Effect of Multiyear Biodegradable Plastic Mulch on Soil Microbial Community, Assembly, and Functioning
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. DNA Extraction, PCR Application, and Sequence Analysis
2.3. Data Analysis
3. Results
3.1. Microbial Diversity
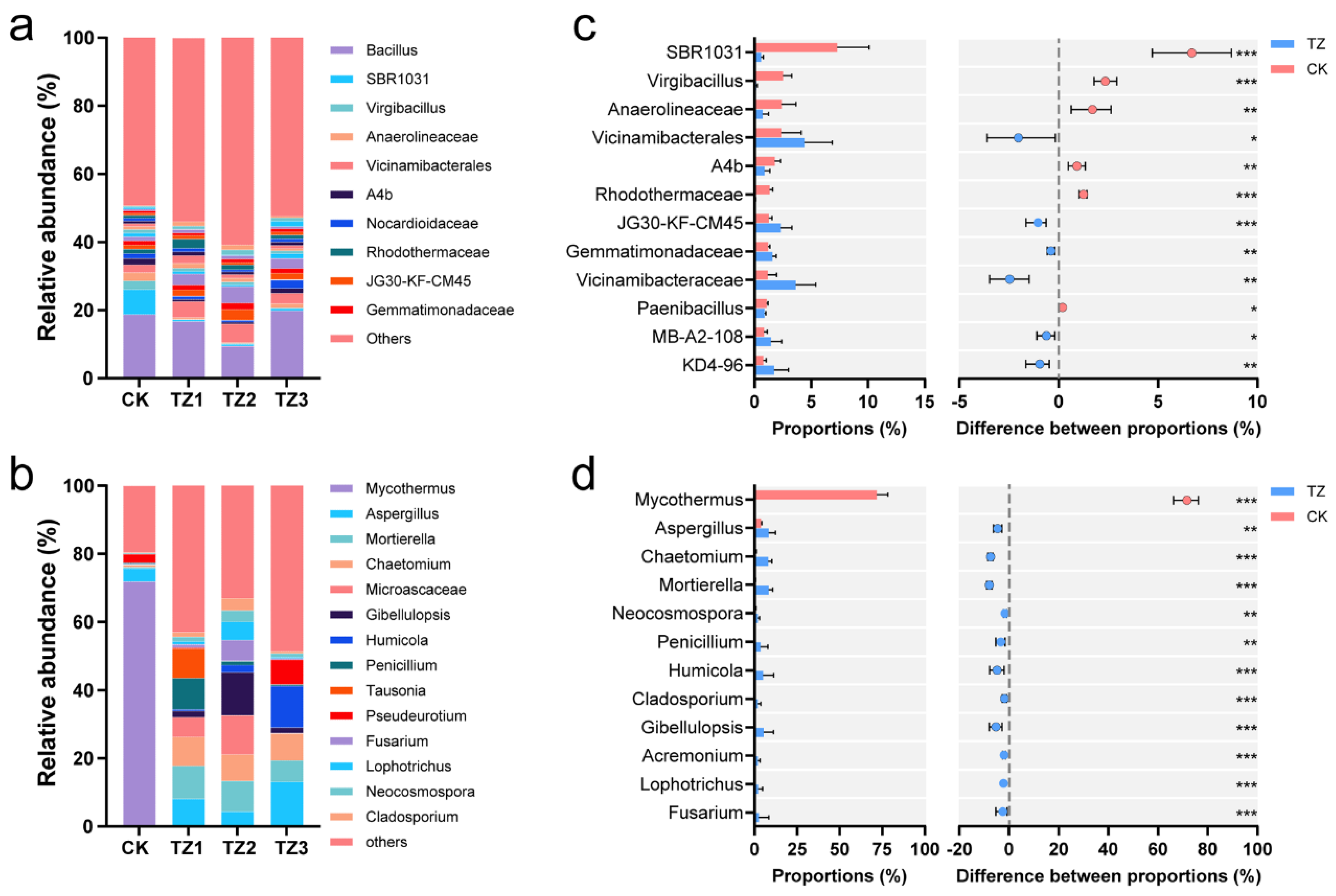
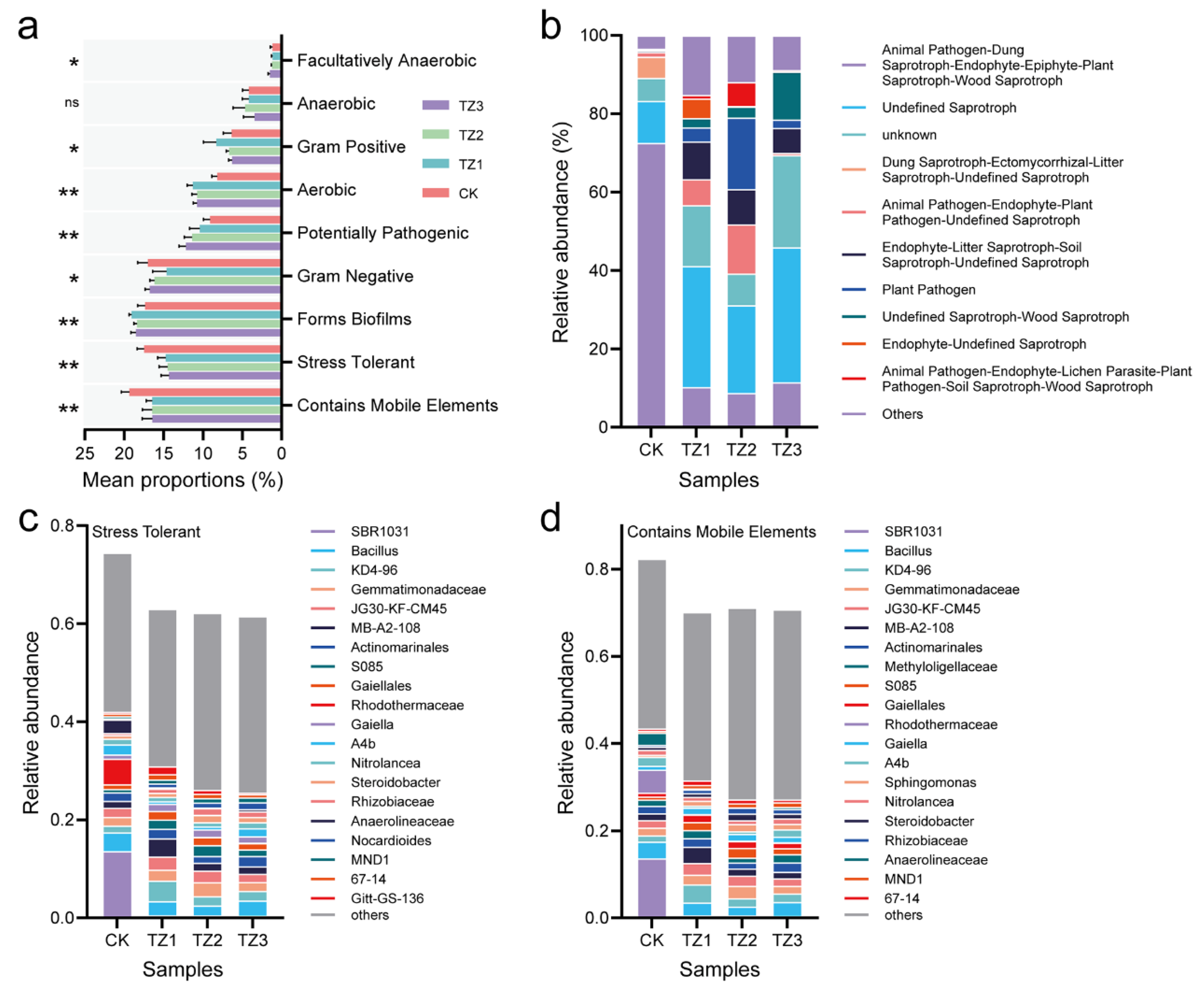
3.2. Microbial Community Composition
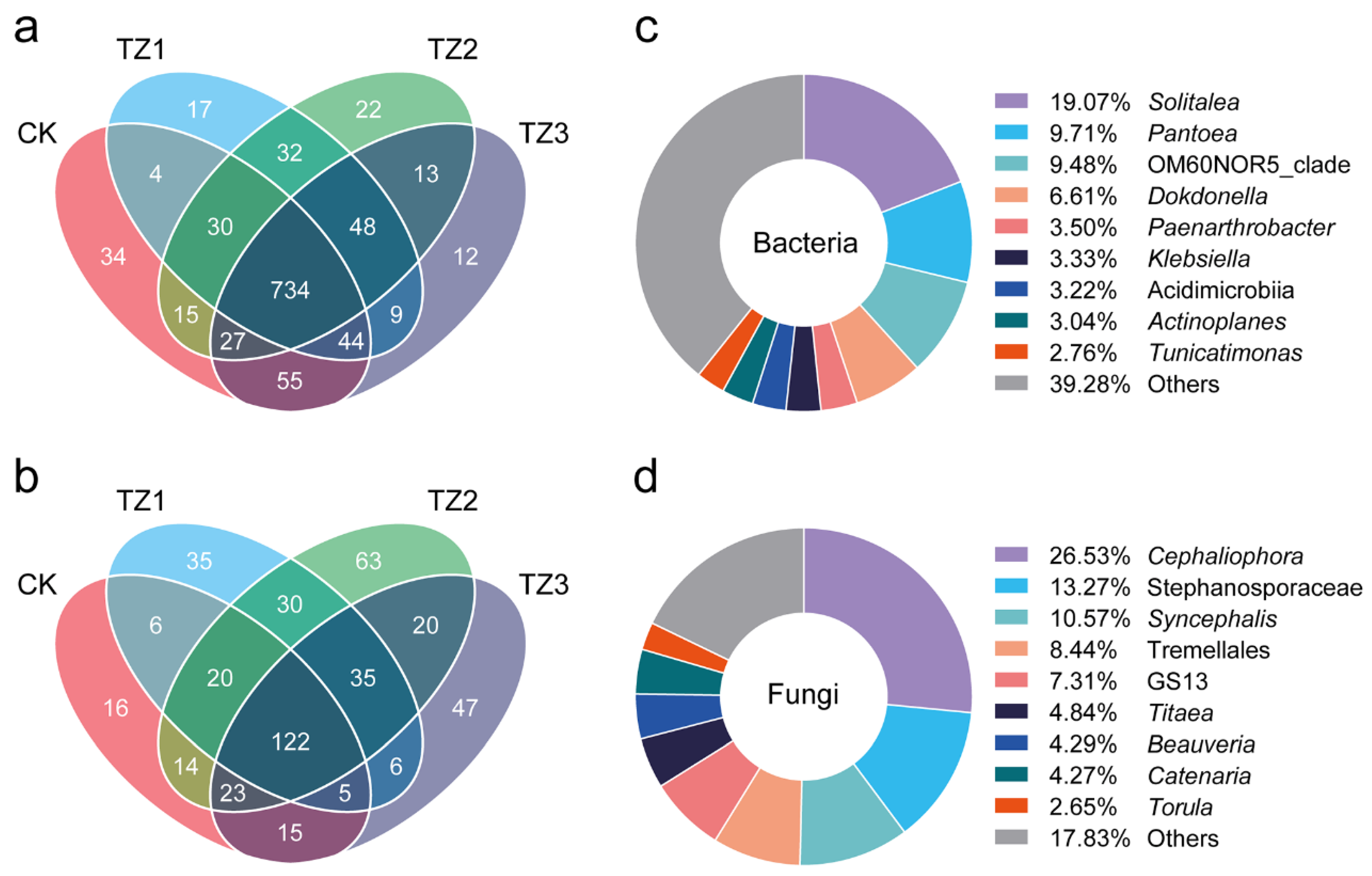
3.3. Assembly of Microbial Community
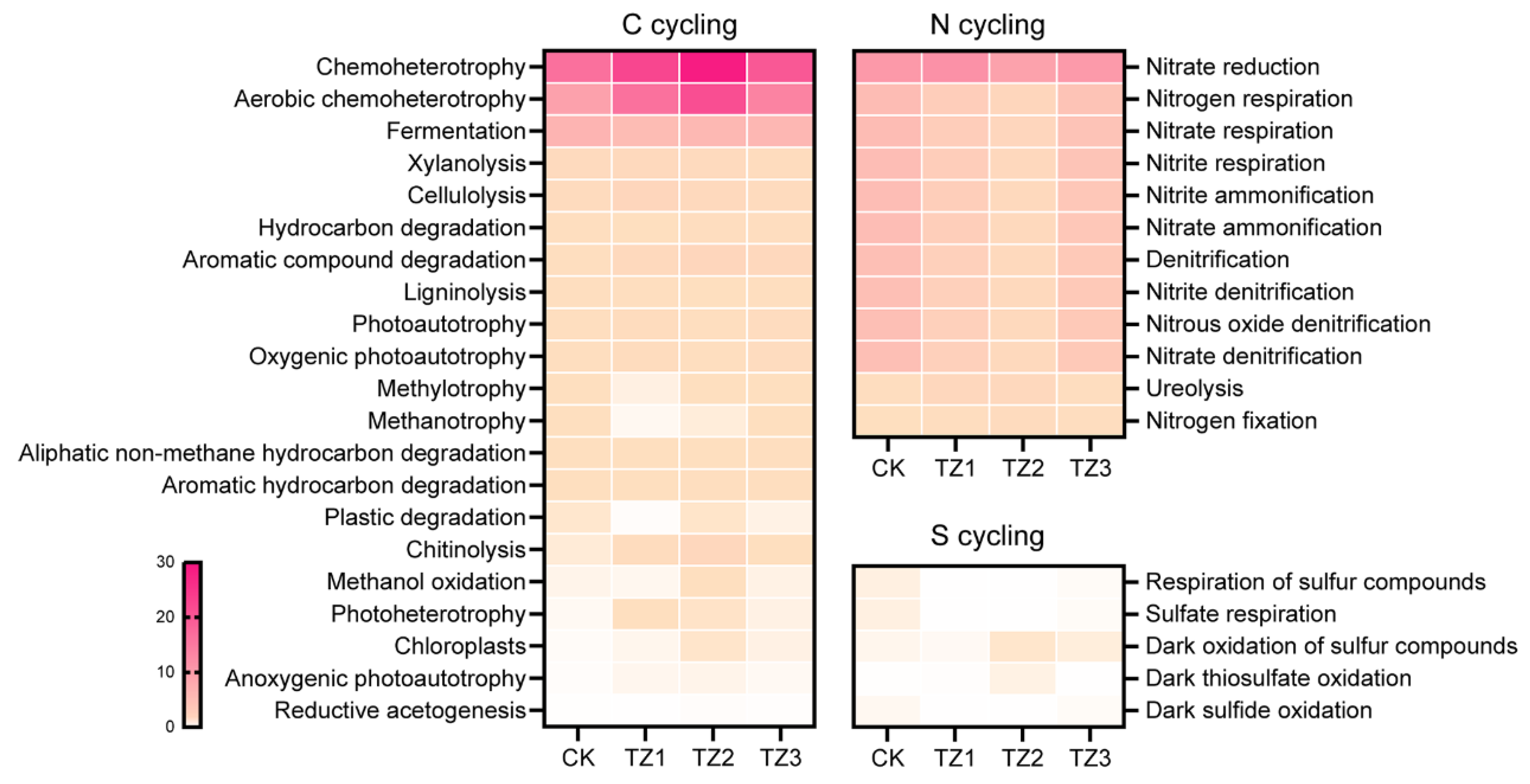
3.4. Potential Microbial Functional Profiling Response to PBAT Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarara, J.M. Microclimate modification with plastic mulch. Hortscience 2000, 35, 169–180. [Google Scholar] [CrossRef]
- Li, R.; Hou, X.Q.; Jia, Z.K.; Han, Q.F.; Ren, X.L.; Yang, B.P. Effects on soil temperature, moisture, and maize yield of cultivation with ridge and furrow mulching in the rainfed area of the Loess Plateau, China. Agric. Water Manag. 2013, 116, 101–109. [Google Scholar] [CrossRef]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy considerations for limiting unintended residual plastic in agricultural soils. Environ. Sci. Policy 2017, 69 (Suppl. SC), 81–84. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.W.; Jiao, J.; Yang, Z.M.; Lv, M.Z.; Li, Y.Z.; Zhou, C.; Wang, C.; He, Z.Y.; Liu, Y.H.; et al. Renewable sourced biodegradable mulches and their environment impact. Sci. Hortic. 2020, 268, 109375. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Divya, V.U.; Sarkar, N.C. Plastic mulch pollution and introduction of biodegradable plastic mulches: A review. Agric. Rev. 2019, 40, 314–318. [Google Scholar] [CrossRef]
