Abstract
It was previously discovered that, in the Gram-negative bacterium Escherichia coli growing on a minimal medium with sulfate, stress-induced growth arrest is accompanied by the release of hydrogen sulfide. The source of the sulfide is the desulfurization of intracellular cysteine as one of the ways of maintaining it at a safe level. The danger of excess cysteine is associated with its participation in the Fenton reaction, leading to the formation of highly toxic hydroxyl radicals. Using electrochemical sensors, we identified stress-induced sulfide production in the Gram-positive bacteria Bacillus subtilis and Bacillus megaterium, growing on a minimal medium with sulfate, and changes in physiological parameters such as Eh, pH, and oxygen and potassium consumption. Sulfide production was observed during growth arrest due to the depletion of glucose, ammonium or antibiotic action. The use of sensors allowed to continuously record, in growing cultures, even small changes in parameters. There were significant differences in the amount and kinetics of sulfide production between Bacillus and E. coli. These differences are thought to be due to the lack of glutathione in Bacillus. It is suggested that stress-induced sulfide production by Bacillus under the described conditions may be one of the previously unknown sources of hydrogen sulfide in nature.
1. Introduction
A sharp drop in the redox potential (Eh jump), measured using a platinum electrode, was previously discovered during stress-induced growth arrest of the Gram-negative bacterium Escherichia coli, growing on a minimal medium with sulfate as the sole sulfur source. A close correlation was found between the stress-induced changes in Eh and the level of low-molecular-weight thiols (LWTs) in the medium [1]. Our further studies showed that, in E. coli, stress-induced Eh jumps are the result of hydrogen sulfide (H2S) excretion. Sulfide production is induced during growth arrest caused by glucose depletion, isoleucine starvation, or antibiotic exposure [2,3]. The production of sulfide has been observed in E. coli growing on M9 medium containing thiosulfate as the sole inorganic sulfur source in response to glucose starvation [4].
In E. coli, the formation of sulfide by cysteine desulfurization may be one of the ways of maintaining cysteine homeostasis under stress-induced conditions [2,3]. L-cysteine is used for protein and glutathione synthesis and as a source of reduced sulfur in other organic molecules. However, even at subtoxic concentrations, cysteine can inhibit several enzymes of amino acid synthesis and impair bacterial growth [5]. Cysteine can also promote the Fenton reaction in the presence of H2O2 to form toxic hydroxyl radicals that cause oxidative damage to critical biomolecules, including the DNA [6]. Therefore, the intracellular level of cysteine is strictly regulated by various pathways, including biosynthesis, transport, and degradation [7].
In mammals, H2S is an important endogenous gasotransmitter and signaling molecule [8]. There are increasing data on the important role of endogenously produced H2S in bacterial physiology. In cultures growing on cysteine-containing media, H2S can protect bacteria from antibiotic-induced damage [9] and oxidative stress [10], and it is involved in a bacterial defense mechanism against the host immune response [11]. Modulation of endogenous hydrogen sulfide levels can significantly alter the sensitivity of bacteria, including pathogens, to antibiotics [12].
In aerobic cultures of Gram-positive bacteria Bacillus subtilis and Bacillus megaterium growing on a minimal medium, characteristic Eh jumps to negative values upon growth cessation caused by glucose and ammonium exhaustion have also been observed. As with E. coli, a correlation between the stress-induced changes in Eh and the level of low-molecular-weight thiols in the medium was found [1].
Bacillus are widespread in nature and play an active part in the decomposition of organic matter. The ecological niches of B. subtilis and B. megaterium are located in soil, and they do not produce sulfide during normal growth on sulfate as a sulfur source. It was of interest to test whether Bacillus produces sulfide under stressful conditions. In this work, using five electrochemical sensors, for the first time, we identified stress-induced sulfide production in B. subtilis and B. megaterium, as well as changes in physiologically important parameters, such as Eh, pH, and oxygen and potassium consumption, which accompany sulfide leakage. There were significant differences in the amount and kinetics of sulfide production between Bacillus and E. coli. These differences are thought to be due to the lack of the tripeptide glutathione in Bacillus.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
The strains of Bacillus subtilis VKM428 and Bacillus megaterium VKM512 from the Russian collection of microorganisms (VKM) and Escherichia coli BW25113 from the Keio collection (E. coli Genetic Stock Center) were used in this study. Overnight cultures were grown aerobically at 37 °C in 250 mL flasks with shaking at 150 rpm in M9 minimal medium [13] supplemented with glucose (5 g L−1) as the sole carbon and energy sources. In the experiments with glucose starvation, after centrifugation, the cells were transferred to 100 mL of M9 medium containing 0.25 g L−1 glucose to a final optical density at 600 nm (OD600) of 0.1 and cultured as indicated above. For the experiments with nitrogen starvation, the amount of NH4Cl in the M9 medium was reduced to 0.05 g L−1, and the amount of glucose was 10 g L−1. Substrate exhaustion and the onset of starvation were confirmed by the cessation of growth by measuring OD600. The antibiotics chloramphenicol (25 µg mL−1), kanamycin, tetracycline, and erythromycin (30–120 µg mL−1) were added to the culture growing on M9 medium with 10 g L−1 glucose when the OD600 reached 0.4.
