affecting the personnel at risk in fish farms, the food industry, or within the broader community.
1. Introduction
Several species belonging to the genus Lactococcus including Lactococcus garvieae, Lactococcus plantarum, Lactococcus piscium, and Lactococcus raffinolactis have been identified as clinically significant fish pathogens, [1,2]. In recent years, the new species Lactococcus petauri and Lactococcus formosensis, which are closely related to the Lactococcus garvieae species, have been described [3].
Lactococcus garvieae (L. garvieae, previously known as Enterococcus seriolicidae) was initially isolated in 1974 from the Japanese amberjack (Seriola quinqueradiata) in Japan [4]. Later, this pathogen has been isolated from mastitis in cows [5]. Lactococcus garvieae is a Gram-positive, facultative anaerobe, arranged in pairs or chains. On nutrient media, it grows with small, rounded colonies with smooth borders and a convex profile. It exhibits a catalase-negative reaction and primarily demonstrates alpha hemolysis on blood agar, resembling enterococci and alpha-hemolytic streptococci. As a part of the genus Lactococcus, it is able to produce lactic acid from glucose [2,6]. This pathogen has three serological types based on geographical differences: the European capsular serotype, the Japanese capsular serotype, and the non-capsulated serotype from both regions—Europe and Japan. Numerous studies on the pathogenicity of L. garvieae have established that encapsulated isolates (KG− serotype) exhibit greater virulence compared to non-encapsulated strains (KG+ serotype) [1].
In routine microbiological practice, lactococci are often misidentified as enterococci (Enterococcus faecalis and Enterococcus faecium) or streptococci even when commercial biochemical identification panels are used. The precise microbiological diagnosis of L. garvieae is challenging because of the close homology of the species belonging to the genus Lactococcus. Importantly, L. garvieae, L. petauri, and L. formosensis cannot be discriminated using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF), 16S sequencing, or biochemical tests [3,7]. In recent years, some relatively new approaches have been developed to identify these species: whole-genome sequencing, intergenic spacer 16S–23S sequencing, gyrB gene sequencing, multiplex PCR, and serotype analysis using PCR [7,8,9,10].
L. garvieae has emerged as a significant global bacterial pathogen in the aquaculture industry, affecting both freshwater and marine fish, particularly at water temperatures exceeding 15 °C, causing significant economic loss [2,11,12,13]. Lactococcosis has been reported in various aquaculture species, including Nile tilapia (Oreochromis niloticus), rainbow trout (Oncorhynchus mykiss), yellowtail (Seriola quinqueradiata), amberjack (Seriola dumerili), cobia (Rachycentron canadum), barramundi (Lates calcarifer), and catfish (Pseudoplatystoma sp.) [13,14,15,16,17,18,19,20]. L. garvieae has been isolated from subclinical inflammations in cattle, poultry, pigs, canine, and feline tonsils [21,22,23,24,25,26,27]. Outbreaks among those animals may serve as a significant source with the potential to affect humans due to the various routes of transmission involved [28,29]. Recently, L. garvieae has been recognized as an opportunistic pathogen in humans, attributed to a rising incidence of infections that result in considerable morbidity and mortality [6,30,31,32,33]. Risk factors include exposure to or ingestion of raw fish, along with the presence of concurrent gastrointestinal disease. Even immunocompetent individuals could be susceptible when they consume undercooked fish [6,34]. The first human case of L. garvieae infection was reported in 1991 in a patient with endocarditis [28]. Bacterial endocarditis is one of the clinical manifestations, predominantly observed in elderly patients [29,35]. A limited number of cases of this rare condition has been reported globally, most notably in Europe, Turkey, North America, Latin America, and Asia [31,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Bacteremia and sepsis have been observed in both children and adults [33,50,51,52,53,54]. Other reported diseases associated with L. garvieae include infective endocarditis, septicemia, peritonitis, hepatic abscess, meningitis, tonsillitis, osteomyelitis, empyema, and urinary tract infections [6,42,47,49,55,56,57,58,59,60,61,62,63,64]. Also, the pathogen has been frequently isolated from stools [65]. In many patients infected with L. garvieae, the source of infections remains unknown [6,32,57].
Considering all of the above, this study aimed to test classical and molecular biology methods to identify L. garvieae and differentiate it from closely related species, perform epidemiological typing, and determine the antimicrobial resistance and factors of virulence of bacterial strains isolated in the period of 2002–2019 from various clinical specimens. It is crucial to identify the causative agent promptly and implement measures to restrict the dissemination of pathogens, thereby preventing outbreaks among fish, other species, and humans, as well as mitigating potential economic losses.
2. Materials and Methods
2.1. Sample Collection
A total of 24 strains were obtained over an eighteen-year period from 2002 to 2019, 21 (n = 21) of which were confirmed as L. garvieae. The other three were identified as L. lactis, S. iniae, and E. faecalis and subsequently used as negative controls. Isolates were collected from different cities in Bulgaria and Greece (Table 1). The specimens were from various organs of rainbow trout fish (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar), including the heart, kidney, brain, small and large intestines, lungs, liver, and spleen.

Table 1.
Strains of L. garvieae and other bacterial species included in the study.
2.2. L. garvieae Bacterial Isolates
L. garvieae was initially identified by colony and Gram staining morphology, alpha-hemolysis on blood agar, the positive pyrrolidonyl arylamidase (PYR) test, and negative catalase, oxidase, and motility tests. Strains were growth on Trypticase Soy Agar (TSA), Tryptic Soy Blood Agar (TSBA), Brain Heart Infusion Agar (BHIA), 5% sheep blood agar, and MRS agar. Growth in the presence of 6.5% NaCl and pH 9.6 on Brain Heart Infusion Broth (BHIB) was tested. Detailed biochemical identification was performed with BD BBL™ Crystal™ Gram-Positive (GP) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Strains that correspond biochemically to the genus Lactococcus were additionally tested with Antony staining (for the demonstration of capsule production with India ink) and the slide agglutination test using specific anti-Lactococcus garvieae serum (NRL “FMCR”, NDRRIMI, Sofia, Bulgaria) against a capsular reference strain 331/8066/Belgium. The presumed identification of the species was conducted by MALDI-TOF MS (VITEK MS system) according to the manufacturer’s instructions (bioMerieux, Craponne, France).
The isolates were stored in tryptic soy broth (Sigma-Aldrich, Bengaluru, India) supplemented with 10% glycerin at −80 °C until the beginning of the tests. Prior to testing, they were subcultivated on tryptic soy blood agar (TSBA, Himedia, Thane, India) for 24 h at 37 °C under aerobic conditions.
Lactococcus garvieae ATCC 43921, confirmed isolates of Lactococcus garvieae from Serbia, Streptococcus iniae, Lactococcus lactis, and Enterococcus faecalis were used as positive and negative control strains in the experiments.
2.3. DNA Extraction
Pure cultures of all strains included in the study were used for genomic DNA extraction, which was performed using an extraction kit (NucleoSpin Tissue, Macherey-Nagel, Düren, Germany) in accordance with the manufacturer’s instructions. All extracted DNA was stored at −80 °C until testing
2.4. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed using the Kirby–Bauer disk diffusion method. Eight antibiotic disks were used to determine antimicrobial susceptibility: ampicillin (2 µg), ceftriaxone (30 µg), erythromycin (15 µg), tetracycline (30 µg), levofloxacin (5 µg), chloramphenicol (30 µg), vancomycin (5 µg), and linezolid (30 µg). Examination took place on Mueller Hinton Fastidious Agar (Horse blood 5% + 20 mg/L beta-NAD; Liofilchem, Roseto degli Abruzzi, Italy).
