Host–Microbe Interactions in Healthy and CSOM-Affected Middle Ears
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Sample Collection
2.3. Culture from Swabs
2.4. Enumeration of Host Inflammatory Cells
2.5. Gram Staining
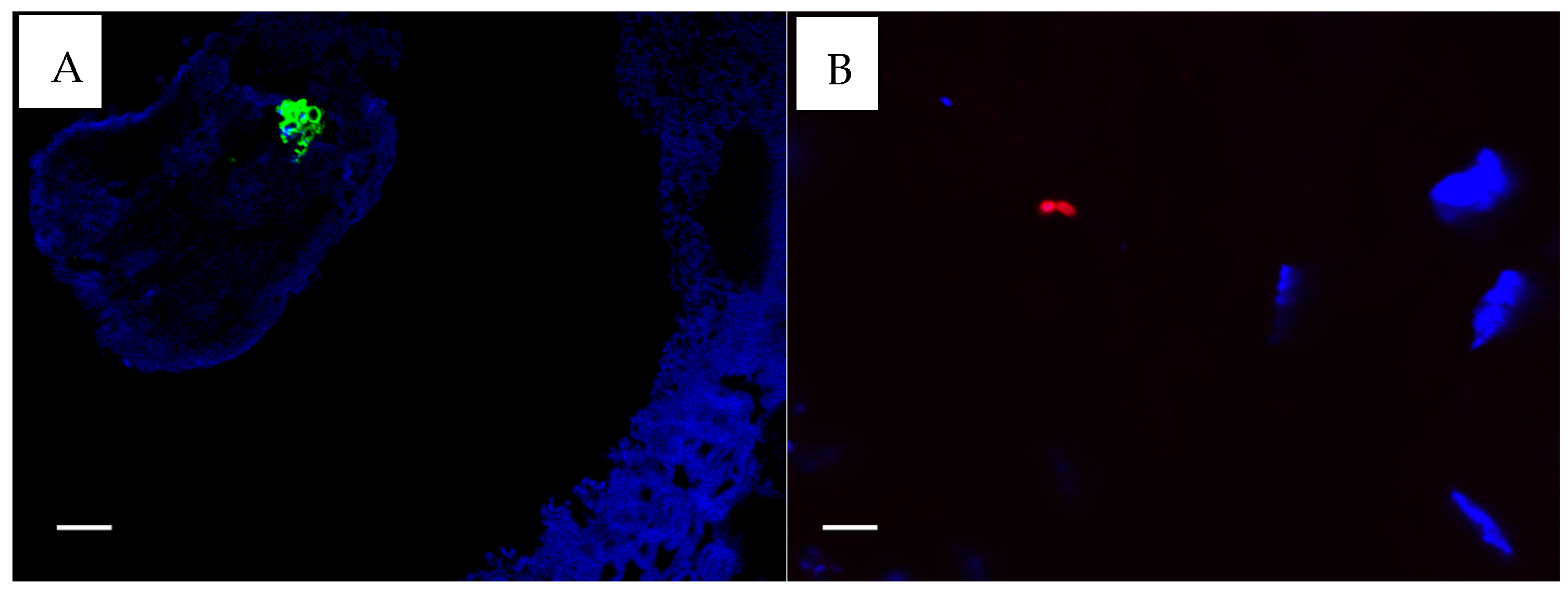
2.6. Immunofluorescence Staining for P. aeruginosa and S. aureus
2.7. Data Analyses
3. Results
3.1. Participant Characteristics
3.2. Swab Culture Data
3.3. Differences in Host Immune Cells
3.3.1. Comparison by Disease Status
3.3.2. Comparison by Anatomical Location
3.3.3. Comparison Based on Pathology
3.4. Histological Characterisation
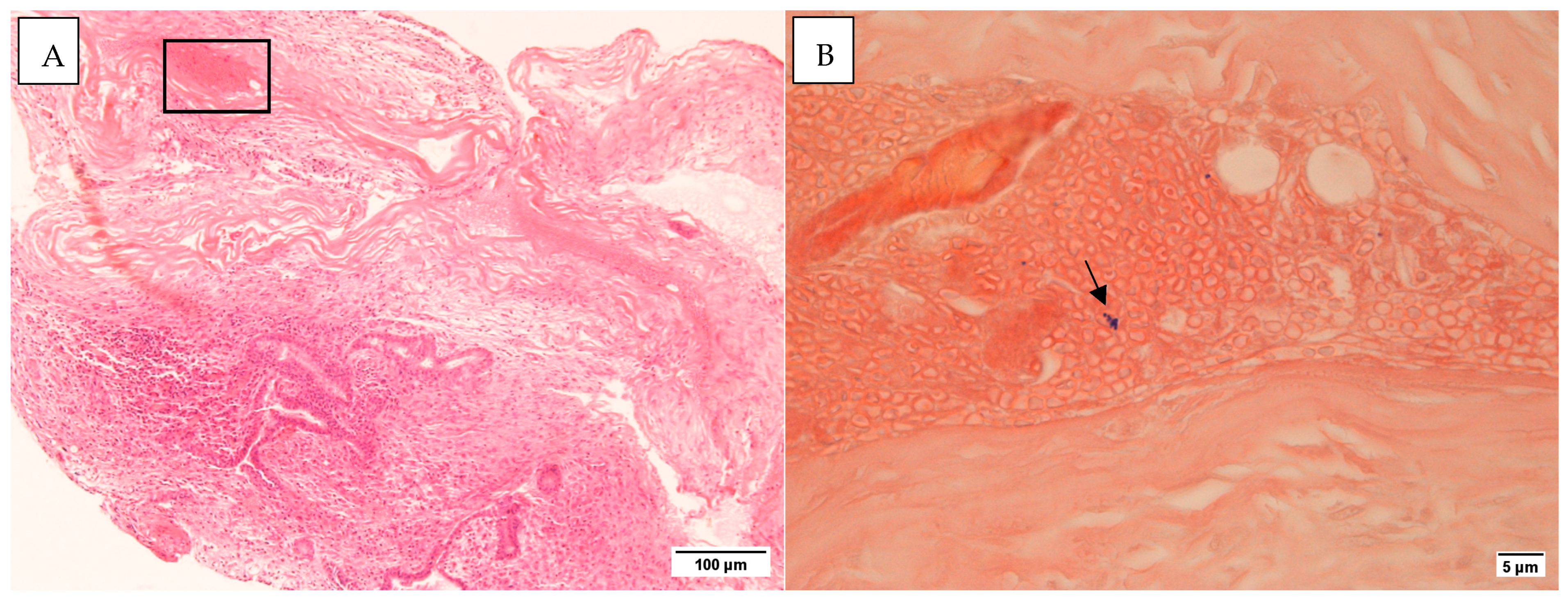
3.4.1. Gram and H&E Staining
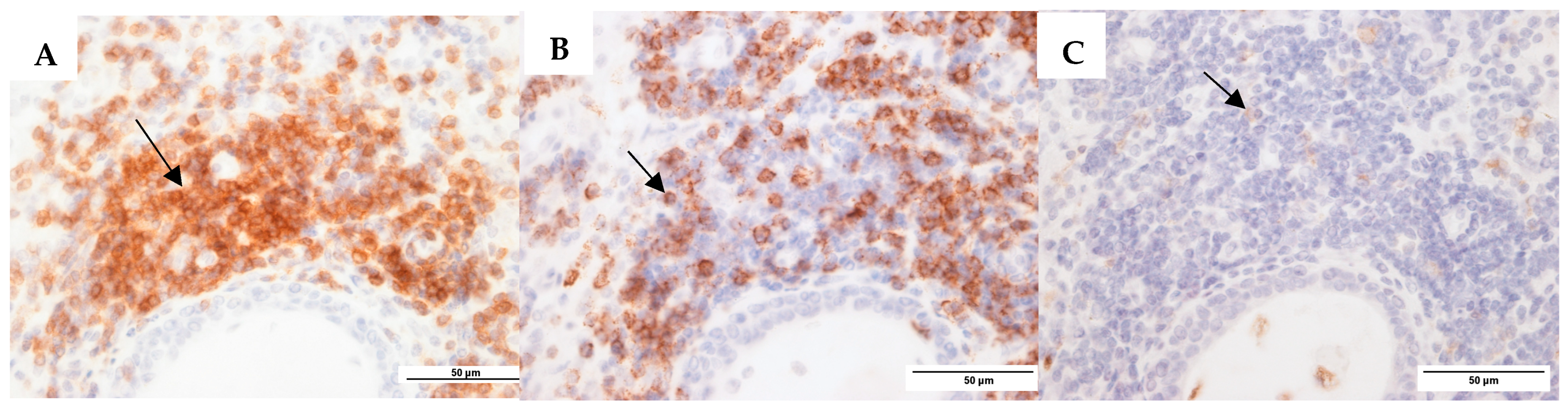
3.4.2. Immunohistochemistry: Antibody-Based S. aureus and P. aeruginosa Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acuin, J. Chronic Suppurative Otitis Media: Burden of Illness and Management Options; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Verhoeff, M.; van der Veen, E.L.; Rovers, M.M.; Sanders, E.A.M.; Schilder, A.G.M. Chronic suppurative otitis media: A review. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, A.O.; Olaniyan, F.A.; Muibi, S.A.; Azeez, I.A.; Abdulwasiu, K.G.; Lasisi, T.J.; Imam, Z.O.; Yekinni, T.O.; Olayemi, O. Clinical and demographic risk factors associated with chronic suppurative otitis media. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; Incesulu, A.; Trabalzini, F.; et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Acuin, J. Chronic suppurative otitis media. BMJ Clin. Evid. 2007, 2007, 0507. [Google Scholar] [PubMed]
- Li, M.G.; Hotez, P.J.; Vrabec, J.T.; Donovan, D.T. Is chronic suppurative otitis media a neglected tropical disease? PLoS Negl. Trop. Dis. 2015, 9, e0003485. [Google Scholar] [CrossRef] [PubMed]
- Coticchia, J.M.; Chen, M.; Sachdeva, L.; Mutchnick, S. New paradigms in the pathogenesis of otitis media in children. Front. Pediatr. 2013, 1, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittal, R.; Lisi, C.V.; Gerring, R.; Mittal, J.; Mathee, K.; Narasimhan, G.; Azad, R.K.; Yao, Q.; Grati, M.; Yan, D.; et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J. Med. Microbiol. 2015, 64, 1103–1116. [Google Scholar] [CrossRef]
