Molecular Detection of Trypanosomatids in Rodents and Marsupials in the State of Amapá, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
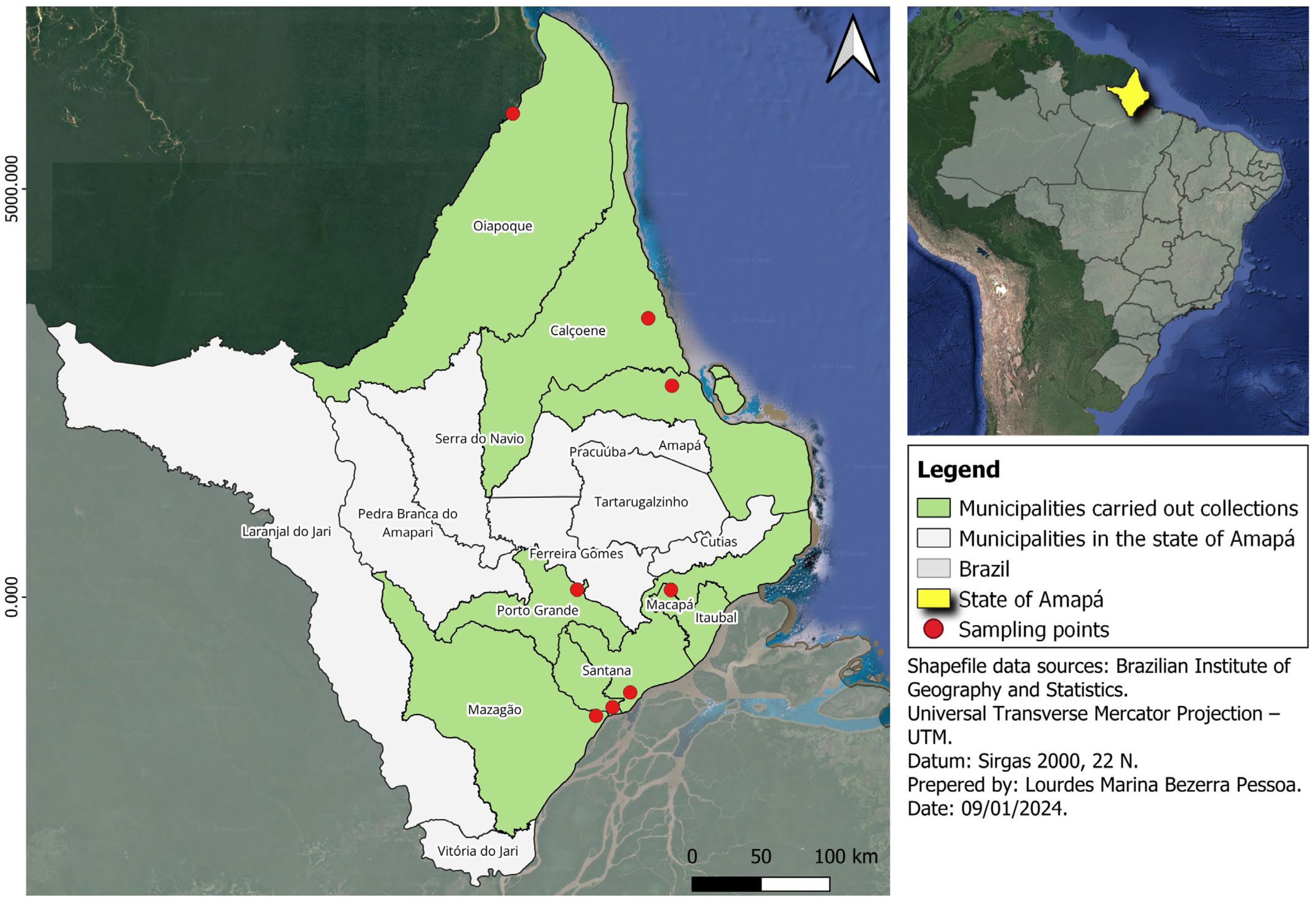
2.2. Animals and Study Area
2.3. DNA Extraction, Nested Polymerase Chain Reaction (Nested-PCR), and Amplicon Purification
2.4. Sequencing of Positive Samples
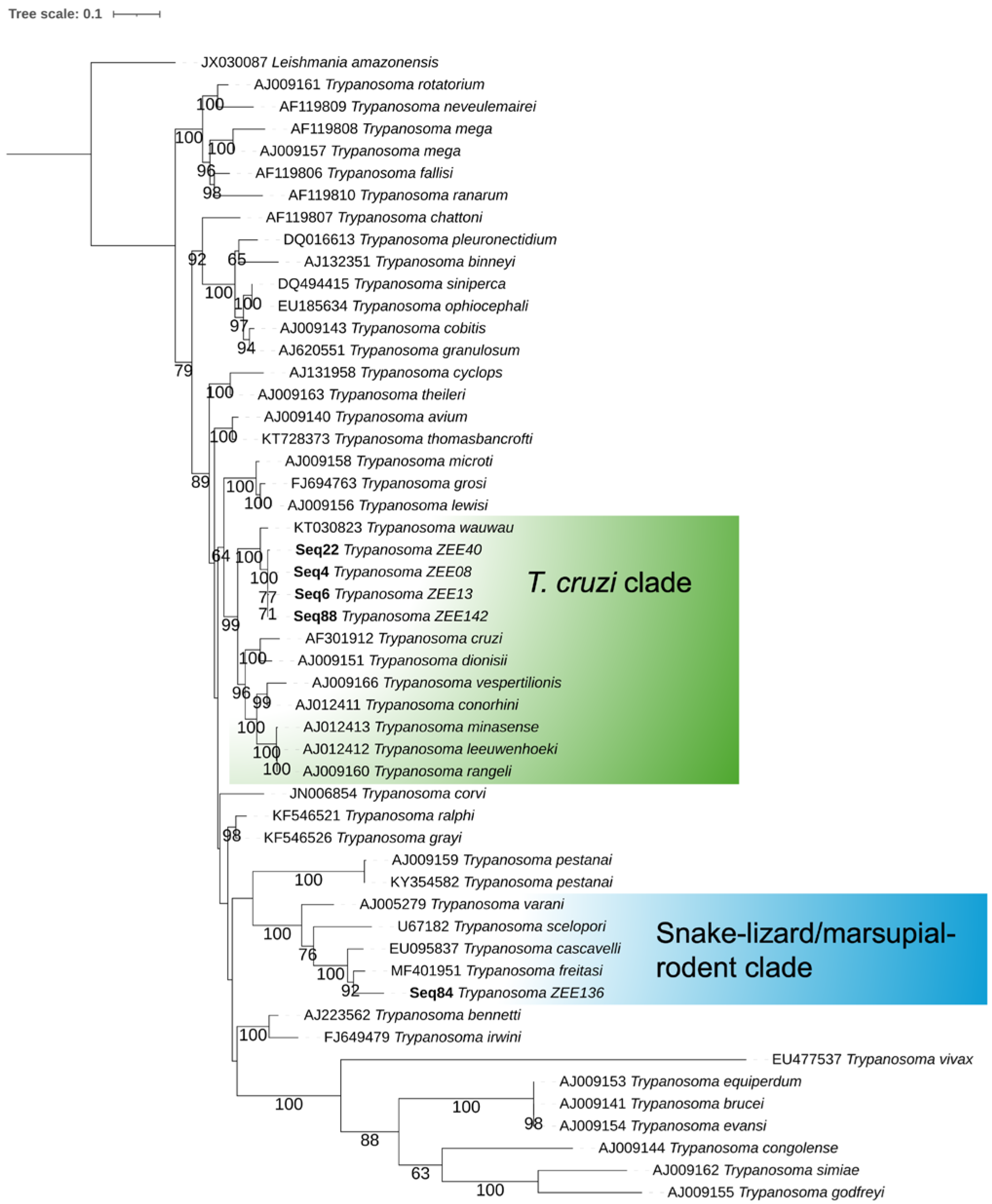
2.5. Phylogenetic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufer, A.; Ellis, J.; Stark, D.; Barratt, J. The evolution of trypanosomatid taxonomy. Parasit. Vectors. 2017, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Maslov, D.A.; Lukeš, J.; Jirků, M.; Simpson, L. Phylogeny of trypanosomes as inferred from the small and large subunit rRNAs: Implications for the evolution of parasitism in the trypanosomatid protozoa. Mol. Biochem. Parasitol. 1996, 75, 197–205. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Cucunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramírez, J.D.; Velásquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health Am. 2024, 37, 100881. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.K.; Lejon, V.; Barrett, M.P.; Blumberg, L.; Bukachi, S.A.; Chancey, R.J.; Edielu, A.; Matemba, L.; Mesha, T.; Mwanakasale, V.; et al. New WHO guidelines for treating rhodesiense human African trypanosomiasis: Expanded indications for fexinidazole and pentamidine. Lancet Infect. Dis. 2024, 24, 00581–00584. [Google Scholar] [CrossRef] [PubMed]
- WHO. Neglected Tropical Diseases. 2024. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 4 November 2024).