For interpretations of the results from antibiotic testing, EUCAST recommendations for Viridans group streptococci were used (EUCAST 2024) [66]. In the absence of criteria, the guidelines provided by the Clinical and Laboratory Standards Institute (CLSI) were applied [67].
2.5. Polymerase-Chain Reaction (PCR)
All collected strains were tested with PCR using forward and reverse primers, which amplify a fragment of 1100 bp, previously used for identification by Zlotkin et al. in 1998 [68]. A final confirmation test to differentiate L. garvieae from closely related species was conducted using primers that were previously described by Dang et al. in 2012 and Saticioglu et al. in 2023 [9,69] (Table 2). The MyTaq PCR mix was used (Bioline, Meridian Bioscience, Memphis, TN, USA). The reaction conditions for PCR were initial denaturation at 95 °C for 1 min followed by 35 cycles consisting of denaturation at 95 °C for 1 min, annealing for 45 sec, elongation at 72 °C for 1 min, and final elongation at 72 °C for 7 min. Gradient PCR with different annealing temperatures (53–63 °C) was performed in order to optimize the protocol of Zlotkin et al. [68] and to inhibit non-specific reactions [68]. The study used Streptococcus iniae, Enterococcus fecalis, and deionized water as negative controls.

Table 2.
Primer sequences and amplification conditions for PCR of genes used in the current study.
The products before and after purification were controlled by gel electrophoresis in 2% agarose gel (Bioline, Meridian Bioscience, Memphis, TN, USA), 1× TAE buffer, a 10 pmol/mL concentration of ethidium bromide (Sigma-Aldrich, Steinheim, Germany), and DNA markers 100 bp (New England Biolabs, Ipswich, MA, USA) and 100 bp (Bioline, Meridian Bioscience, Memphis, TN, USA).
2.6. Sequencing of 16S rDNA Gene and Capsular Gene
The 16S rDNA gene was identified by sequencing using universal primers described by Lane et al. in 1991 (Table 2) [70]. The PCR conditions were similar to those described above. L. lactis, S. iniae, and E. fecalis were used as control strains.
To detect the presence of the 17 322 bp pathogenicity-associated island (capsule cluster) and a 750 bp capsule gene, the primers described by Miyauchi et al. in 2012 were used [71] (Table 2). The reaction was performed using the LongAmp Taq 2x Master Mix (New England Biolabs, Ipswich, MA, USA) and the following protocol: activation of polymerase for 30 s at 94 °C, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing for 30 s, elongation at 65 °C for 3.5 min, and a final step at 65 °C for 10 min. The final products were prepared for sequencing by purification through NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. The purified products were sequenced at Macrogen (Seoul, Republic of Korea). The obtained product was controlled as described above.
Obtained sequential data were processed and equalized by MEGA X software [72], using the MUSCLE algorithm [73], and were analyzed in BLAST (Basic Local Alignment Search Tool) NCBI (National Center for Biotechnology Information, U.S. National Library of Medicine, 8600 Rockville Pike, Bethesda, MD 20894, USA).
2.7. Epidemiological Typing by Random Amplification of Polymorphic DNA (RAPD Analysis)
Three primers were used to perform RAPD analysis: E1 (CCCAAGGTCC) [74], P2 (GTTTCGCTCC) [75], and RAPD 4 (AAGACGCCGT) [76]. Reactions with individual primers were performed in volumes of 50 µL including 4 µL of DNA, 4 µL of primer, 25 µL of the Kapa Ready PCR mix (KapaBiosystems), and 17 µL of deionized water. Two programs were tested with the following temperature parameters:
1st variant: activation of polymerase at 95 °C for 1 min, followed by 45 cycles: denaturation at 95 °C for 1 min, annealing at 36 °C for 1 min (modification from Ravelo et al., 54/2003 (35 °C) [75]), and elongation at 72 °C for 2 min.
2nd variant: activation of polymerase at 94 °C for 5 min, followed by: 4 cycles at 94 °C—45 s, 30 °C—2 min, and 72 °C—30 s; 10 cycles at 94 °C—45 s, 36 °C—30 s, 72 °C—30 s; 10 cycles at 94 °C—45 s, 36 °C—30 s, 72 °C—40 s; 10 cycles at 94 °C—45 s, 36 °C—30 s, 72 °C—40 s; 10 cycles at 94 °C—45 s, 36 °C—30 s, 72 °C—50 s; 10 cycles at 94 °C—45 s, 36 °C—30 s, 72 °C—1 min; final extension at 72 °C for 10 min.
The resulting fragments from the reactions with the three primers were compared visually in 2% agarose gel with 1× TAE buffer and 10 pmol/mL ethidium bromide (Sigma-Aldrich, Steinheim, Germany), and subsequent phylogenetic analysis was performed.
The photo documentation system “Syngene GelVue” model No. GVM20 (Synoptics Ltd., Cambridge, UK) was used.
2.8. Data Analysis
The following programs were used to analyze the data:
The software GeneTools v. 4.1 (ChemiGenius, Syngene, Cambridge, UK) was used for the similarity matrices calculations on the basis of the electrophoretic gel images and the construction of the unweighted pair group method with arithmetic (UPGMA) dendrograms using the “profile” and “band position” options. Calculations were performed by means of the Jaccard algorithm [77], accounting not only for their positions but the peak surfaces of the fluorescent bands as well.
The evolutionary history of the 16s rDNA gene was inferred by using the Maximum Likelihood method and the Kimura 2-parameter model [78]. The tree with the highest log likelihood (−3008.43) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with the superior log likelihood value. The bootstrap 1000 replicates were used. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.5289)). The proportion of sites where at least 1 unambiguous base is present in at least 1 sequence for each descendent clade is shown next to each internal node in the tree. Evolutionary analyses were conducted in MEGA X [72].
3. Results
3.1. Cultivation and Identification
The cultural characteristics of the isolates presented small, rounded shapes with smooth edges and a convex profile when grown on MRS agar. They demonstrated α-hemolysis on blood agar. Biochemical analysis revealed that they were catalase-negative and -positive for the PYR reaction. Gram and Anthony staining procedures were performed, revealing the presence of Gram-positive ovoid cocci, occurring either singly or in groups of 2–3 cells, as well as in short chains (Supplementary Figure S1) with capsules (Supplementary Figure S2).
All tested isolates showed positive agglutination with rabbit anti-L. garvieae serum, except for strains 472 and 332 (Supplementary Figure S3).
Further biochemical identification was conducted with BD BBL™ Crystal™ Gram-positive (GP) (Becton, Dickinson and Company, USA). Nineteen strains were identified as L. garvieae. L. garvieae strains in the BBL Crystal Gram-positive ID System showed variability in 2 tests: sucrose and 4-methyl umbelliferyl-N-acethyl-b-D-glucosaminide (FGA) in combinations S+/FGA− and S−/FGA+.
Using MALDI-TOF MS spectrophotometric analysis, two more strains with positive agglutination were identified as L. garvieae. Isolate 472 was identified as L. lactis, isolate 330 as S. iniae, and isolate 332 as E. faecalis.
3.2. Antimicrobial Susceptibility
All L. garvieae strains were resistant to clindamycin and susceptible to ampicillin, ceftriaxone, erythromycin, tetracycline, levofloxacin, chloramphenicol, vancomycin, and linezolid.
3.3. Molecular-Genetic Methods
After DNA had been extracted from the strains, all DNA was tested by PCR to detect L. garvieae using the protocol of Zlotkin et al. [68]. The gel electrophoresis demonstrated the presence of PCR products not only from L. garvieae but also from the other three isolates that tested negative for L. garvieae (S. iniae, Enterococcus faecalis, and L. lactis). However, there were minor differences in both fragment length and signal intensity (Figure 1).