- Xia, A.; Thai, A.; Cao, Z.; Chen, X.; Chen, J.; Bacacao, B.; Bekale, L.A.; Schiel, V.; Bollyky, P.L.; Maria, P.L.S. Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss. J. Neuroinflammation 2022, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.F.; Thornton, R.B.; Kirkham, L.-A.S.; Kerschner, J.E.; Cheeseman, M.T. Understanding the aetiology and resolution of chronic otitis media from animal and human studies. Dis. Model. Mech. 2017, 10, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, Y.; Xu, M. Characterization of the T-cell subpopulations in the granulation tissues of chronic suppurative otitis media. Biomed. Rep. 2016, 4, 694–698. [Google Scholar] [CrossRef]
- Agarwal, A.C.; Srivastava, A.; Sen, M. Chronic suppurative otitis media and microbial flora: Adult versus pediatric population. Indian J. Otol. 2021, 27, 22–25. [Google Scholar] [CrossRef]
- Wan Draman, W.N.A.; Md Daud, M.K.; Mohamad, H.; Hassan, S.A.; Abd Rahman, N. Evaluation of the current bacteriological profile and antibiotic sensitivity pattern in chronic suppurative otitis media. Laryngoscope Investig. Otolaryngol. 2021, 6, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Alam, K.M.F.; Naznin, M.; Salam, M.A. Microbiology of chronic suppurative otitis media: An update from a tertiary care hospital in Bangladesh. Pakistan J. Med. Sci. 2021, 37, 821–826. [Google Scholar] [CrossRef]
- Attallah, M.S. Microbiology of chronic suppurative otitis media with cholesteatoma. Saudi Med. J. 2000, 21, 924–927. [Google Scholar] [PubMed]
- Leach, A.; Wood, Y.; Gadil, E.; Stubbs, E.; Morris, P. Topical ciprofloxin versus topical framycetin-gramicidin-dexamethasone in Australian Aboriginal children with recently treated chronic suppurative otitis media. Pediatr. Infect. Dis. J. 2008, 27, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.-Y.; Head, K.; Webster, K.E.; Daw, J.; Richmond, P.; Snelling, T.; Bhutta, M.F.; Schilder, A.G.; Burton, M.J.; Brennan-Jones, C.G. Topical versus systemic antibiotics for chronic suppurative otitis media. Cochrane Database Syst. Rev. 2021, 2021, CD013053. [Google Scholar] [CrossRef]