2.2. Real-Time Monitoring of Eh, Dissolved Oxygen (dO2), pH, and Ions of Extracellular Sulfide (S2−) and Potassium (K+)
This work used highly sensitive electrochemical sensors immersed directly in the culture medium, which made it possible to record the measured parameters continuously and simultaneously and detect even small changes, which is difficult when using other methods. The use of electrochemical sensors is described in more detail in our previous works [2,3]. Four electrochemical sensors were used to monitor the physiological state of the bacterial cultures. The redox potential (Eh) in the bacterial cultures was measured using platinum and reference electrodes and Mettler Toledo SevenCompact™ pH/Ionmeters S220 (Mettler Toledo, Greifensee, Switzerland). The dissolved oxygen (dO2) and pH were measured using a Clarke oxygen electrode InPro 6800 (Mettler Toledo, Greifensee, Switzerland) and a combined pH electrode InLab Expert ProISM (Mettler Toledo, Greifensee, Switzerland), respectively. The dO2/pH controller of a BioFlo 110 fermentor (New Brunswick Scientific Co., Edison, NJ, USA) was used for data recording. Changes in the levels of extracellular K+ were registered using the system of K+-selective (ELIS121K) (IT Company, Moscow, Russia) and reference electrodes and a computer pH/ion meter cpX-2 (IBI, Pushchino, Russia). For the K+ measurements, cells were grown as described above, except for the medium, which contained a low K+ concentration (0.2 mM).
The extracellular sulfide levels were detected using the system of sulfide-specific ion-selective electrodes XC-S2—001 (Sensor Systems Company, St. Petersburg, Russia), a reference electrode, and a computer pH/ion meter cpX-2 (IBI, Pushchino, Russia). The electrode had a sensitivity threshold of 10 nM, operated in a wide pH range (6 ÷ 12), and did not respond to changes in oxygen in the cultivation environment. The sulfide concentration in the medium was calculated using a standard curve prepared with known amounts of Na2S. For the calculations, the value of the difference between the maximum drop in the potential of the sulfide electrode after exposure to the studied factor and its value in the absence of any effects was used. The synchronous processing of all primary data from the sensor system was carried out using the RS-232 and Modbus protocols and the Advantech OPC Server v3.0 software package.
The H2S levels in the gas phase were estimated using lead acetate [Pb(Ac)2], which reacts specifically with H2S to form a brown lead sulfide stain [10]. In our conditions, this method had a sensitivity of 0.1 μM. Lead acetate-soaked paper strips were affixed in culture flasks above the level of the liquid culture. To determine the total H2S, the paper strip was left for the entire duration of the experiment. The spots were scanned and then quantified using ImageJ1.54g. The results were expressed as arbitrary units.
2.3. Determination of Intracellular and Extracellular Cysteine
For the intracellular L-cysteine assays, 40 mL of culture (20 mL each from two identical flasks) was centrifuged (8000× g for 5 min), suspended in 4 mL of 0.1 M Tris-HCl pH 8.6, and lysed by sonification at 0 °C, using a 30 s pulse for six cycles. Perchloric acid (final concentration of 0.5 M) was added to the lysate to precipitate proteins. After 30 min, the suspension was centrifuged (8000× g for 5 min), the supernatant was adjusted to a pH of 8.6 with KOH, frozen, centrifuged to eliminate the potassium perchlorate, and evaporated using a rotary evaporator RV10 (IKA, Staufen, Germany) at 65 °C to 0.57 mL and then treated with 0.25 mL of dithiothreitol (10 mM) for 10 min. Reduced samples (0.5 mL) were used to determine the amount of cysteine according to the Gaitonde method, as described previously [14]. Standard curves were generated with known amounts of cysteine, which were treated as samples of cell suspensions.