- WHO. Leishmaniasis. 2023. Health-Topics/Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 4 November 2024).
- Maia-Elkhoury, A.N.; Yadón, Z.E.; Díaz, M.I.S.; Lucena, F.F.A.L.; Castellanos, L.G.; Sanchez-Vazquez, M.J. Exploring spatial and temporal distribution of cutaneous Leishmaniasis in Americas, 2001–2011. PloS Negl. Trop. Dis. 2016, 10, e0005086. [Google Scholar] [CrossRef] [PubMed]
- Portella, T.P.; Kraenkel, R.A. Spatial-temporal pattern of cutaneous leishmaniasis in Brazil. Infect. Dis. Poverty. 2021, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Belo, V.S.; Bruhn, F.R.P.; Barbosa, D.S.; Câmara, D.C.P.; Simões, T.C.; Buzanovsky, L.P.; Duarte, A.G.S.; de Melo, S.N.; Cardoso, D.T.; Donato, L.E.; et al. Temporal patterns, spatial risks, and characteristics of tegumentary leishmaniasis in Brazil in the first twenty years of the 21st Century. PLoS Negl. Trop. Dis. 2023, 17, e0011405. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.S.; Vermeij, D.; Ramos, A.N.J.R.; Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- BRASIL. Casos de Doença de Chagas Aguda (DCA) Segundo Unidade Federada de Infecção e ano de Início de Sintomas, Brasil, 2010 a 2020. 2022. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/doenca-de-chagas/arquivos/casos-de-doenca-de-chagas-aguda-dca-segundo-unidade-federada-de-infeccao-e-ano-de-inicio-de-sintomas-brasil-2010-a-2020.pdf (accessed on 5 November 2024).
- Roque, A.L.R.; Xavier, S.C.C.; Gerhardt, M.; Silva, M.F.O.; Lima, V.S.; D’Aandrea, P.S.; Jansen, A.M. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Pará State, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013, 193, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cassia-Pires, R.; Boite, M.C.; D’Andrea, P.S.; Herrera, H.M.; Cupolillo, E.; Jansen, A.M.; Roque, L.R. Distinct Leishmania Species Infecting Wild Caviomorph Rodents (Rodentia: Hystricognathi) from Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3389. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Jansen, A. Wild and synanthropic reservoirs of Leishmania species in the Americas. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Caldart, E.T.; Freire, R.L.; Ferreira, F.P.; Ruffolo, B.B.; Sbeghen, M.R.; Mareze, M.; Garcia, J.L.; Mitsuka-Breganó, R.; Navarro, I.T. Leishmania in synanthropic rodents (Rattus rattus): New evidence for the urbanization of Leishmania (Leishmania) amazonensis. Braz. J. Vet. Parasitol. 2017, 26, 17–27. [Google Scholar] [CrossRef]
- Morales, E.A.; Mayor, P.; Bowler, M.; Aysanoa, E.; Pérez-Velez, E.S.; Pérez, J.; Ventocilla, J.A.; Baldeviano, C.C.; Lescano, A.G. Prevalence of Trypanosoma cruzi and Other Trypanosomatids in Frequently-Hunted Wild Mammals from the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2017, 97, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors. 2018, 11, 502. [Google Scholar] [CrossRef]