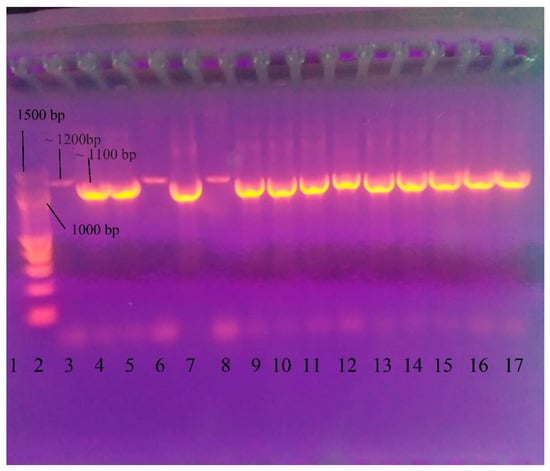
Figure 1.
Gel electrophoresis after PCR for identification of L. garvieae using the amplification protocol of Zlotkin et al. [68]. 1—negative control (double distilled water); 2—DNA Ladder 100 bp (New England Biolabs, Ipswichm, MA, USA); 3—472 L. lactis (~1200 bp); 4—331 L. garvieae (~1100 bp); 5—412 L. garvieae; 6—330 Streptococcus iniae (~1200 bp); 7—443 L. garvieae; 8—332 Enterococcus faecalis (~1200 bp); 9—17 L. garvieae isolates—322, 329, 386, 400, 418, 443, 459, 467, and 470.
The results of PCR performed following the protocol of Zlotkin et al. [68] showed that the method needed optimization [68]. Gradient PCR was conducted using a range of annealing temperatures from 55 °C to 62.9 °C to optimize the protocol and remove products from strains that were not L. garvieae. Annealing temperatures of 60.8 °C and 61.8 °C resulted only in L. garvieae products (Figure 2). The lower temperature (60.8 °C) was selected for the optimized protocol.
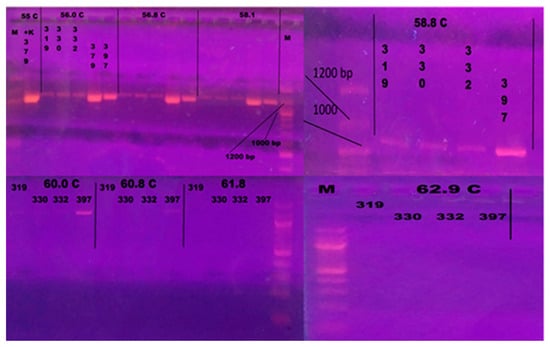
Figure 2.
Gel electrophoresis after gradient PCR for optimization of annealing temperature. The following isolates were included: 319—L. lactis; 330—Streptococcus iniae, 332—Enterococcus faecalis; 379 L. garvieae; 397—L. garvieae. M—100 bp Ladder (New England Biolabs, Ipswich, MA, USA). Annealing temperatures of 60.8 °C and 61.8 °C resulted in products specific to L. garvieae. The lower temperature (60.8 °C) was selected for the optimized protocol.
A final confirmation test for differentiation from closely related species in the genus Lactococcus was performed. PCR with primers LG_IBS_F (132), LG_IBS_R (132), LP_IBS_F (583), LG_IBS_R (583), ITS 30F (290), and ITS 319R (290) showed that 21 isolates were L. garvieae [9,69].
PCR for the identification of a 17,322 bp pathogenicity island (capsular cluster) was performed in L. garvieae strains and no such pathogenicity island was found. All of the strains, except for 468, demonstrated the presence of a ~750 bp product (Figure 3).
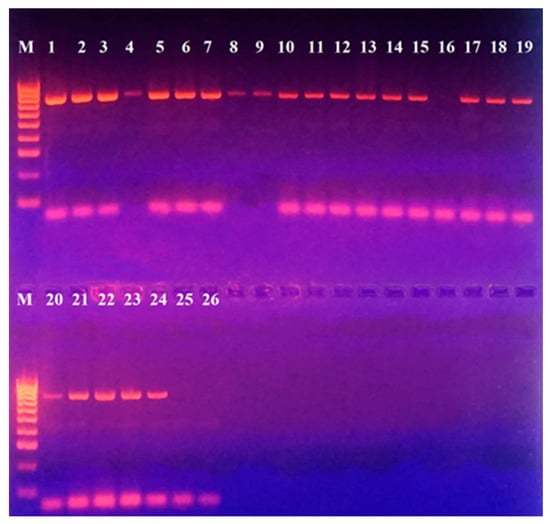
Figure 3.
PCR for detection of a pathogenicity island with included capsule production gene in L. garvieae strains included in this study. M—DNA Ladder 100 bp (Bio-line, Meridian Bioscience, TN, USA); Positions: 1—331; 2—322; 3—386; 4—329; 5—397; 6—400; 7—415; 8—412; 9—465; 10—417; 11—418; 12—443; 13—456; 14—459; 15—466; 16—468; 17—467; 18—469; 19—470; 20—471; 21—473 Serbian control L. garvieae; 22—474 Serbian control L. garvieae; 23—442; 24—444; 25—472 L. lactis; 26—Negative control (double-distilled water).
Epidemiological typing with both programs and three primers showed the largest number of fragments with primers P2 and E1. Typing with the primer RAPD4 showed too high homogeneity of the results in most strains (Figure 4 and Figure S4). These results were also reflected in the dendrogram based on the resulting fragments. Accordingly, the most appropriate primer for epidemiological typing was selected based on the position of strain L. lactis (472) included in this analysis. Namely, the selected primer was P2, which positioned it as an external group (Figure 5).
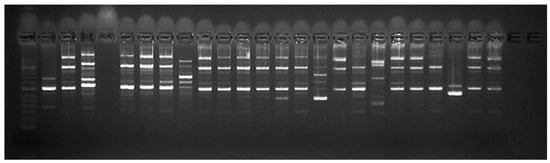
Figure 4.
Gel electrophoresis after epidemiological typing with primer P2; Position 1—Control ladder; Position 2—331/2002/Belgium; Position 3—322/2002/Dospat; Position 4—negative control; Position 5—386/2006/Dospat; Position 6—397/2008/Dospat; Position 7—400/2008/Dospat; Position 8—412/2010/Dospat; Position 9—415/2010/Greece; Position 10—417/2013 Dospat; Position 11—418/2013/Dospat; Position 12—443/2016/salmon; Position 13—456/2017/Dospat; Position 14—459/2017/Dospat; Position 15—465 brain of a cooled trout bought in 2017 from a fish stall at ‘Krasno selo’ market; Position 16—466 brain of a cooled trout bought in 2017 from a fish stall at supermarket; Position 17—467 heart of a trout from a fish farm in the town of Pirdop, 2019; Position 19—469 brain of a trout before therapy, town of Pirdop, 2019; Position 20—470 spleen of a trout, town of Pirdop, 2019; Position 21—471 after florfenicole treatment, town of Pirdop, 2019; Position 22—472 town of Pirdop, 2019; Position 24—473/2019/Serbian control L. garvieae; Position 25—474/2019/Serbian control L. garvieae.
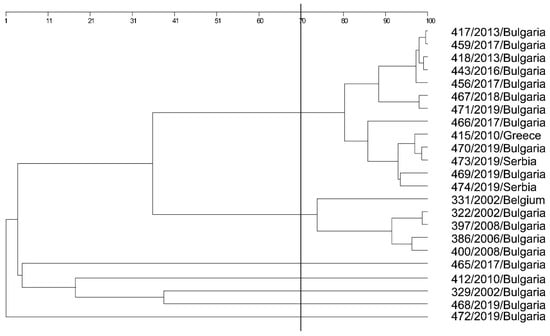
Figure 5.