2.4. Determination of Total Catalase Activity
The total catalase activity was measured by a spectrophotometric method [15]. To prepare the extracts, E. coli samples (20 mL) were centrifuged (5 min, 8000× g) and resuspended in 4 mL of 5 mM potassium phosphate buffer (pH 7.0), containing 5 mM EDTA, 10% glycerol, and 25 μmol phenylmethylsulfonyl fluoride, and then disrupted by sonication on ice (four rounds of five 5 s pulses). The soluble cell fraction was separated by centrifugation (10 min at 12,000× g) at 4 °C. The total protein concentration in the supernatant was measured by the method of Lowry. The specific catalase activity was expressed in micromoles of H2O2 decomposed per minute per milligram of total protein.
2.5. Statistical Analysis of the Data
Each result is indicated as the mean value of three-to-five independent experiments ± the standard error of the mean (SEM). Significant differences were analyzed by Student’s t-test. A p-value of 0.05 was used as the cut-off for statistical significance. The results were analyzed by means of the program packet Statistica 8.0.360 (StatSoft Inc., Tulsa, OK, USA, accessed on 27 August 2007).
3. Results
3.1. Glucose and Ammonium Depletion in B. subtilis and E. coli
It has previously been shown that, in E. coli and B. subtilis growing on aerobic media with sulfate as a sulfur source, the transition from exponential growth to glucose starvation is accompanied by a rapid drop in the potential of the platinum electrode measuring Eh. In E. coli, this Eh jump has later been shown to be associated with sulfide excretion [2]. In our study, it was of interest to check whether there was a relationship between the changes in Eh and extracellular sulfide in this situation in B. subtilis.
The growth arrest of B. subtilis due to glucose depletion (Figure 1) was accompanied by a rapid irreversible drop in the potential of the platinum and sulfide sensors, indicating an increase in extracellular sulfide (Figure 2a,b). The largest deviation in the potential of the sulfide sensor from the baseline was 73 ± 6 mV, which corresponded to 510 nM sulfide. The test using Pb(Ac)2 strips showed an increase in sulfide production during glucose starvation by 21 ± 4 conventional units (Figure 3a and Figure S1).
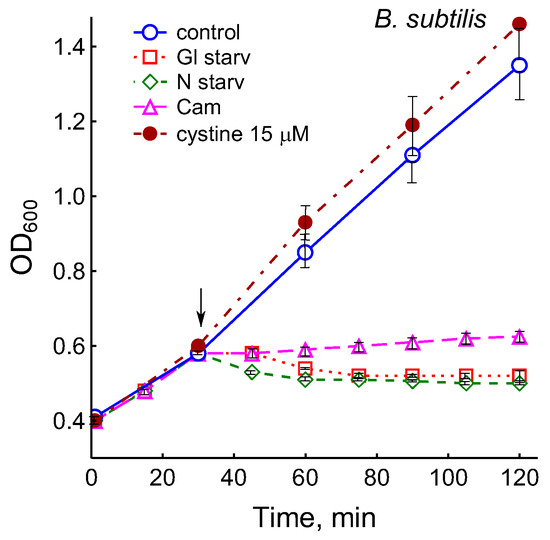
Figure 1.
Treatment with 25 µg mL−1 chloramphenicol (CAM) and glucose or ammonium depletion (but not cystine addition) causes B. subtilis growth arrest.
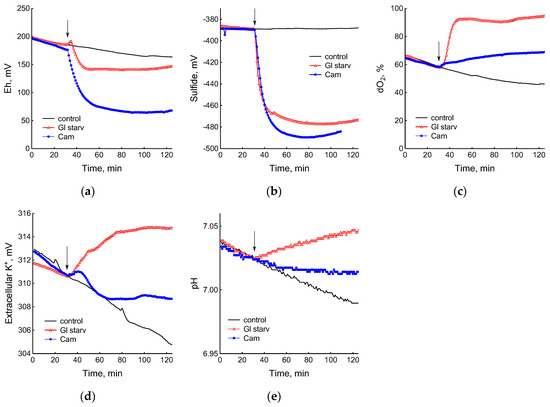
Figure 2.
Changes in platinum (a) or sulfide-selective (b) electrode potential, dissolved oxygen (dO2) level (c), potassium-selective electrode potential (d), and pH (e) in B. subtilis in response to glucose depletion or the addition of chloramphenicol (25 µg mL−1). The arrows indicate the time of CAM addition or the onset of glucose starvation.