- Berbigier, A.P.; Barros, J.H.D.S.; Pontes, E.S.; Lisboa, C.V.; Gentile, R.; Xavier, S.C.D.C.; Jansen, A.M.; Roque, A.L.R. Trypanosomatid Richness in Wild and Synanthropic Small Mammals from a Biological Station in Rio de Janeiro, Brazil. Pathogens 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Sabioni, L.; Seixas, M.; Souza Filho, J.; Marcelino, A.; Shimabukuro, P. Evidence of a sylvatic enzootic cycle of Leishmania infantum in the State of Amapá, Brazil. Rev. Soc. Bras. Med. Trop. 2019, 53, e20190169. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.L.; Pardiñas, U.F.; D’Elía, G. Mammals of South America. v. 2. Rodents; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2. [Google Scholar]
- Weksler, M.; Percequillo, A.R. Key to the genera of the Tribe Oryzomyini (Rodentia: Cricetidae: Sigmodontinae). Mastozool. neotrop. 2011, 18, 281–292. [Google Scholar]
- Weksler, M.; Percequillo, A.R.; Voss, R.S. Ten New Genera of Oryzomyine Rodents (Cricetidae: Sigmodontinae). Am. Mus. Novit. 2006, 3537, 1–29. [Google Scholar] [CrossRef]
- Voss, R.S.; Jansa, S.A. Phylogenetic relationships and classification of Didelphis marsupials, an extant radiation of new world metatherian mammals. Bull. Am. Mus. Nat. Hist. 2009, 322, 1–177. [Google Scholar] [CrossRef]
- Voss, R.S.; Lunde, D.P.; Simmons, N.B. The mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna. Part-2. Nonvolant species. Bull. Am. Mus. Nat. Hist. 2001, 263, 3–236. [Google Scholar] [CrossRef]
- Abreu, E.F.; Casali, D.; Costa-Araújo, R.; Garbino, G.S.T.; Libardi, G.S.; Loretto, D.; Loss, A.C.; Marmontel, M.; Moras, L.M.; Nascimento, M.C.; et al. Lista de Mamíferos do Brasil (2022-1); Zenodo: Geneve, Switzerland, 2022. [Google Scholar]
- Silva, C.R.; Martins, A.M.; Castro, I.J.; Bernard, E.; Cardoso, E.M.; Lima, D.S.; Gregorin, R.; Rossi, R.V.; Percequillo, A.R.; Castro, K.C. Mammals of Amapá State, eastern Brazilian Amazonia: A revised taxonomic list with comments on species distributions. Mammalia 2013, 4, 409–424. [Google Scholar] [CrossRef]
- Noyes, H.A.; Stevens, J.R.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 2, 331–339, Erratum in Int. J. Parasitol. 2000, 2, 228. [Google Scholar] [CrossRef]
- Smith, A.; Clark, P.; Averis, S.; Lymbery, A.J.; Wayne, A.F.; Morris, K.D.; Thompson, R.C. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillate (Marsupialia: Potoroidae). Parasitology 2008, 11, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Seward, E.A.; Votýpka, J.; Kment, P.; Lukeš, J.; Kelly, S. Description of Phytomonas oxycareni n. sp. from the salivary glands of Oxycarenus lavaterae. Protist 2017, 168, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Engstler, M.; Wolf, M. 18S rRNA gene sequence-structure phylogeny of the Trypanosomatida (Kinetoplastea, Euglenozoa) with special reference to Trypanosoma. Eur. J. Protistol. 2021, 81, 125824. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 5, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Filho, S.P.; Brito, M.E.; Carvalho, F.G.; Ishikawa, E.A.; Cupolillo, E.; Floeter-Winter, L.; Shaw, J.J. Wild and synanthropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 3, 291–296. [Google Scholar] [CrossRef]
- Lima, B.S.; Dantas-Torres, F.; de Carvalho, M.R.; Marinho-Junior, J.F.; de Almeida, E.L.; Brito, M.E.; Gomes, F.; Brandão-Filho, S.P. Small mammals as hosts of Leishmania spp. in a highly endemic area for zoonotic leishmaniasis in North-Eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2013, 9, 592–597. [Google Scholar] [CrossRef]