- Muggeo, A.; Coraux, C.; Guillard, T. Current concepts on Pseudomonas aeruginosa interaction with human airway epithelium. PLoS Pathog. 2023, 19, e1011221. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, M.-N.; An, Y.S.; Moon, B.J. Preoperative, intraoperative, and postoperative results of bacterial culture from patients with chronic suppurative otitis media. Otol. Neurotol. 2012, 33, 54–59. [Google Scholar] [CrossRef]
- Albert, R.R.A.; Job, A.; Kuruvilla, G.; Joseph, R.; Brahmadathan, K.N.; John, A. Outcome of bacterial culture from mastoid granulations: Is it relevant in chronic ear disease? J. Laryngol. Otol. 2005, 119, 774–778. [Google Scholar] [CrossRef]
- Yang, J.A.; Kim, J.Y.; Yoon, Y.K.; Kim, S.; Park, D.W.; Sohn, J.W.; Sim, H.S.; Kim, M.J. Epidemiological and genetic characterization of methicillin-resistant Staphylococcus aureus isolates from the ear discharge of outpatients with chronic otitis media. J. Korean Med. Sci. 2008, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lee, S.-H.; Choi, J.; Im, G.J.; Jung, H.H. Nasopharynx as a microbiologic reservoir in chronic suppurative otitis media: Preliminary study. Clin. Exp. Otorhinolaryngol. 2011, 4, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, M.S.; Jung, S.Y.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Antimicrobial resistance of Pseudomonas aeruginosa from otorrhea of chronic suppurative otitis media patients. Otolaryngol. Neck Surg. 2010, 143, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Dohar, J.E.; Hebda, P.A.; Veeh, R.; Awad, M.; Costerton, J.W.; Hayes, J.; Ehrlich, G.D. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope 2005, 115, 1469–1472. [Google Scholar] [CrossRef]
- Byrd, M.S.; Pang, B.; Hong, W.; Waligora, E.A.; Juneau, R.A.; Armbruster, C.E.; Weimer, K.E.D.; Murrah, K.; Mann, E.E.; Lu, H.; et al. Direct evaluation of Pseudomonas aeruginosa biofilm mediators in a chronic infection model. Infect. Immun. 2011, 79, 3087–3095. [Google Scholar] [CrossRef]
- Iglewski, B.H. Pseudomonas; University of Texas: Austin, TX, USA, 1996; ISBN 0963117211. [Google Scholar]
- Homøe, P.; Bjarnsholt, T.; Wessman, M.; Sørensen, H.C.F.; Johansen, H.K. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202. [Google Scholar] [CrossRef]