- Rauscher, A.; Meyer, N.; Jakobs, A.; Bartnick, R.; Lueders, T.; Lehndorff, E. Biodegradable microplastic increases CO2 emission and alters microbial biomass and bacterial community composition in different soil types. Appl. Soil Ecol. 2023, 182, 104714. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Obayomi, O.; Edelstein, M.; Safi, J.; Mihiret, M.; Ghazaryan, L.; Vonshak, A.; Bernstein, N.; Gillor, O. The combined effects of treated wastewater irrigation and plastic mulch cover on soil and crop microbial communities. Biol. Fert. Soils 2020, 56, 729–742. [Google Scholar] [CrossRef]
- Bonanomi, G.; Chiurazzi, M.; Caporaso, S.; Del Sorbo, G.; Moschetti, G.; Felice, S. Soil solarization with biodegradable materials and its impact on soil microbial communities. Soil Biol. Biochem. 2008, 40, 1989–1998. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.M.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; O’Connor, P.; Zhu, Y.G. Microbial communities on biodegradable plastics under different fertilization practices in farmland soil microcosms. Sci. Total Environ. 2022, 809, 152184. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biot. 2015, 99, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, C.; Cao, N.; Ding, C.; Huang, Y.; Wang, J. Biodegradable and conventional microplastics exhibit distinct microbiome, functionality, and metabolome changes in soil. J. Hazard. Mater. 2022, 424 Pt A, 127282. [Google Scholar] [CrossRef]