Phylogenetic analysis (epidemiological typing) based on primer P2 and 23 L. garvieae strains, and a single L. lactis strain—472/2019.
The results of capsular gene sequencing were not suitable for processing due to background noise. Electropherograms from the 16S rRNA gene showed no background noise and were processed.
Multiple alignments of processed sequential data were performed, and L. garvieae sequences were chosen from GeneBank (NCBI). The most appropriate model for phylogenetic analysis was described by Kimura et al. [78]. The sequenced 16s rRNA genes of the included strains were processed and analyzed (Blast-NCBI) and showed 100% identity to the six L. garvieae strains stored in GeneBank (NCBI). A phylogenetic analysis was performed (Figure 6).
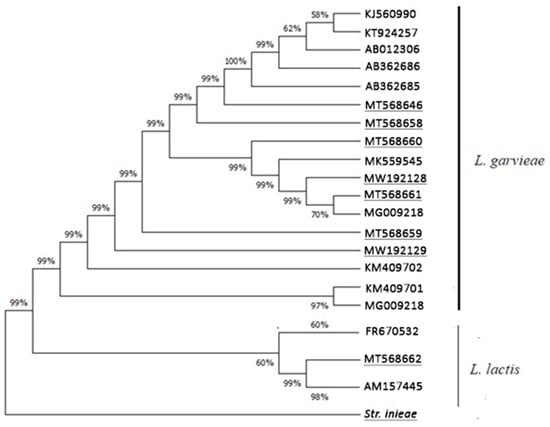
Figure 6.
The evolutionary history. This analysis involved 21 nucleotide sequences with standard 27F и 1492R primers for 16s rDNA gene. Sequences from our isolates (underlined) and the NCBI database were used. As an external group, S. iniae was used. There were a total of 1294 positions in the final dataset. Data were visualized via MEGA X [72].
4. Discussion
Lactococcosis is a bacterial disease that usually presents as acute hemorrhagic septicemia in fish, causing morbidity and mortality at rates between 20% and 50%. Infections have been reported in a wide range of hosts—fish, reptiles, marine mammals, cows, dogs, birds, and humans [6]. L. garvieae is considered the most important pathogen associated with lactococcosis. This microorganism is considered an emerging opportunistic agent, affecting primarily immunosuppressed individuals [30,31,32,33]. Genera with similar cultural and biochemical characteristics, as well as closely related species (Lactococcus petauri and Lactococcus formosensis), additionally complicate microbiology diagnosis, which requires a combination of morphological, physiological, and genetic methods [3].
The morphology of strains used in the current study corresponds to the typical features of L. garvieae described by other authors [9,79,80]. The colonies exhibited α-hemolysis on blood agar, which is generally more common than β-hemolysis observed in atypical isolates from water buffalo mastitis [13,75,80,81].
All L. garvieae isolates exhibited negative catalase activity and positive PYR tests; however, these tests are insufficient to differentiate them from enterococci. BD BBL™ Crystal™ Gram-positive (GP) (Becton, Dickinson and Company, USA) misdiagnosed two of the strains, while MALDI-TOF identified them as L. garvieae.
On the one hand, some authors have applied the method of Zlotkin et al. [68] to identify and/or confirm L. garvieae in clinical specimens from humans and fish, without detecting amplification in other bacterial species [2,24,38,39,40,68,75]. On the other hand, non-specific reactions, yet with small differences in the fragment length (100 bp), have been found by other authors [13]. In our study, positive results from other species (L. lactis, E. faecalis, and S. iniae) were found when the annealing temperature was up to 60 °C. Both different sizes and weaker signal intensities due to various product quantities were observed. These contradictory data about the presence or absence of non-specific reactions in the same microorganisms are possibly due to various factors such as strain specifications, method execution, types and sources of the reagents (whether the Taq polymerase is hot start or not), and the operator. It is well known that higher annealing temperatures may increase the specificity of PCR, which is the basis for optimizing the protocol and eliminating the non-specific reaction with the previously mentioned microorganisms, thus improving the diagnostic power of the method. This is useful in cases where no controls from other bacterial species are loaded as markers for non-specific reactions [11]. In this study, a combination of methods was suggested to eliminate the disadvantages of the Zlotkin protocol [68].
Antimicrobial susceptibility testing of lactococci was conducted in accordance with the established criteria for Viridans group streptococci. The decision was based on the fact that they both belong to the same family of Streptococcaceae, as well as on their common cultural characteristics. All examined isolates were resistant to clindamycin. This feature was used to differentiate L. lactis from L. garvieae [28].
Some authors divide L. garvieae serologically into three groups based on geographical differences, while others divide them into two groups, but both classifications have KG− (capsular) and KG+ (non-encapsulated), with KG− being more virulent [82,83,84]. When testing for the presence of a pathogenicity island, the presence of a cluster was not detected, but the presence of a capsule gene was observed in three of the isolates, showing poor capsule production and one isolate lacking a capsule gene. This result showed that with the exception of a single KG+ isolate, all other strains belonged to the European capsular serotype KG−, meaning that highly virulent strains were predominant in our study [1,84].
The results from capsular gene sequencing were not suitable for processing due to the presence of too many signaling peaks. These cannot be identified as ‘background noise’, but as rather overlapping peaks, which could be caused by various factors during the PCR processes, purification, and sequencing or the gene being non-homogeneous in the bacterial population [85]. However, as the same bacterial cultures were used and the results from 16s rDNA gene sequences were indisputable, the most probable reason was a non-homogeneous gene. The weaker visualization of the capsular gene on the agarose gel was likely due to the non-homogeneous bacterial population. Some bacterial cells may produce capsules while others may not. Such heterogeneity of the bacterial population with regard to the capsular gene was (to some extent) confirmed by the capsular staining performed where various-size capsules were observed in pure bacterial culture (the same cultures were used in other methods in this study).
Ravelo et al. tested a series of primers (P1–P6) for RAPD analysis of L. garvieae from various hosts (yellowtail, rainbow trout, and catfish) and various geographic regions and determined primers P5 and P6 as the most appropriate for both features—geographic origin and host [75]. The resulting fragments were from 7 up to 14. In this study, L. garvieae strains were collected from rainbow trout and Atlantic salmon. L. lactis was used as an external group control. Therefore, tests were conducted on alternative primers—E1, P2, and RAPD4—using different amplification protocols. Variant II of our program demonstrated the best representation (5–12 fragments) with primer P2. The difference between our results and those of Ravelo et al. was most likely due to the different amplification programs, as the authors used 30 amplification cycles with the same parameters: 95 °C–35 °C–72 °C in the different steps [75]. The program is very similar to variant I described in the Section 2. In our study, the annealing temperature was 36 °C, as higher temperatures may increase the reaction specificity, which indicates that a greater number of DNA fragments is likely to be present at lower temperatures [85].
Some authors applied other primers for RAPD, such as M13 and P1–P6 [11,13,75]. Similarly, the P2 primer in our study ensured a sufficient number of fragments for the subsequent phylogenetic analysis of the epidemiological typing. The closely related L. lactis, which provided non-specific PCR cross-reactions with L. garvieae, was well distinguished in epidemiological typing, so the method is appropriate to differentiate those two species.