- Thornton, R.B.; Rigby, P.J.; Wiertsema, S.P.; Filion, P.; Langlands, J.; Coates, H.L.; Vijayasekaran, S.; Keil, A.D.; Richmond, P.C. Multi-species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr. 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Thornton, R.B.; Wiertsema, S.P.; Kirkham, L.-A.S.; Rigby, P.J.; Vijayasekaran, S.; Coates, H.L.; Richmond, P.C. Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media—A potential treatment target. PLoS ONE 2013, 8, e53837. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, M.; Waldvogel-Thurlow, S.; Zoing, M.; Chang, K.; Radcliff, F.J.; Wagner Mackenzie, B.; Biswas, K.; Douglas, R.G.; Taylor, M.W. Inflammatory endotypes and microbial associations in chronic rhinosinusitis. Front. Immunol. 2018, 9, 2065. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Neeff, M.; Biswas, K.; Hoggard, M.; Taylor, M.W.; Douglas, R. Molecular microbiological profile of chronic suppurative otitis media. J. Clin. Microbiol. 2016, 54, 2538–2546. [Google Scholar] [CrossRef] [PubMed]
- Neeff, M.; Broderick, D.; Douglas, R.G.; Biswas, K. Anaerobic bacteria dominate the cholesteatoma tissue of chronic suppurative otitis media patients. Microb. Pathog. 2024, 196, 106935. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Moore, A.; Colville-Nash, P.; Gilroy, D. Resolution of inflammation. Int. J. Immunopharmacol. 2000, 22, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Fleit, H.B. Chronic inflammation. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 300–314. [Google Scholar]
- Si, Y.; Chen, Y.B.; Chen, S.J.; Zheng, Y.Q.; Liu, X.; Liu, Y.; Jiang, H.L.; Xu, G.; Li, Z.H.; Huang, Q.H.; et al. TLR4 drives the pathogenesis of acquired cholesteatoma by promoting local inflammation and bone destruction. Sci. Rep. 2015, 5, 16683. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Bezema, T.; Calder, P.C.; Charloux, A.; Frossard, N.; Garssen, J.; Haworth, O.; Dilevskaya, K.; Levi-Schaffer, F.; Lonsdorfer, E.; et al. Activation of resolution pathways to prevent and fight chronic inflammation: Lessons from asthma and inflammatory bowel disease. Front. Immunol. 