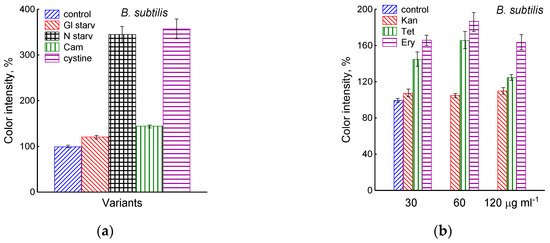
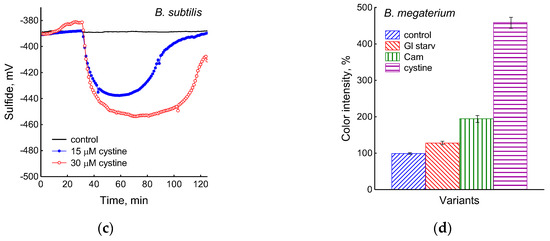
Figure 3.
Extracellular H2S accumulation in B. subtilis was determined using Pb(Ac)2 strips after incubation for 120 min under the tested conditions. Ammonium or glucose starvation and CAM or cystine supplementation (a), treatment with various concentrations of kanamycin, tetracycline, or erythromycin (b). Changes in sulfide electrode potential upon treatment of B. subtilis with cystine (c). Extracellular H2S accumulation in B. megaterium was determined using Pb(Ac)2 after the addition of chloramphenicol (25 µg mL−1) or cystine (15 µM) and glucose depletion (d).
The comparison of the profiles of changes in the potentials of the platinum and S2−-selective electrodes (Figure 2a,b) indicates that, as in E. coli [2], changes in Eh during glucose starvation in B. subtilis are also associated with sulfide leakage. At the same time, unlike E. coli, in B. subtilis the excretion of sulfide upon glucose depletion is irreversible. In both bacteria, under normal growth conditions (control) extracellular sulfide is not detected by either of the methods used.
Data obtained using electrochemical sensors show that the growth arrest of B. subtilis caused by the exhaustion of glucose was accompanied by a decrease in metabolic activity, as evidenced by a rapid and almost complete decrease in oxygen consumption and the release of potassium from the cells (Figure 2c,d). Acidification of the medium was replaced by alkalization (Figure 2e), which may be due to the consumption of organic acids accumulated during glucose catabolism.
In response to glucose starvation, the cysteine levels in B. subtilis increased by 33% in the cells and 30% in the medium (Figure S2). Notably, glucose depletion in E. coli growing under the same conditions was not accompanied by extracellular cysteine accumulation [2].
It has previously been shown that, as in the case of glucose, the growth arrest of B. subtilis and E. coli under ammonium depletion is accompanied by a rapid drop in Eh [1]. In this study, it was of interest to test whether B. subtilis produced sulfide during ammonium starvation and simultaneously compare the responses of B. subtilis and E. coli to this stress. Whether E. coli produced sulfide during ammonium starvation was also unknown.
The depletion of ammonium in the medium led to a rapid and complete cessation of growth of B. subtilis and E. coli (Figure 1). In response to growth arrest, a drop in the potential of the platinum and sulfide electrodes was observed in both B. subtilis and E. coli (Figure 4a,b). The high agreement between the time profiles of changes in Eh and the sulfide sensor for each of the two bacteria indicates the contribution of sulfide to the change in Eh in response to stress. The largest deviation of the sulfide electrode potential from the baseline was 48 ± 3 mV in B. subtilis and 35 ± 2 mV in E. coli (corresponding to 260 and 80 nM sulfide). Thus, under ammonium depletion, sulfide production in B. subtilis was more than three times higher than in E. coli. As in the case of glucose [2], the leakage of sulfide under ammonium depletion in E. coli is reversible and includes a phase of rapid leakage lasting about 10 min and a phase of slower return to baseline values. In B. subtilis, sulfide leakage is irreversible and proceeds much slower than in E. coli (Figure 4a,b). An independent test using Pb(Ac)2 strips showed a simultaneous increase in H2S over the culture medium in B. subtilis (Figure 3a and Figure S1).
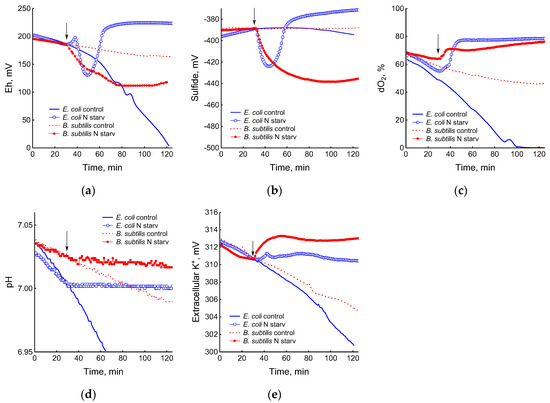
Figure 4.
Changes in platinum (a) or sulfide-selective (b) electrode potential, dissolved oxygen (dO2) level (c), pH (d), and potassium-selective electrode potential (e) in the response of B. subtilis and E. coli to ammonium depletion. The arrows indicate the time of the onset of ammonium starvation.