Using a closely related species as an external group in epidemiological typing is an important index for the specificity of the PCR products. This is important because, afterward, the studied species will be differentiated from each other based on the external group—the root of the tree. L. lactis was precisely positioned as an external group in the phylogenetic analysis of L. garvieae strains. Moreover, by using the P2 primer, information about the genetic and phenotypic changes in the species through the years was gained. It followed a logical pattern: two major groups were formed based on the strain history and similarity confidence threshold (SCT) 70. Strains 468, 329, 412, and 465 were individual, separate from all other strains, and the closest to the external group (L. lactis), yet these could not be defined as separate branches (SCT < 40). Strain 468/2019 was the closest to the external group. As several strains were isolated from the same farm, the L. lactis (472) strain was isolated after therapy against L. garvieae. Strain 468 was non-capsular (like 472 L. lactis). In addition, all three dendrograms showed that 468 was a separate branch. This is an indication that the method using the P2 primer includes the region of the capsular gene and carries important information about the strain’s virulence. Strain 329 was isolated from fish brains; the clinical manifestation was mild, as was the production of capsular polysaccharides (Figure 6). Strain 412/2010 was a vaccinal strain from the Greek clinical isolate 415/2010. The last strain (465/2017) from this group was isolated from a rainbow trout purchased from a store; the fish exhibited no pathological changes in its internal organs. Thus, it can be concluded that a separate group of non-virulent strains was formed. All other strains were clinical (except for strain 466), capsular, and highly virulent and formed two major groups (SCT > 70).
Strain 466/2010 was additionally isolated from a retail store located in a different region, yet, unlike strain 465, it was in the group of clinically high virulent capsular isolates.
These results show that the methodology for epidemiological typing of L. garvieae strains by using P2 was informative and suitable for prognoses and studies.
Sequenced 16s rDNA of the included strains were processed and analyzed in Blast-NCBI and showed 100% identity with L. garvieae sequences deposited in NCBI. Thus, 16s rDNA sequencing analysis confirmed the microbiological, biochemical, and molecular identification of the isolated bacteria as belonging to the species L. garvieae. The phylogenetic analysis conducted formed groups of L. lactis and L. garvieae; the latter was represented by two major branches. The phylogenetic analysis based on 16s defined our isolates in an L. garvieae group together with isolates from India, South Africa, and Japan, thus confirming the identification as L. garvieae [86,87]. Some authors [32,88] applied phylogenetic analysis based on high-sensitivity methods, such as detecting a housekeeping gene or whole-genome sequencing. In contrast, the use of only 16s provides limited epidemiological information and is suitable as a complementary method.
In recent years, new species closely related to Lactococcus garvieae have been described that cannot be discriminated using MALDI-TOF, 16S sequencing, or biochemical tests [3,7]. This is why new approaches have been developed to identify these species: whole-genome sequencing, intergenic spacer 16S–23S sequencing, gyrB gene sequencing, multiplex PCR, and serotype analysis using PCR [7,8,9,10]. In the current study, the final identification of L. garvieae was performed using multiplex PCR as described by Dang et al. and Saticioglu et al. [9,69].
The strains included in this study belonged to the European capsular and non-capsular (strain 468) types. Epidemiological typing with the P2 primer formed groups of less virulent (non-capsular or weak capsule producers) and capsulated virulent strains. Moreover, the virulent capsular strains were grouped into two major branches, showing a change in the L. garvieae genome over the years: namely, group I comprised the period of 2002–2008 and group II included the period of 2013–2019. In group II, a relationship between a Greek clinical strain from 2010 and clinical strains from Bulgaria and Serbia isolated in 2019 was confirmed. This is proof that genetically similar strains are circulating in the Balkan region, which may be associated with the commercial exchange of breeding fish and ready-to-use products.
5. Conclusions
Due to morphological, phenotypical, and genetic similarities between different species of cocci and among the representatives of the genus Lactococcus, a combination of methods is necessary to discriminate L. garvieae. Its importance is linked to possible outbreaks in fish and other animals, which can cause significant economic losses and possible transmission to immunocompromised patients acting as an opportunistic agent. Additionally, further investigation into the genetic basis of the observed virulence and resistance patterns, especially regarding the capsular gene region identified through the RAPD method, could provide deeper insight into the pathogenicity of L. garvieae. Such studies could also explore the potential for targeted prophylaxis based on the specific virulence factors of the strains. Future research should encompass a broader range of fish species, particularly those that are commonly consumed by humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13020436/s1, Supplementary File S1 with four Figures S1–S4. Figure S1: Gram stain method of isolate 443; Figure S2: Anthony stain method for capsule of isolate 443; Figure S3: Gruber type agglutination reaction with specific rabbit anti-L. garvieae serum against capsular isolate 443; Figure S4: Gel electrophoresis after epidemiological typing with primers: Ist row (top)—E1; IInd row (middle)—P2; and III row (bottom)—RAPD4; Position 1—Control ladder; Position 2—331/2002/Belgium; Position 3—322/2002/Dospat; Position 4—negative control; Position 5—386/2006/Dospat; Position 6—397/2008/Dospat; Position 7—400/2008/Dospat; Position 8—412/2010/Dospat; Position 9—415/2010/Greece; Position 10—417/2013 Dospat; Position 11—418/2013/Dospat; Position 12—443/2016/salmon; Position 13—456/2017/Dospat; Position 14—459/2017/Dospat; Position 15—465 brain of a cooled trout bought in 2017 from a fish stall at “Krasno selo”market; Position 16—466 brain of a cooled trout bought in 2017 from a fish stall at “Fantastiko” supermarket; Position 17—467 heart of a trout from a fish farm in the town of Pirdop, 2019; Position 19—469 brain of a trout before therapy, town of Pirdop, 2019; Position 20—470 spleen of a trout, town of Pirdop, 2019; Position 21—471 after florfenicole treatment, town of Pirdop, 2019; Position 22—472 town of Pirdop, 2019; Position 23—473/2019/Serbian control L. garvieae; Position 24—474/2019/Serbian control L. garvieae.
Author Contributions
Conceptualization, I.M. and I.S.; methodology, I.M., I.S., T.V.S. and P.O.; samples collection, I.M., P.O. and V.R.; software, I.S., S.G.D. and V.S.B.; validation, I.S., I.M. and P.O.; formal analysis, I.M., I.S., P.O. and N.R.; investigation, I.S., I.M., P.O., T.V.S., R.G., D.Y., B.S. and S.G.D.; resources, I.M., I.S., P.O., V.R., V.S.B. and L.B.; data curation, I.S., I.M., N.R., T.V.S., V.S.B., B.S., D.Y. and P.O.; writing—original draft preparation, I.S., V.S.B. and I.M.; writing—review and editing, I.S., R.G., V.R., T.V.S., V.S.B., P.O., S.G.D., N.R., L.B., B.S. and I.M.; visualization, I.S., V.S.B., L.B., P.O. and S.G.D.; supervision, I.M., I.S., T.V.S. and R.G.; project administration, I.S. and I.M.; funding acquisition, I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded under Project No. 8250/20.11.2018 and CONTRACT No. D-74/23.04.2019 by the Board of Medical Sciences, Medical University of Sofia, Bulgaria. APC was paid by Medical University-Sofia, according to Rector’s order № PK-36-811/02.04.2021 and Decision of Academic Council from 29 March 2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
In Memoriam Corr. Member. Ivan Mitov, DSci., 2000–2020 Head of Department “Medical Microbiology”, 2008–2016 Vice Dean of Faculty of Medicine, 2016–2020 Dean of Faculty of Medicine, Medical University—Sofia.