2019, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Kılıçkaya, M.; Aynali, G.; Tuz, M.; Bagcı, Ö. Is there a systemıc inflammatory effect of cholesteatoma? Int. Arch. Otorhinolaryngol. 2016, 21, 42–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prakash, R.; Juyal, D.; Negi, V.; Pal, S.; Adekhandi, S.; Sharma, M. Neelam Sharma Microbiology of chronic suppurative otitis media in a tertiary care setup of Uttarakhand state, India. N. Am. J. Med. Sci. 2013, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pestrak, M.J.; Chaney, S.B.; Eggleston, H.C.; Dellos-Nolan, S.; Dixit, S.; Mathew-Steiner, S.S.; Roy, S.; Parsek, M.R.; Sen, C.K.; Wozniak, D.J. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLOS Pathog. 2018, 14, e1006842. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Fraser, J.D.; Swift, S.; Patterson-Emanuelson, E.A.C.; Amirapu, S.; Douglas, R.G. Intramucosal bacterial microcolonies exist in chronic rhinosinusitis without inducing a local immune response. Am. J. Rhinol. Allergy 2012, 26, 265–270. [Google Scholar] [CrossRef]
- Murphy, E.C.; Frick, I.-M. Gram-positive anaerobic cocci—commensals and opportunistic pathogens. FEMS Microbiol. Rev. 2013, 37, 520–553. [Google Scholar] [CrossRef] [PubMed]
- Harimaya, A.; Takada, R.; Himi, T.; Yokota, S.; Fujii, N. Evidence of local antibody response against Alloiococcus otitidis in the middle ear cavity of children with otitis media. FEMS Immunol. Med. Microbiol. 2007, 49, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Cavubati, R.; Gunaratna, S.; Hoggard, M.; Waldvogel-Thurlow, S.; Hong, J.; Chang, K.; Wagner Mackenzie, B.; Taylor, M.W.; Douglas, R.G. Comparison of subtyping approaches and the underlying drivers of microbial signatures for chronic rhinosinusitis. mSphere 2019, 4, e00679-18. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Controls (n = 22) | CSOM (n = 24) |
|---|---|---|
| Female, n (%) | 7 (33) | 8 (33) |
| Age, mean (SD) | 33 (29) | 33 (26.3) |
| Ethnicity | ||
| European | 13 | 9 |
| Asian | 9 | 5 |
| Polynesian and Māori | 3 | 10 |
| Other (African) | 1 | 0 |
| Host Immune Cells | Controls (n = 15) | CSOM (n = 24) | CSOM | |
|---|---|---|---|---|
| Cholesteatoma (n = 16) | Non-Cholesteatoma (n = 8) | |||
| Mastoid | ||||