- Achilles, G.R.; Kautzmann, R.P.; Chagas, H.D.F.; Pereira-Silva, J.W.; Almeida, J.F.; Fonseca, F.R.; Da Silva, M.N.F.; Pessoa, F.A.C.; Nava, A.F.D.; Ríos-Velásquez, C.M. Presence of trypanosomatids, with emphasis on Leishmania, in Rodentia and Didelphimorphia mammals of a rural settlement in the central Amazon region. Mem. Inst. Oswaldo Cruz 2021, 116, e200427. [Google Scholar] [CrossRef]
- Andrade, M.S.; Courtenay, O.; Brito, M.E.F.; Carvalho, F.G.; Carvalho, A.W.S.; Soares, F.; Carvalho, S.M.; Costa, P.L.; Zampieri, R.; Floeter-Winter, L.M.; et al. Infectiousness of Sylvatic and Synanthropic Small Rodents Implicates a Multi-host Reservoir of Leishmania (Viannia) braziliensis. PLoS Negl. Trop. Dis. 2015, 10, e0004137. [Google Scholar] [CrossRef]
- Shaw, J.J.; Marinho-Júnior, J.F.; Courtenay, O.; Brandão-Filho, S.P. Assessing reservoir host status in leishmaniasis with special reference to the infectiousness of Leishmania (Viannia) braziliensis infections in wild rodents. Rev. Soc. Bras. Med. Trop. 2023, 56, e0503-2023. [Google Scholar] [CrossRef]
- Marinho-Júnior, J.F.; Monteiro, J.F.C.L.S.; Sales De Carvalho, A.W.; De Carvalho, F.G.; De Paiva Cavalcanti, M.; Shaw, J.; Courtenay, O.; Brandão-Filho, S.P. High levels of infectiousness of asymptomatic Leishmania (Viannia) braziliensis infections in wild rodents highlights their importance in the epidemiology of American Tegumentary Leishmaniasis in Brazil. PLoS. Negl. Trop. Dis. 2023, 1, e0010996. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Marinho-Júnior, J.F.; Brito, M.E.F.; Monteiro, J.F.C.L.S.; Shaw, J.J.; Brandão-Filho, S.P. Incidence of Human and Free-Ranging Wild Rodent Infections with Leishmania (Viannia) braziliensis, Aetiological Agent of Cutaneous Leishmaniasis. Pathogens 2023, 12, 1395. [Google Scholar] [CrossRef]
- Almeida, A.N.F.; Nascimento, L.C.S.D.; Sousa, E.S.M.M.; Oliveira, A.J.D.; Sena, M.G.; Resende, B.M.; Chaves, R.C.G.; Garcez, L.M. Surveillance of cutaneous leishmaniasis in clinical samples: Distribution of Leishmania guyanensis in the state of Amapá, Brazil, 2018. Epidemiol. Serv. Saude. 2020, 17, e2018504. [Google Scholar]
- López, M.; Erazo, D.; Hoyos, J.; Léon, C.; Fuya, P.; Lugo, L.; Cordovez, J.M.; González, C. Measuring spatial co-occurrences of species potentially involved in Leishmania transmission cycles through a predictive and fieldwork approach. Sci. Rep. 2021, 11, 6789. [Google Scholar] [CrossRef] [PubMed]
- Bonvicino, C.R.; Oliveira, J.A.; D’andrea, P.S. Guia dos Roedores do BRASIL, Com Chaves Para Gêneros Baseadas em Caracteres Externos; Centro Pan-Americano de Febre Aftosa—OPAS/OMS: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Nantes, W.A.G.; Santos, F.M.; De Macedo, G.C.; Barreto, W.T.G.; Gonçalves, L.R.; Rodrigues, M.S.; Chulli, J.V.M.; Rucco, A.C.; Assis, W.O.; Porfírio, G.E.O.; et al. Trypanosomatid species in Didelphis albiventris from urban forest fragments. Parasitol. Res. 2021, 120, 223–231. [Google Scholar] [CrossRef]