The growth arrest of B. subtilis and E. coli caused by the depletion of ammonium was accompanied by a decrease in oxygen consumption (Figure 4c) and the cessation of medium acidification (Figure 4d). As in the case of glucose, in response to ammonium starvation, B. subtilis lost some of the intracellular potassium. It is remarkable that E. coli cells retained potassium at a level close to that which was in the growing cells up until the onset of ammonium starvation (Figure 4e).
Importantly, in B. subtilis, the addition of glucose or ammonium during the starvation response quickly restores growth and metabolic activity, as evidenced by renewed oxygen consumption and a decrease in pH. An increase in the potential of the sulfide sensor indicated a decrease in the sulfide content in the medium, which may have been due to its involvement in the sulfur metabolism via growing cells (Figure S3).
3.2. Exposure of B. subtilis to Antibiotics
The treatment of growing B. subtilis with 25 μg mL−1 chloramphenicol caused a rapid cessation of growth (Figure 1) and an irreversible decrease in the potential of Eh and sulfide sensors (Figure 2a,b). The largest deviation from the baseline was 108 ± 3 mV, which corresponded to 1.35 µM sulfide (Figure 2b). The Pb(Ac)2 strip test also showed an increase in the H2S content over the culture medium after the addition of Cam. Besides chloramphenicol, H2S excretion in B. subtilis was induced by erythromycin, kanamycin, and tetracycline (Figure 3a,b and Figure S3). H2S excretion was previously observed by us when E. coli, growing on M9 medium with glucose, was treated with several antibiotics, including chloramphenicol (Cam) and tetracycline (Tet) [2].
The use of electrochemical sensors made it possible to identify significant differences in the response of B. subtilis to glucose starvation and the effect of chloramphenicol. Although in both cases there was a complete cessation of growth, the cells treated with the antibiotic showed noticeable metabolic activity, continuing to consume glucose and oxygen and retain potassium, but at a lower intensity than the untreated cells (Figure 2c–e).
The exposure of B. subtilis to chloramphenicol did not cause significant changes in free intracellular cysteine, but stimulated the accumulation of extracellular cysteine to 0.95 µM/OD600, more than twice its level in the control (Figure S2). Under similar conditions, E. coli responded to treatment with chloramphenicol in the same way, accumulating 1.05 µM/OD600 cysteine in the medium [3]. It should be noted that M9 medium containing 10 g L−1 glucose was used when studying the effect of chloramphenicol on the level of extra- and intracellular cysteine in growing B. subtilis. This prevented growth arrest due to exhaustion of the carbon and energy source. In experiments with glucose starvation, M9 medium containing 0.25 g L−1 glucose was used (Section 2.1) This may explain why, in the latter case, the basal level of extracellular cysteine was significantly lower than in the medium with a high glucose content. The absence of glucose could also have been the reason for the slower accumulation of extracellular cysteine during starvation than after chloramphenicol treatment (Figure S2). Like sulfide, cysteine has a high redox activity, and an increase in its extracellular concentration may contribute to a decrease in the redox potential of the platinum electrode during glucose starvation and chloramphenicol treatment.
Remarkably, the continuous and simultaneous recording of changes in the sulfide electrode potential and other parameters (Eh, dO2, pH, K+) indicated that the cells quickly responded to all the stresses tested. The release of sulfide from the cells was recorded a few seconds after exposure to a stress-inducing factor and occurred synchronously with changes in other parameters.
3.3. Response of B. subtilis to the Addition of Cystine
It was previously reported that the addition of cysteine or cystine to B. subtilis and E. coli growing on a minimal medium with sulfate leads to the release of hydrogen sulfide [16]. In another study, cystine treatment of E. coli growing on a medium with glycerol and sulfate also led to the release of sulfides [17]. In their work, hydrogen sulfide was determined using the methylene blue method.
It was of interest in this study to test this effect in B. subtilis under our conditions and investigate the action of exogenous cystine on the growth and other physiological parameters in these bacteria. Monitoring changes in the potential of the sulfide-selective electrode showed that the addition of cystine to B. subtilis growing on M9 medium with sulfate resulted in reversible sulfide release. The amplitude changes in pS2− upon the addition of 15 or 30 μM cystine were 50 ± 14 (295 nM) and 103 ± 11 mV (1.3 μM), respectively (Figure 3c). The Pb(Ac)2 strip test confirmed H2S excretion after the addition of cystine to growing B. subtilis (Figure 3a). The addition of cystine at the indicated concentrations did not stop the growth (Figure 1) and did not affect the rate of oxygen consumption, in line with the data reported earlier for E. coli [17]. There was also no effect of cystine on parameters such as the pH and the content of potassium ions.