| CD3 T cells | 0 (0–0) | 19.8 (3.1–86.3) * | 12.6 (3.1–72.9) | 46.9 (13.1–88.9) |
| CD20 B cells | 0 (0–0) | 18.6 (0.4–52.3) * | 18.6 (0.4–52) | 20.7 (1.5–72.4) |
| CD68 macrophages | 0 (0–0) | 9 (2.4–23.5) * | 19.8 (3.9–35.5) | 7.4 (0.35–13.8) |
| Lymphocytes | 0 (0–0) | 44.7 (11–75.2) * | 44.7 (10.8–74.8) | 54.4 (21–78.4) |
| Histiocytes | 0 (0–0) | 24.3 (11.3–62.8) * | 31.3 (12.2–67.2) | 22.8 (11–61.2) |
| Eosinophils | 0 (0–0) | 0 (0–0.3) | 0 (0–0) | 0.3 (0–0.6) |
| Neutrophils | 0 (0–0) | 0.3 (0–1.3) | 0.3 (0.2–1.1) | 0.3 (0–3) |
| Plasma cells | 0 (0–0) | 0.6 (0–1.3) | 0.7 (0–1.3) | 0.3 (0–1.3) |
| Middle ear | ||||
| CD3 T cells | 0 (0–0) | 6 (0.2–54.4) * | 18.2 (0.7–52.2) | 4.2 (1.2–93.1) |
| CD20 B cells | 0 (0–0) | 17.8 (0–107) * | 18 (3.1–97.3) | 6.4 (0–130) |
| CD68 macrophages | 0 (0–0) | 4.2 (1.4–9.9) * | 4.6 (2.1–9.8) | 3 (0.8–8) |
| Lymphocytes | 0 (0–0) | 41 (10.4–67.2) * | 50.4 (11.4–66.5) | 30 (14.6–60.2) |
| Histiocytes | 0 (0–0) | 27.5 (3.0–61.2) * | 27 (4.5–46.5) | 28 (4.3–67.4) |
| Eosinophils | 0 (0–0) | 0 (0–0) | 1 (0–0) | 2 (0–0) |
| Neutrophils | 0 (0–0) | 0.3 (0–3.0) | 0.3 (0–3.0) | 0.8 (0.2–2.2) |
| Plasma cells | 0 (0–0) | 0.5 (0–2.0) | 0.3 (0–1.9) | 0.7 (0.4–4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neeff, M.; Kimita, W.; Waldvogel-Thurlow, S.; Douglas, R.G.; Biswas, K. Host–Microbe Interactions in Healthy and CSOM-Affected Middle Ears. Microorganisms 2025, 13, 339. https://doi.org/10.3390/microorganisms13020339
Neeff M, Kimita W, Waldvogel-Thurlow S, Douglas RG, Biswas K. Host–Microbe Interactions in Healthy and CSOM-Affected Middle Ears. Microorganisms. 2025; 13(2):339. https://doi.org/10.3390/microorganisms13020339
Chicago/Turabian StyleNeeff, Michel, Wandia Kimita, Sharon Waldvogel-Thurlow, Richard G. Douglas, and Kristi Biswas. 2025. "Host–Microbe Interactions in Healthy and CSOM-Affected Middle Ears" Microorganisms 13, no. 2: 339. https://doi.org/10.3390/microorganisms13020339
APA StyleNeeff, M., Kimita, W., Waldvogel-Thurlow, S., Douglas, R. G., & Biswas, K. (2025). Host–Microbe Interactions in Healthy and CSOM-Affected Middle Ears. Microorganisms, 13(2), 339. https://doi.org/10.3390/microorganisms13020339