- Lima, L.; Espinosa-Alvarez, O.; Pinto, C.M.; Cavazzana, M.; Pavan, A.C.; Carranza, J.C.; Lim, B.K.; Campaner, M.; Takata, C.S.A.; Camargo, E.P.; et al. New insights into the evolution of the Trypanosoma cruzi clade provided by a new trypanosome species tightly linked to Neotropical Pteronotus bats and related to na Australian lineage of trypanosomes. Parasit. Vectors 2015, 8, 657. [Google Scholar] [CrossRef]
- Da Costa, A.P.; Nunes, P.H.; Leite, B.H.S.; Ferreira, J.I.G.D.S.; Tonhosolo, R.; Da Rosa, A.R.; Rocha, P.A.; Aires, C.C.; Gennari, S.M.; Marcili, A. Diversity of bats trypanosomes in hydroeletric area of Belo Monte in Brazilian Amazonia. Acta Trop. 2016, 164, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.A.; Lisboa, C.V.; Novaes, R.L.M.; Silva, B.A.; Souza, R.D.F.; Jansen, A.M.; Moratelli, R.; Roque, A.L.R. Isolation and characterization of trypanosomatids, including Crithidia mellificae, in bats from the Atlantic Forest of Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2019, 7, e0007527. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Lima, L.; Xavier, S.C.C.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Santos, F.M.; Barreto, W.T.G.; Macedo, G.C.; Barros, J.H.S.; Xavier, S.C.C.; Garcia, C.M.; Mourão, G.; Oliveira, J.; Rimoldi, A.R.; Porfírio, G.E.O.; et al. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large Neotropical wetland. Acta Trop. 2019, 199, 105098. [Google Scholar] [CrossRef]
- Agosta, S.J. On ecological fitting, plant–insect associations, herbivore host shifts, and host plant selection. Oikos 2006, 114, 556–565. [Google Scholar] [CrossRef]
- Câmara, A.C.; Varela-Freire, A.A.; Valadares, H.M.; Macedo, A.M.; D’Avila, D.A.; Machado, C.R.; Lages-Silva, E.; Chiari, E.; Galvão, L.M. Genetic analyses of Trypanosoma cruzi isolates from naturally infected triatomines and humans in northeastern Brazil. Acta Trop. 2010, 115, 205–211. [Google Scholar] [CrossRef]
- Cordon-Obras, C.; Cano, J.; González-Pacanowska, D.; Benito, A.; Navarro, M.; Bart, J.M. Trypanosoma brucei gambiense adaptation to different mammalian sera is associated with VSG expression site plasticity. PLoS ONE 2013, 12, e85072. [Google Scholar] [CrossRef] [PubMed]
- Drini, S.; Criscuolo, A.; Lecha, T.P.; Imamura, H.; Skalický, T.; Rachidi, N.; Lukeš, J.; Dujardin, J.C.; Späth, G.F. Species- and strainspecific adaptation of the HSP70 super family in pathogenic Trypanosomatids. Genome Biol. Evol. 2016, 6, 1980–1995. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Cantillo-Barraza, O.; Triana-Chávez, O.; Mejia-Jaramillo, A.M. Molecular surveillance reveals bats from eastern Colombia infected with Trypanosoma theileri and Trypanosoma wauwau-like parasites. Prev. Vet. Med. 2020, 84, 105159. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.T.; Barreto, R.F.S.M.; Pavan, M.G.; Pereira, C.S.; Roque, A.L.R. Trypanosoma janseni n. sp. (Trypanosomatida: Trypanosomatidae) isolated from Didelphis aurita (Mammalia: Didelphidae) in the Atlantic Rainforest of Rio de Janeiro, Brazil: Integrative taxonomy and phylogeography within the Trypanosoma cruzi clade. Memórias Inst. Oswaldo Cruz 2018, 113, 45–55. [Google Scholar] [CrossRef]