3.4. Catalase Activity in B. subtilis and E. coli
In the mid-exponential growth phase, the catalase activity of B. subtilis growing under aerobic conditions on M9 medium was 5.2 times higher than in E. coli under the same conditions, amounting to 107 ± 13 and 20.7 ± 2.3 μmol H2O2/min × mg protein, respectively.
3.5. H2S Excretion in B. megaterium
Using the Pb(Ac)2 strip test, it was found that the stress-induced excretion of H2S was also detected in another Gram-positive bacteria, B. megaterium. The amount of H2S excreted by B. megaterium under conditions of glucose exhaustion, treatment with 25 μg mL−1 chloramphenicol, or the addition of 30 μM cystine was equal to 28, 95, and 360 arbitrary units, respectively (Figure 3d and Figure S4). Compared with B. subtilis, B. megaterium cells excreted twice as much H2S under the treatment with chloramphenicol and almost the same under glucose exhaustion. No growth inhibition was observed with cystine.
4. Discussion
We previously reported that, in E. coli growing on minimal M9 medium with sulfate, stress-induced growth arrest is accompanied by sulfide production. Sulfide formation was a consequence of the desulfurization of intracellular cysteine, as one of the ways to reduce its excess, resulting from the inhibition of protein synthesis [2,3].
There are significant differences in the physiology of bacteria growing on media with cysteine or sulfate as the sulfur sources. The first case can occur in the life cycle of bacteria such as E. coli, for which the intestinal tract is the main ecological niche. For soil bacteria like B. subtilis, growth on sulfate media can happen quite often.
When E. coli grows in an aerobic environment containing cystine, cells import and reduce it to cysteine, which is then consumed for protein synthesis and other needs. To prevent the accumulation of cytoplasmic cysteine above dangerous levels, an inducible cysteine/cystine shuttle system is in place. Some part of cysteine is degraded by enzymes with desulfhydrase and desulfidase activity, followed by the release of sulfide [17,18,19]. In E. coli growing on a medium with sulfate as the sole source of sulfur, sulfate is transported into the cell, reduced to sulfide, and reacts with O-acetylserine to form cysteine [7]. In the present work, all studied bacteria were grown in minimal medium M9 with sulfate and did not produce sulfide during normal growth. Here, we showed that, as in E. coli, in aerobic B. subtilis grown on sulfate minimal medium, sulfide production is induced during growth arrest in response to glucose or ammonium depletion and antibiotic exposure.
In B. subtilis, the pathways involved in the synthesis of cysteine from sulfate and the subsequent metabolism are well characterized and largely similar to those found in E. coli [20,21,22,23]. As in other bacteria, in B. subtilis, cysteine desulfurization can be catalyzed by several enzymes [20] and be one of the sources of the stress-induced sulfide production observed here. At the same time, significant differences are observed in the kinetics of production and the amount of sulfide formed between B. subtilis and E. coli. Unlike B. subtilis, stress-induced sulfide release in E. coli is a short-term process, and, accordingly, the amount of sulfide accumulated in the medium is much less.
One possible explanation for the observed effect may be related to the use of different types of low-molecular-weight (LMW) thiols by these bacteria. E. coli, as other Gram-negative bacteria, contains tripeptide glutathione as the main LMW thiol and cysteine buffer, while in most Gram-positive bacteria it is absent [24]. In aerobic E. coli, glutathione is present in the millimolar range [25] and plays a number of important roles in the cellular metabolism [26]. When E. coli grows on a minimal medium with sulfate, the stress-induced excess of cysteine is mainly incorporated into glutathione (up to 90%), 7–9% of free cysteine is exported from the cells, and the share of hydrogen sulfide does not exceed 1–3% [3].
B. subtilis, as a functional analog of glutathione, can utilize bacillithiol (BSH) (α-anomeric glycoside of L-cysteinyl-D-glucosamine with L-malic acid) [27,28]. If, in growing E. coli, the intracellular concentration of GSH is two orders of magnitude higher than that of cysteine [3,29], then, in B. subtilis, the BSH concentration is close to that of cysteine, and the BSH content is about 30 times lower than GSH in bacteria producing this tripeptide [27]. Considering these data, BSH is not a very suitable buffer for cysteine. B. megaterium, as an LMW thiol, contains 0.4 mM coenzyme A (CoA), 0.1 mM BSH, and 0.17 mM cysteine and does not synthesize GSH [27]. CoA is considered an antioxidant cofactor, though not as a protected form of cysteine [30].