Conflicts of Interest
Author Bilyana Sirakova was employed by the company “AIPPMPDM”, Ltd., 2800 Sandanski. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Vendrell, D.; Balcazar, J.L.; Ruiz-Zarzuela, I.; de Blas, I.; Girones, O.; Muzquiz, J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Baldisserotto, B.; Hosseini, S.P.; Shafiei, S.; Bashiri, M. Lactococcosis a Re-Emerging Disease in Aquaculture: Disease Significant and Phytotherapy. Vet. Sci. 2021, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.X.; Cao, H.; Jiang, S.; Li, X.; Fung, K.K.; Lee, C.H.; Sridhar, S.; Chen, J.H.K.; Ho, P.L. Genomic investigation of Lactococcus formosensis, Lactococcus garvieae, and Lactococcus petauri reveals differences in species distribution by human and animal sources. Microbiol. Spectr. 2024, 12, e0054124. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, R. A new pathogenic bacterium belonging to the genus Streptococcus isolated from an epizootic of cultured yellowtail. Nippon Suisan Gakkai Shi 1976, 42, 1345–1352. [Google Scholar] [CrossRef]
- Collins, M.D.; Farrow, J.A.; Phillips, B.A.; Kandler, O. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. Microbiology 1983, 129, 3427–3431. [Google Scholar] [CrossRef]
- Gibello, A.; Galán-Sánchez, F.; Blanco, M.M.; Rodríguez-Iglesias, M.; Domínguez, L.; Fernández-Garayzábal, J.F. The zoonotic potential of Lactococcus garvieae: An overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Res. J. Vet. Sci. 2016, 109, 59–70. [Google Scholar] [CrossRef]
- Heckman, T.I.; Yazdi, Z.; Older, C.E.; Griffin, M.J.; Waldbieser, G.C.; Chow, A.M.; Medina Silva, I.; Anenson, K.M.; García, J.C.; LaFrentz, B.R.; et al. Redefining piscine lactococcosis. Appl. Environ. Microbiol. 2024, 90, e0234923. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Abdelsalam, M.; Kawato, S.; Harakawa, S.; Kawakami, H.; Hirono, I.; Kondo, H. Comparative genome analyses of three serotypes of Lactococcus bacteria isolated from diseased cultured striped jack (Pseudocaranx dentex). J. Fish. Dis. 2023, 46, 829–839. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Onuk, E.E.; Ay, H.; Ajmi, N.; Demirbas, E.; Altun, S. Phenotypic and molecular differentiation of Lactococcus garvieae and Lactococcus petauri isolated from trout. Aquaculture 2023, 577, 739933. [Google Scholar] [CrossRef]
- Stoppani, N.; Colussi, S.; Pastorino, P.; Prearo, M.; Sciuto, S.; Altinok, I.; Öztürk, R.Ç.; Ture, M.; Vela, A.I.; Blanco, M.D.M.; et al. 16S-23S rRNA Internal Transcribed Spacer Region (ITS) Sequencing: A Potential Molecular Diagnostic Tool for Differentiating Lactococcus garvieae and Lactococcus petauri. Microorganisms 2023, 11, 1320. [Google Scholar] [CrossRef]
- Duman, M.; Buyukekiz, A.G.; Saticioglu, I.B.; Cengiz, M.; Sahinturk, P.I.; Altun, S.O. Epidemiology, genotypic diversity, and antimicrobial resistance of Lactococcus garvieae in farmed rainbow trout (Oncorhynchus mykiss). Iran. J. Fish. Sci. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Radosavljević, V.; Radanović, O.; Zdravković, N.; Savić, B.; Stanković, M.; Zorić, J.M.; Veljović, L.; Nešić, K. The first outbreak of lactococcosis caused by Lactococcus garvieae in Serbia. AVM 2020, 13, 53–68. [Google Scholar] [CrossRef]
- Rao, S.; Pham, T.H.; Poudyal, S.; Cheng, L.W.; Nazareth, S.C.; Wang, P.C.; Chen, S.C. First report on genetic characterization, cell-surface properties and pathogenicity of Lactococcus garvieae, emerging pathogen isolated from cage-cultured cobia (Rachycentron canadum). Transbound. Emerg. Dis. 2022, 69, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.J.; Klesius, P.H.; Shoemaker, C.A. First isolation and characterization of Lactococcus garvieae from Brazilian Nile tilapia, Oreochromis niloticus (L.), and pintado, Pseudoplathystoma corruscans (Spix & Agassiz). J. Fish. Dis. 2009, 32, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Nishiki, I.; Furukawa, M.; Matui, S.; Itami, T.; Nakai, T.; Yoshida, T. Epidemiological study on Lactococcus garvieae isolates from fish in Japan. Fish. Sci. 2011, 77, 367–373. [Google Scholar] [CrossRef]
- Sebastião, F.; Furlan, L.; Hashimoto, D.; Pilarski, F. Identification of Bacterial Fish Pathogens in Brazil by Direct Colony PCR and 16S rRNA Gene Sequencing. Adv. Microbiol. 2015, 5, 409–424. [Google Scholar] [CrossRef]
- Fukushima, H.C.; Leal, C.A.; Cavalcante, R.B.; Figueiredo, H.C.; Arijo, S.; Moriñigo, M.A.; Ishikawa, M.; Borra, R.C.; Ranzani-Paiva, M.J. Lactococcus garvieae outbreaks in Brazilian farms Lactococcosis in Pseudoplatystoma sp.—development of an autogenous vaccine as a control strategy. J. Fish. Dis. 2017, 40, 263–272. [Google Scholar] [CrossRef]
- Tavares, L.M.; de Jesus, L.C.L.; da Silva, T.F.; Barroso, F.A.L.; Batista, V.L.; Coelho-Rocha, N.D.; Azevedo, V.; Drumond, M.M.; Mancha-Agresti, P. Novel Strategies for Efficient Production and Delivery of Live Biotherapeutics and Biotechnological Uses of Lactococcus lactis: The Lactic Acid Bacterium Model. Front. Bioeng. Biotechnol. 2020, 8, 517166. [Google Scholar] [CrossRef]
- Karami, E.; Alishahi, M.; Molayemraftar, T.; Ghorbanpour, M.; Tabandeh, M.R.; Mohammadian, T. Study of pathogenicity and severity of Lactococcus garvieae isolated from rainbow trout (Oncorhynchus mykiss) farms in Kohkilooieh and Boyerahmad province. Fish. Aquat. Sci. 2019, 22, 21. [Google Scholar] [CrossRef]
- Bwalya, P.; Hang’ombe, B.M.; Gamil, A.A.; Munang’andu, H.M.; Evensen, Ø.; Mutoloki, S. A whole-cell Lactococcus garvieae autovaccine protects Nile tilapia against infection. PLoS ONE 2020, 15, e0230739. [Google Scholar] [CrossRef]
- Kawanishi, M.; Yoshida, T.; Kijima, M.; Yagyu, K.; Nakai, T.; Okada, S.; Endo, A.; Murakami, M.; Suzuki, S.; Morita, H. Characterization of Lactococcus garvieae isolated from radish and broccoli sprouts that exhibited a KG+ phenotype, lack of virulence and absence of a capsule. Lett. Appl. Microbiol. 2007, 44, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, J.L.; Vela, A.I.; Gibello, A.; Casamayor, A.; Domínguez, L.; Fernández-Garayzábal, J.F. A genetic comparison of pig, cow and trout isolates of Lactococcus garvieae by PFGE analysis. Lett. Appl. Microbiol. 2011, 53, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Mami, Z.; Meune, C. Lactococcus garvieae endocarditis. Arch. Cardiovasc. Dis. 2011, 104, 138–139. [Google Scholar] [CrossRef][Green Version]