- Legey, A.P.; Pinho, A.P.; Xavier, S.C.C.; Leon, L.; Jansen, A.M. Humoral immune response kinetics in Philander opossum and Didelphis marsupialis infected and immunized by Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 1999, 94, 371–373. [Google Scholar] [CrossRef]
- Barros, J.H.S.; Xavier, S.C.C.; Bilac, D.; Lima, V.S.; Dario, M.A.; Jansen, A.M. Identification of novel mammalian hosts and Brazilian biome geographic distribution of Trypanosoma cruzi TcIII and TcIV. Acta Trop. 2017, 172, 173–179. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Ramos, R.A.N.; Campos, A.K.; Dantas-Torres, F.; Otranto, D. Didelphis spp. opossums and their parasites in the Americas: A One Health perspective. Parasitol. Res. 2021, 12, 4091–4111. [Google Scholar] [CrossRef] [PubMed]
- Martinez Ibarra, J.A.; Martinez, B.O.; Rodas Martinez, A.Z.; Flores, R.A.; Garcia, C.I.M.; Franco, E.R.; Villalobos, G.; Martinez Hernandez, F. Trypanosoma cruzi in Wild and Synanthropic Mammals in Two Regions of Mexico: A Fieldwork and Genetic Discrete Typing Unit Review. Vector Borne Zoonotic Dis. 2024, 8, 499–509. [Google Scholar] [CrossRef]
- Carvalho, G.H.F.; Medeiros, G.G.; Magalhães, R.L.B. Subnotificação de doença de Chagas no Estado do Amapá no período da pandemia de COVID-19. Cad. Pedagógico 2024, 21, e7609. [Google Scholar] [CrossRef]
- Ardente, N.C.; Ferreguetti, A.C.; Gettinger, D.; Leal, P.; Mendes-Oliveira, A.C.; Martins-Hatano, F.; Bergallo, H.G. Diversity and impacts of mining on the nonvolant small mammal communities of two vegetation types in the Brazilian Amazon. PLoS ONE 2016, 11, e0167266. [Google Scholar] [CrossRef] [PubMed]
| Order | Species | Total Tested | Number of Positive (Type of Sample) | Positivity (%) |
|---|---|---|---|---|
| Didelphimorphia | Cryptonanus sp. | 6 | 0 | 0.0 |
| Didelphis imperfecta | 1 | 1 (pooled spleen and liver) | 100.0 | |
| Didelphis marsupialis | 9 | 0 | 0.0 | |
| Gracilinanus emiliae | 2 | 0 | 0.0 | |
| Hyladelphys kalynowskii | 1 | 0 | 0.0 | |
| Marmosa demerarae | 3 | 1 (liver) | 33.3 | |
| Marmosa murina | 15 | 2 (both liver) | 13.3 | |
| Metachirus nudicaudatus | 1 | 1 (pooled spleen and liver) | 100.0 | |
| Monodelphis touan | 8 | 0 | 0.0 | |
| Philander opossum | 5 | 1 (pooled spleen and liver) | 20.0 | |
| Rodentia | Dactylomys dactylinus | 2 | 0 | 0.0 |
| Hylaeamys megacephalus | 19 | 2 (both pooled spleen and liver) | 10.5 | |
| Mesomys hispidus | 3 | 1 (pooled spleen and liver) | 33.3 | |
| Neacomys paracou | 8 | 4 (1 liver, 3 pooled spleen and liver) | 50.0 | |
| Oecomys auyantepui | 1 | 0 | 0.0 | |
| Oecomys bicolor | 2 | 1 (pooled spleen and liver) | 50.0 | |
| Oecomys rutilus | 2 | 0 | 0.0 | |
| Oecomys sp. | 1 | 0 | 0.0 | |
| Proechimys cuvieri | 15 | 1 (pooled spleen and liver) | 6.7 | |
| Proechimys guyannensis | 20 | 1 (pooled spleen and liver) | 5.0 | |
| Rattus rattus | 1 | 0 | 0.0 | |
| Rhypidomys nitela | 1 | 0 | 0.0 | |
| Sigmodon alstoni | 2 | 0 | 0.0 | |
| Zygodontomys brevicauda | 9 | 3 (1 spleen, 2 pooled spleen and liver) | 33.3 | |
| Total | 137 | 19 (4 liver, 1 spleen, 14 pooled spleen and liver) | 13.9 |
| Species | Common Name | Municipality | Sample ID | Sample | Sequence Size (Our Genbank Accession Number) and BLASTn Results, with Highest Percent Identity and Species (GenBank Accession Number) |