The data obtained in this study indicate that, in the absence of an LMW thiol similar to glutathione, B. subtilis cells resolve the problem of cysteine homeostasis by increasing desulfhydrase activity to a higher level than in E. coli. The response of B. subtilis to chloramphenicol suggests that cysteine export may make some contribution to maintaining its intracellular homeostasis, but, as in E. coli, this pathway is probably of minor importance. Interestingly, this mode of action is manifested in response to stress in E. coli mutants deficient in glutathione synthesis. In response to amino acid starvation and chloramphenicol treatment, these mutants increase H2S production, the level of intracellular cysteine, and the excretion of cysteine [3]. It appears that, under stress conditions, Bacillus behave, at least in part, like GSH-deficient E. coli mutants. A proposed scheme explaining the differences in the mechanisms of the stress-induced excretion of low-molecular-weight thiols in Bacillus and E. coli is shown in Figure 5.
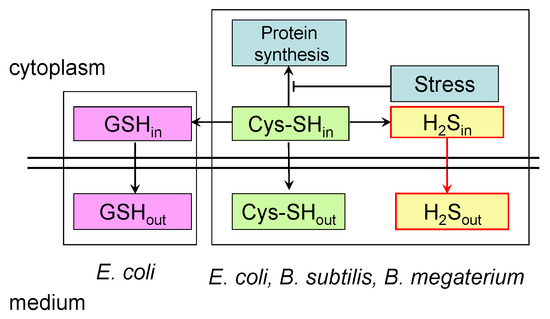
Figure 5.
Proposed scheme of stress-induced excretion of low-molecular-weight thiols by Bacillus and E. coli. The stress-induced inhibition of protein synthesis leads to an increase in intracellular cysteine pools. In B. subtilis, B. megaterium, and E. coli, to restore cysteine homeostasis, one part of it is excreted into the medium, and the other part undergoes desulfurization with the formation of hydrogen sulfide, which diffuses into the medium. In E. coli, part of the cysteine is also incorporated into glutathione. It is assumed that this is the reason for the increased production of stress-induced sulfide in Bacillus compared to E. coli. GSHin and GSHout are intra- and extracellular glutathione pools; Cys-SHin and Cys-SHout are intra- and extracellular cysteine pools; H2Sin and H2Sout are intra- and extracellular hydrogen sulfide.
In the course of this work, we identified another case in which the behavior of B. subtilis and mutants of E. coli lacking glutathione is phenotypically similar. The catalase activity of B. subtilis was found to be more than five times greater than that of wild-type E. coli under identical conditions (Section 3.4). We previously reported that total catalase activity and the expression of the katG and katE genes encoding HPI and HPII catalases were significantly higher in the GSH-deficient E. coli strain than in wild-type cells [26]. Thus, B. subtilis can compensate for the lack of glutathione in several ways.
Another factor that distinguishes B. subtilis from E. coli and may affect sulfide production is the ability of B. subtilis to sporulate. This factor can be ruled out, since, under our conditions, ammonium or glucose remained in the medium during starvation stress. It is known that the presence of these compounds in B. subtilis growing on minimal media prevents spore formation [31].
It is assumed that, in nature, hydrogen sulfide is generated mainly in the processes of dissimilatory sulfate reduction by sulfate-reducing bacteria. These microorganisms are ubiquitous in anoxic habitats and produce hydrogen sulfide during normal growth [32,33]. The ecological niches of B. subtilis and B. megaterium can be found in soil; they live primarily in an aerobic environment and do not produce sulfide during normal growth on sulfate as a sulfur source. The data obtained in this study, together with those we reported earlier for E. coli, indicate that the production of sulfide under the described conditions may be a universal reaction, induced under different types of stresses by different bacterial species, and one of the potential sources of sulfide in nature.
In the natural environment, Bacillus bacteria are constantly exposed to various stresses, including starvation and antibiotics. In these cases, conditions can be created for the production of sulfide in a wide range of concentrations. High levels of stress-induced sulfide can accumulate on the surface of bacterial biofilms growing on sulfate in oxygenated environments. The resulting sulfide may contribute to the adaptation of Bacillus to growth-inhibitory stresses and also play the role of a signal molecule in intercellular and inter-organismal communications.