- Ferrario, C.; Ricci, G.; Borgo, F.; Rollando, A.; Fortina, M.G. Genetic investigation within Lactococcus garvieae revealed two genomic lineages. FEMS Microbiol. Lett. 2012, 332, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.A.; Wang, P.C.; Liaw, L.L.; Yoshida, T.; Chen, S.C. Comparison of genetic characteristics and pathogenicity of Lactococcus garvieae isolated from aquatic animals in Taiwan. Dis. Aquat. Org. 2012, 102, 43–51. [Google Scholar] [CrossRef]
- Sirakov, I.; Orozova, P.; Strateva, T.; Mitov, I. Lactococcus garvieae—zoonotic pathogen with commercial importance. Vet. Pract. 2021, 6, 26–33. [Google Scholar]
- Dimov, S.G. The Controversial Nature of Some Non-Starter Lactic Acid Bacteria Actively Participating in Cheese Ripening. BioTech 2023, 12, 63. [Google Scholar] [CrossRef]
- Elliot, J.A.; Collins, M.D.; Pigott, N.E.; Facklam, R.R. Differentiation of Lactococcus lactis and Lactococcus garvieae from humans by comparison of whole-cell protein patterns. J. Clin. Microbiol. 1991, 29, 2731–2734. [Google Scholar] [CrossRef]
- Fefer, J.J.; Ratzan, K.R.; Sharp, S.E.; Saiz, E. Lactococcus garvieae endocarditis: Report of a case and review of the literature. Diagn. Microb. Infect. Dis. 1998, 32, 127–130. [Google Scholar] [CrossRef]
- Chan, J.F.; Woo, P.C.; Teng, J.L.; Lau, S.K.; Leung, S.S.; Tam, F.C.; Yuen, K.Y. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection 2011, 39, 259–264. [Google Scholar] [CrossRef]
- Russo, G.; Iannetta, M.; D’Abramo, A.; Mascellino, M.T.; Pantosti, A.; Erario, L.; Tebano, G.; Oliva, A.; D’Agostino, C.; Trinchieri, V.; et al. Lactococcus garvieae endocarditis in a patient with colonic diverticulosis: First case report in Italy and review of the literature. New Microbiol. 2012, 35, 495. [Google Scholar]
- Lin, Y.S.; Kweh, K.H.; Koh, T.H.; Lau, Q.C.; Rahman, N.B. Genomic analysis of Lactococcus garvieae isolates. Pathology 2020, 52, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Colagrossi, L.; Costabile, V.; Scutari, R.; Agosta, M.; Onori, M.; Mancinelli, L.; Lucignano, B.; Onetti Muda, A.; Del Baldo, G.; Mastronuzzi, A.; et al. Evidence of pediatric sepsis caused by a drug resistant Lactococcus garvieae contaminated platelet concentrate. Emerg. Microbes Infect. 2022, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Ishikawa, N.; Ono, R. Infective endocarditis caused by Lactococcus garvieae: A case report and review of the literature. IDCases 2024, 36, e01941. [Google Scholar] [CrossRef]
- Rösch, R.M.; Buschmann, K.; Brendel, L.; Schwanz, T.; Vahl, C.F. Lactococcus garvieae Endocarditis in a Prosthetic Aortic Valve: A Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619832052. [Google Scholar] [CrossRef]
- Cañas, V.H.; Ramirez, M.P.; Jiménez, F.B.; Martin, M.R.; Casas, C.M.; Arriaza, M.M.; Marí, J.N. Lactococcus garvieae endocarditis in a native valve identified by MALDI-TOF MS and PCR-based 16s rRNA in Spain: A case report. New Microbes New Infect. 2015, 5, 13–15. [Google Scholar] [CrossRef][Green Version]
- Backes, Y.; Gruteke, P.; Branger, J. Lactococcus garvieae endocarditis. Ned. Tijdschr. Voor Geneeskd. 2015, 159, 8738. [Google Scholar]
- Sunnerhagen, T.; Hammarlund, P.; Rasmussen, M. A case of suspected infective endocarditis with Lactococcus garvieae: Lack of in vitro synergy between ampicillin and gentamicin. JMM Case Rep. 2015, 2, e000018. [Google Scholar] [CrossRef]
- Rasmussen, M.; Björk, J.; Werner, J.; Dolk, M.; Christensson, B. Lactococcus garvieae endocarditis presenting with subdural haematoma. BMC Cardiovasc. Disord. 2014, 1, 13–14. [Google Scholar] [CrossRef][Green Version]
- Ortiz, C.; López, J.; del Amo, E.; Sevilla, T.; García, P.E.; San Román, J.A. Lactococcus garvieae infective endocarditis: Report of 2 cases and review of the literature. Rev. Esp. Cardiol. 2014, 67, 776–778. [Google Scholar] [CrossRef]
- Gönüllü, N.; Yenıdünya, G.; Çelık, S.; Güney, N.; Midilli, K.; Bavunoğlu, I.; Öngen, Z. Lactococcus garvieae endokarditi turkiye klinikleri. J. Med. Sci. 2012, 32, 588. [Google Scholar]
- Tessier, S.; Emengo, I.; Yoder, N.; Longo, S.; Ido, F. Massive empyema due to Lactococcus garvieae. Germs 2022, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Rawling, R.A.; Granato, P.A. Lactococcus garvieae native valve endocarditis. Clin. Microbiol. Newsl. 2014, 36, 182–183. [Google Scholar] [CrossRef]
- Sharngoe, C.; Fazili, T.; Javaid, W.; Endy, T.; Polhemus, M. 923 Lactococcus garvieae infective endocarditis requiring valve replacement: First case in the United States. InOpen Forum Infect. Dis. 2014, 1, S267. [Google Scholar] [CrossRef]
- Navas, M.E.; Hall, G.; El Bejjani, D. A case of endocarditis caused by Lactococcus garvieae and suggested methods for identification. J. Clin. Microbiol. 2013, 51, 1990–1992. [Google Scholar] [CrossRef]
- Hirakawa, T.F.; Costa, F.A.; Vilela, M.C.; Rigon, M.; Abensur, H.; Araújo, M.R. Lactococcus garvieae endocarditis: First case report in Latin America. Arq. Bras. Cardiol. 2011, 97, 108–110. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.H.; Yeo, M.H.; Chang, K.S. First case of urinary tract infection by Lactococcus garvieae in Korea. Korean J. Clin. Lab. Sci. 2021, 53, 277–283. [Google Scholar] [CrossRef]
- Tariq, E.F.; Irshad, Y.; Khalil, H.B.; Khakwani, A.S.; Khan, U.A. Urinary tract infection caused by the novel pathogen, Lactococcus garvieae: A case report. Cureus 2020, 12, e9462. [Google Scholar] [CrossRef]
- Watanabe, Y.; Naito, T.; Kikuchi, K.; Amari, Y.; Uehara, Y.; Isonuma, H.; Hisaoka, T.; Yoshida, T.; Yaginuma, K.; Takaya, N.; et al. Infective endocarditis with Lactococcus garvieae in Japan: A case report. J. Med. Case Rep. 2011, 5, 356. [Google Scholar] [CrossRef]
- Wang, C.Y.; Shie, H.S.; Chen, S.C.; Huang, J.P.; Hsieh, I.C.; Wen, M.S.; Lin, F.C.; Wu, D. Lactococcus garvieae infections in humans: Possible association with aquaculture outbreaks. Int. J. Clin. Pract. 2007, 61, 68–73. [Google Scholar] [CrossRef]
- Mitra, N.; Kumar, P. Lactococcus garvieae: An emerging pathogen. Indian. Pediatr. 2015, 52, 814. [Google Scholar] [PubMed]
- Jo, W.; Bae, I.G.; Kim, B.R. A case of Lactococcus garviaea bacteremia in patient with infectious colitis after the ingestion of ascidians. Korean J. Intern. Med. 2014, 29, 376. [Google Scholar]
- Aguado-Urda, M.; López-Campos, G.H.; Blanco, M.M.; Fernández-Garayzábal, J.F.; Cutuli, M.T.; Aspiroz, C.; López-Alonso, V.; Gibello, A. Genome sequence of Lactococcus garvieae 21881, isolated in a case of human septicemia. J. Bacteriol. 2011, 193, 4033–4034. [Google Scholar] [CrossRef] [PubMed]
- Aspiroz, C.; Saez-Nieto, J.A.; Ruiz-Zarzuela, I.; Vela, A.I. Bacteriemia por Lactococcus garvieae y Citrobacter freundii. Casos De Microbiolgía Clínica Caso 2007, 387. Available online: http://www.f-soria.es/admfsoria/casos/img/387.pdf (accessed on 10 October 2019).