|---|---|---|---|---|---|
| Didelphis imperfecta | Guianan white-eared opossum | Macapá | RAM 07 (21) | Spleen and liver (pooled) | 802 bp (PQ766626), 99.4% identity with Trypanosoma wauwau (KR653210) |
| Hylaeamys megacephalus | Large-headed rice rat | Oiapoque | ZEE 132 (83) | Spleen and liver (pooled) | 523 bp (PQ766617), 100% identity with Leishmania (Viannia) spp. (JX030135, GQ332355, JN003595) |
| Marmosa demerarae | Woolly mouse opossum | Mazagão | ZEE 08 (4) | Liver | 540 bp (PQ766614), 99.4% identity with Trypanosoma sp. (MN196493) |
| Marmosa murina | Linnaeus’s mouse opossum | Mazagão | ZEE 13 (6) | Liver | 532 bp (PQ766615), 99.8% identity with Trypanosoma sp. (MN196493) |
| Mesomys hispidus | Ferreira’s spiny tree-rat | Macapá | RAM 11 (26) | Spleen and liver (pooled) | 894 bp (PQ766627), 99.9% identity with different Leishmania (Viannia) spp. (JX030135, GQ332355, JN003595) |
| Neacomys paracou | Paracou bristly mouse | Oiapoque | ZEE 136 (84) | Spleen and liver (pooled) | 450 bp (PQ799498), 99.1% identity with Trypanosoma freitasi (MF401951) |
| Neacomys paracou | Paracou bristly mouse | Oiapoque | ZEE 142 (88) | Spleen and liver (pooled) | 534 bp (PQ766619), 99.8% identity with Trypanosoma sp. (MN196493) |
| Philander opossum | Gray four-eyed opossum | Macapá | RAM 15 (30) | Spleen and liver (pooled) | 882 bp (PQ766628), 100% identity with Trypanosoma cruzi (KX007998) |
| Zygodontomys brevicauda | Short-tailed cane mouse | Mazagão | ZEE 16B (8) | Spleen | 525 bp (PQ766616), 99.6% identity with Leishmania (Viannia) spp. (JX030135, GQ332355, JN003595) |
| Zygodontomys brevicauda | Short-tailed cane mouse | Calçoene | ZEE 40 (22) | Spleen and liver (pooled) | 532 bp (PQ766618), 99.6% identity with Trypanosoma sp. (MN196493) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, L.M.B.; Silva, C.R.; Sales, K.G.d.S.; Souza, D.C.d.; Bonifácio, L.L.N.; Luna, R.L.N.d.; Dantas-Torres, F.; Viana, L.A. Molecular Detection of Trypanosomatids in Rodents and Marsupials in the State of Amapá, Brazil. Microorganisms 2025, 13, 242. https://doi.org/10.3390/microorganisms13020242
Pessoa LMB, Silva CR, Sales KGdS, Souza DCd, Bonifácio LLN, Luna RLNd, Dantas-Torres F, Viana LA. Molecular Detection of Trypanosomatids in Rodents and Marsupials in the State of Amapá, Brazil. Microorganisms. 2025; 13(2):242. https://doi.org/10.3390/microorganisms13020242
Chicago/Turabian StylePessoa, Lourdes Marina Bezerra, Claudia Regina Silva, Kamila Gaudêncio da Silva Sales, Darlison Chagas de Souza, Lucas Lisboa Nunes Bonifácio, Rafaela Lira Nogueira de Luna, Filipe Dantas-Torres, and Lúcio André Viana. 2025. "Molecular Detection of Trypanosomatids in Rodents and Marsupials in the State of Amapá, Brazil" Microorganisms 13, no. 2: 242. https://doi.org/10.3390/microorganisms13020242
APA StylePessoa, L. M. B., Silva, C. R., Sales, K. G. d. S., Souza, D. C. d., Bonifácio, L. L. N., Luna, R. L. N. d., Dantas-Torres, F., & Viana, L. A. (2025). Molecular Detection of Trypanosomatids in Rodents and Marsupials in the State of Amapá, Brazil. Microorganisms, 13(2), 242. https://doi.org/10.3390/microorganisms13020242