- Li, K.; Jia, W.; Xu, L.; Zhang, M.; Huang, Y. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. J. Hazard. Mater. 2023, 442, 130011. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Prewitt, L.; Kang, Y.; Kakumanu, M.L.; Williams, M. Fungal and bacterial community succession differs for three wood types during decay in a forest soil. Microb. Ecol. 2014, 68, 212–221. [Google Scholar] [CrossRef]
- Tian, L.X.; Cao, X.X.; Zhang, L.; Yang, T.Y.; Feng, B.L. Plastic film mulching regime altered fungal, but not bacterial community structure at the regional scale. Agric. Ecosyst. Environ. 2023, 347, 108356. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Chen, M.; Zhao, X.; Liu, H.; Ge, M.; Li, N.; Ning, Z.; Gao, W.; Fan, C.; et al. Molecular insights into effects of PBAT microplastics on latosol microbial diversity and DOM chemodiversity. J. Hazard. Mater. 2023, 450, 131076. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Cao, N.; Duan, C.; Ding, C.; Huang, Y.; Wang, J. Biodegradable microplastics enhance soil microbial network complexity and ecological stochasticity. J. Hazard. Mater. 2022, 439, 129610. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome. Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef]
- Asnicar, F.; Weingart, G.; Tickle, T.L.; Huttenhower, C.; Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 2015, 3, e1029. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Tonya, W.; Jake, L.; Jeremy, M.; Ben, H.; Joshua, L.; Dimitri, S.; John, R.S.; Greg, C.; Ran, B.; Rob, K.; et al. BugBase predicts organism-level microbiome phenotypes. BioRxiv 2017. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lei, J.; Li, Z.; Tan, Q.; Xie, L.; Xiao, Y.; Liu, T.; Chen, X.; Wen, Y.; et al. Time-dependent effects of microplastics on soil bacteriome. J. Hazard. Mater. 