- Yiu, K.H.; Siu, C.W.; To, K.K.; Jim, M.H.; Lee, K.L.; Lau, C.P.; Tse, H.F. A rare cause of infective endocarditis; Lactococcus garvieae. Int. J. Cardiol. 2007, 114, 286–287. [Google Scholar] [CrossRef]
- Mofredj, A.; Baraka, D.; Cadranel, J.F.; LeMaitre, P.; Kloeti, G.; Dumont, J.L. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am. J. Med. 2000, 109, 513–514. [Google Scholar] [CrossRef]
- Nadrah, K. “Lactococcus garvieae septicaemia in a patient with artificial heart valves”. Wien. Klin. Wochenschr. 2011, 123, 677–679. [Google Scholar] [CrossRef]
- Chao, C.T.; Lai, C.F.; Huang, J.W. Lactococcus garvieae peritoneal dialysis peritonitis. Perit. Dial. Int. 2013, 33, 100–101. [Google Scholar] [CrossRef]
- Lahlou, W.; Bourial, A.; Maaouni, T.; Bensaad, A.; Bensahi, I.; Sabry, M.; Miguil, M. Lactococcus lactis endocarditis and liver abscess in an immunocompetent patient: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 115. [Google Scholar] [CrossRef]
- Tandel, K.; Bhatt, P.; Ranjan, P.; Rathi, K.R. Meningitis caused by Lactococcus garvieae. Med. J. Armed Forces India 2017, 73, 94–96. [Google Scholar] [CrossRef][Green Version]
- Mayo-Yáñez, M.; González-Torres, L. Recurrent Penicillin-Resistant Tonsillitis Due to Lactococcus garvieae, a New Zoonosis from Aquaculture. Zoonotic Dis. 2023, 3, 1–5. [Google Scholar] [CrossRef]
- James, P.R.; Hardman, S.M.; Patterson, D.L. Osteomyelitis and possible endocarditis secondary to Lactococcus garvieae: A first case report. Postgrad. Med. J. 2000, 76, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Woolery, W. Acute lower urinary tract infection caused by Lactococcus garvieae. Now Seek. “Clin. Images” 2015, 33, 33. [Google Scholar]
- Dylewski, J. Urinary tract sepsis caused by Lactococcus garvieae. Clin. Microbiol. Newsl. 2014, 36, 30–31. [Google Scholar] [CrossRef]
- Reguera-Brito, M.; Galán-Sánchez, F.; Blanco, M.M.; Rodríguez-Iglesias, M.; Domínguez, L.; Fernández-Garayzábal, J.F.; Gibello, A. Genetic analysis of human clinical isolates of Lactococcus garvieae: Relatedness with isolates from foods. Infect. Genet. Evol. 2016, 37, 185–191. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 10 October 2019).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Zlotkin, A.; Eldar, A.; Ghittino, C.; Bercovier, H. Identification of Lactococcus garvieae by PCR. J. Clin. Microbiol. 1998, 36, 983–985. [Google Scholar] [CrossRef]
- Dang, H.T.; Park, H.K.; Myung, S.C.; Kim, W. Development of a novel PCR assay based on the 16S-23S rRNA internal transcribed spacer region for the detection of Lactococcus garvieae. J. Fish. Dis. 2012, 35, 481–487. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Miyauchi, E.; Toh, H.; Nakano, A.; Tanabe, S.; Morita, H. Comparative genomic analysis of Lactococcus garvieae strains isolated from different sources reveals candidate virulence genes. Int. J. Microbiol. 2012, 2012, 728276. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Jayamohan, N.S.; Manohar, S.H.; Kumudini, B.S. Genomic and outer membrane protein diversity fingerprints of siderophore producing fluorescent Pseudomonas spp. using RAPD, Rep-PCR and SDS-PAGE profiling. Biologia 2015, 70, 1150–1158. [Google Scholar] [CrossRef]
- Ravelo, C.; Magarinos, B.; López-Romalde, S.; Toranzo, A.E.; Romalde, J.L. Molecular fingerprinting of fish-pathogenic Lactococcus garvieae strains by random amplified polymorphic DNA analysis. J. Clin. Microbiol. 2003, 41, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.; Haesebrouck, F.; Vaneechoutte, M.; Devriese, L.A.; Godard, C.; De Herdt, P. Differentiation between high and low virulence Staphylococcus aureus strains from rabbits by randomly amplified polymorphic DNA (RAPD) analysis. Vet. Microbiol. 2000, 72, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, P. Distribution de la flore alpine dans le Bassin des Drouces et dans quelques regions voisines. Bull. Soc. Vaudoise Sci. Nat. 1901, 37, 241–272. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Altan, E.; Korun, J. Lactococcus garvieae isolates from rainbow trout (Oncorhynchus mykiss, W.) compared by PLG and SA1B10 PCR primer pairs. J. Ilm. PLATAX 2021, 9, 18–28. [Google Scholar] [CrossRef]
- Akayli, T.; Çanak, Ö.; Yardimci, R.; Çiğdem, Ü.R.; Ökmen, D. A mixed Frigoribacterium faeni and Lactococcus garvieae Infection in cultured Rainbow Trout (O. mykiss). KSU J. Agric. Nat. 2020, 23, 1569–1577. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Merquior, V.L.C.; Vianni, M.D.C.E.; Carvalho, M.D.G.S.; Fracalanzza, S.E.; Steigerwalt, A.G.; Brenner, D.J.; Facklam, R.R. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int. J. Syst. Bacteriol. 1996, 46, 664–668. [Google Scholar] [CrossRef]
- Barnes, A.C.; Ellis, A.E. Role of capsule in serotypic differences and complement fixation by Lactococcus garvieae. Fish. Shell Immunol. 2004, 16, 207–214. [Google Scholar] [CrossRef]
- Kanai, K.; Honma, T.; Souda, A.; Shutou, K.; Sugihara, Y. Variation in the Integration Site for Capsule Gene Cluster in the Genome among Strains of Lactococcus garvieae. Fish Pathol. 2018, 53, 19–28. [Google Scholar] [CrossRef][Green Version]
- Kitao, T. The methods for detection of Streptococcus sp., causative bacteria of streptococcal disease of cultured yellowtail (Seriola quinqueradiata) especially, their cultural, biochemical and serological properties. Fish Pathol. 1982, 17, 17–26. [Google Scholar] [CrossRef]
- Sirakov, I.N. Nucleic acid isolation and downstream applications. In Nucleic Acids—From Basic Aspects to Laboratory Tools, 1st ed.; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen Limited: London, UK, 2016; Volume 10, pp. 1–26. [Google Scholar] [CrossRef]
- TanakaN, N.M.; Okada, S. 16S rRNA gene sequences of NRIC Lactic Acid Bacteria strains. Published Only in Database. 2007. Available online: https://www.ncbi.nlm.nih.gov/nuccore/AB362686 (accessed on 10 February 2020).
- Meyburgh, C.M.; Bragg, R.R.; Boucher, C.E. Detection of virulence factors of South African Lactococcus garvieae isolated from rainbow trout, Oncorhynchus mykiss (Walbaum). Onderstepoort J. Vet. Res. 2018, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Francés-Cuesta, C.; Ansari, I.; Fernández-Garayzábal, J.F.; Gibello, A.; González-Candelas, F. Comparative genomics and evolutionary analysis of Lactococcus garvieae isolated from human endocarditis. Microb. Genom. 2022, 8, 000771. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).