2023, 447, 130762. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Meng, K.; Teng, Y.; Ren, W.; Wang, B.; Geissen, V. Degradation of commercial biodegradable plastics and temporal dynamics of associated bacterial communities in soils: A microcosm study. Sci. Total Environ. 2023, 865, 161207. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Liu, Z.; Lin, Y.; Yang, J.; Chen, W.; Wei, G. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Trinh Tan, F.; Cooper, D.G.; Marić, M.; Nicell, J.A. Biodegradation of a synthetic co-polyester by aerobic mesophilic microorganisms. Polym. Degrad. Stabil. 2008, 93, 1479–1485. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Y.; Su, T.; Wang, Z. Enzymatic hydrolysis of poly(butylene adipate-co-terephthalate) by Fusarium solani cutinase. Polym. Degrad. Stabil. 2023, 211, 110335. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Ren, W.; Wang, X.; Luo, Y.; Zhang, H.; Christie, P. Soil type driven change in microbial community affects poly(butylene adipate-co-terephthalate) degradation potential. Environ. Sci. Technol. 2021, 55, 4648–4657. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef]
- Morales Ramos, J.G.; Fernández Tarrillo, L.M.; Guevara Bravo, A.X.; Sánchez-Purihuamán, M.; Carreño Farfán, C.R.; Loayza Estrada, C.S.; Llontop Ynga, E.G.; De La Cruz Silva, H. Efficiency of microorganisms and effectiveness of biodegradation techniques on LDPE plastics: A systematic review. F1000Research 2024, 13, 745. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Kjøller, R.; Rosendahl, S. Cultivated and fallow fields harbor distinct communities of Basidiomycota. Fungal Ecol. 2014, 9, 43–51. [Google Scholar] [CrossRef]
- Sun, X.; Awasthi, M.K.; Syed, A.; Bahkali, A.H. Effect of cyanobacteria biochar addition on humification, fungal dynamics and its mechanism of action in pig manure composting. J. Environ. Chem. Eng. 2024, 12, 113755. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Zhu, B. Drought alleviates the negative effects of microplastics on soil micro-food web complexity and stability. Environ. Sci. Technol. 2023, 57, 11206–11217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and the response of bacterial community in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Gkoutselis, G.; Hink, L.; Weig, A.R.; Obst, M.; Diekmann, A.; Ho, A.; Rambold, G.; Horn, M.A. Microplastic polymer properties as deterministic factors driving terrestrial plastisphere microbiome assembly and succession in the field. Environ. Microbiol. 2023, 25, 2681–2697. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Xiao, Y. Microplastics increase soil microbial network complexity and trigger diversity-driven community assembly. Environ. Pollut. 2023, 333, 122095. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Liquet y González, J.E.; Henderson, K.B.; Anunciado, M.B.; Hayes, D.G.; DeBruyn, J.M. Soil microbial communities associated with biodegradable plastic mulch films. Front. Microbiol. 2020, 11, 587074. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, T.; Mi, L.; Kuang, H.; Xiong, X.; Chen, Z.; Li, S.; Lin, J.D. hnRNPU/TrkB defines a chromatin accessibility checkpoint for liver injury and nonalcoholic steatohepatitis pathogenesis. Hepatology 2020, 71, 1228–1246. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Jin, T.; Liu, H.; Men, J.; Cai, G.; Cernava, T.; Duan, G.; Jin, D. Biodegradable mulch films significantly affected rhizosphere microbial communities and increased peanut yield. Sci. Total Environ. 2023, 871, 162034. [Google Scholar] [CrossRef]
- Ju, Z.; Du, X.; Feng, K.; Li, S.; Gu, S.; Jin, D.; Deng, Y. The succession of bacterial community attached on biodegradable plastic mulches during the degradation in soil. Front. Microbiol. 2021, 12, 785737. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Yu, H.; Xi, B.; Tan, W. A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? Environ. Int. 2022, 163, 107244. [Google Scholar] [CrossRef]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Yang, Q.; Li, D.; Wu, J.H.; Yang, S.; Deng, Y.R.; Luo, C.L.; Jia, W.L.; Zhong, Y.; Peng, P.A. Stable isotopic and metagenomic analyses reveal microbial-mediated effects of microplastics on sulfur cycling in coastal sediments. Environ. Sci. Technol. 2023, 57, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, C.; Lu, J.; Yang, T.; Shen, C.; Li, F.; Wang, J. Lake plastisphere as a new biotope in the Anthropocene: Potential pathogen colonization and distinct microbial functionality. J. Hazard. Mater. 2024, 461, 132693. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, J.; Feng, L.; He, L.; Zhou, C.; Hong, P.; Sun, S.; Zhao, H.; Liang, Y.; Ren, L.; et al. Chemotaxis-selective colonization of mangrove rhizosphere microbes on nine different microplastics. Sci. Total Environ. 2021, 752, 142223. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Korthals, G.; de Boer, W. The hidden potential of saprotrophic fungi in arable soil: Patterns of short-term stimulation by organic amendments. Appl. Soil Ecol. 2020, 147, 103434. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254 Pt A, 112983. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yergeau, É.; Vigani, G.; Geissen, V.; Garbeva, P. Plastic mulch film residues in agriculture: Impact on soil suppressiveness, plant growth, and microbial communities. FEMS Microbiol. Ecol. 2022, 98, fiac017. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wen, Z.; Zhou, W.; Dong, W.; Ren, H.; Liang, G.; Gong, W. Effect of Multiyear Biodegradable Plastic Mulch on Soil Microbial Community, Assembly, and Functioning. Microorganisms 2025, 13, 259. https://doi.org/10.3390/microorganisms13020259
Liu X, Wen Z, Zhou W, Dong W, Ren H, Liang G, Gong W. Effect of Multiyear Biodegradable Plastic Mulch on Soil Microbial Community, Assembly, and Functioning. Microorganisms. 2025; 13(2):259. https://doi.org/10.3390/microorganisms13020259
Chicago/Turabian StyleLiu, Xiaowei, Zongyu Wen, Wei Zhou, Wentao Dong, Huiqing Ren, Gang Liang, and Wenwen Gong. 2025. "Effect of Multiyear Biodegradable Plastic Mulch on Soil Microbial Community, Assembly, and Functioning" Microorganisms 13, no. 2: 259. https://doi.org/10.3390/microorganisms13020259
APA StyleLiu, X., Wen, Z., Zhou, W., Dong, W., Ren, H., Liang, G., & Gong, W. (2025). Effect of Multiyear Biodegradable Plastic Mulch on Soil Microbial Community, Assembly, and Functioning. Microorganisms, 13(2), 259. https://doi.org/10.3390/microorganisms13020259