B. subtilis and B. megaterium are plant-growth-promoting rhizobacteria (PGPR) that exhibit a significant interaction with plant roots and have positive effects on plant growth and the reduction in both biotic and abiotic stresses. These microorganisms enhance stress tolerance in their plant hosts by inducing the expression of stress-response genes, phytohormones, and stress-related metabolites [34]. PGPR have emerged as an alternative solution to the use of synthetic pesticides and fertilizers, which have toxic effects on human health and the environment [35,36]. In plants, the most important role of H2S is as an intermediate in sulfate assimilation. However, recently, evidence has accumulated on H2S functioning as a signaling molecule, participating in the regulation of plant stress response, particularly draught stress [37]. It can be assumed that the stress-induced production of H2S by Bacillus living in the rhizosphere can have a positive effect on plant growth, increasing plants’ resistance to adverse factors. The data obtained in this study may contribute to the understanding of the mechanisms of interaction between rhizosphere Bacillus and plants.
5. Conclusions
Using five electrochemical sensors, we identified stress-induced sulfide production in the Gram-positive bacterium Bacillus subtilis, growing on a minimal medium with sulfate, as well as changes in the physiological parameters that accompany sulfide leakage. The use of sensors made it possible for us to continuously and synchronously record even small changes in the parameters directly in the growing cultures. There were significant differences in the amount and kinetics of sulfide production between B. subtilis and E. coli. These differences are thought to have been due to the lack of the tripeptide glutathione in Bacillus. The data obtained in this study, together with those we reported earlier for E. coli, indicate that the production of sulfide may be a universal reaction, induced under different types of stresses by different bacterial species, and one of the potential sources of sulfide in nature. The stress-induced accumulation of H2S by Bacillus may play the role of a signaling molecule in intercellular and inter-organismal communications and facilitate the adaptation of bacteria and plants to environmental conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12091856/s1: Figure S1: Extracellular H2S accumulation in B. subtilis was determined using Pb(Ac)2 strips after incubation for 120 min under the tested conditions; Figure S2: Changes in intracellular (a) and extracellular (b) cysteine in B. subtilis under glucose starvation and treatment with chloramphenicol; Figure S3: The addition of glucose (10 mM) or NH4Cl (2 mM) to B. subtilis cultures deprived of glucose (a,c) or nitrogen (b,c) is accompanied by renewed oxygen consumption (a,b), a decrease in pH (a,b), and an increase in potential of the sulfide sensor by cessation of H2S production (c); and Figure S4: Extracellular H2S accumulation in B. megaterium was determined using Pb(Ac)2 after incubation for 120 min under the tested conditions.
Author Contributions
Conceptualization, G.S. and O.O.; methodology, G.S., A.T., L.S., V.U. and T.K.; software, A.T.; validation, A.T., L.S., V.U. and T.K.; formal analysis, O.O., G.S., A.T., L.S., V.U. and T.K.; investigation, A.T., L.S., V.U. and T.K.; resources, O.O.; data curation, G.S. and O.O.; writing—original draft preparation, O.O.; writing—review and editing, O.O. and G.S.; visualization, A.T. and G.S.; supervision, O.O.; project administration, G.S.; and funding acquisition, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, with grant number 22-14-00093.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oktyabrskii, O.N.; Smirnova, G.V. Redox potential changes in bacterial cultures under stress conditions. Microbiology 2012, 81, 131–142. [Google Scholar] [CrossRef]
- Tyulenev, A.; Smirnova, G.; Muzyka, N.; Ushakov, V.; Oktyabrsky, O. The role of sulfides in stress-induced changes of Eh in Escherichia coli cultures. Bioelectrochemistry 2018, 121, 11–17. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Tyulenev, A.V.; Bezmaternykh, K.V.; Muzyka, N.G.; Ushakov, V.Y.; Oktyabrsky, O.N. Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells. Amino Acids 2019, 51, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Hatanom, T.; Saito, S.Y.W.; Abe, T.; Kawano, Y.; Ohtsu, I. Generation of hydrogen sulfide from sulfur assimilation in Escherichia coli. J. Gen. Appl. Microbiol. 2019, 65, 234–239. [Google Scholar] [CrossRef]
- Sorensen, M.A.; Pedersen, S. Cysteine, even in low concentrations, induces transient amino acid starvation in Escherichia coli. J. Bacteriol. 1991, 173, 5244–5246. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Imlay, J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003, 185, 1942–1950. [Google Scholar] [CrossRef]
- Kredich, N.M. Biosynthesis of cysteine. EcoSal Plus 2008, 3. [Google Scholar] [CrossRef]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox. Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Mironov, E.; Seregina, T.; Nagornykh, M.; Luhachack, L.G.; Korolkova, N.; Lopes, L.E.; Kotova, V.; Zavilgelsky, G.; Shakulov, R.; Shatalin, K.; et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6022–6027. [Google Scholar] [CrossRef]
- Toliver-Kinsky, T.; Cui, W.; Törö, G.; Lee, S.-J.; Shatalin, K.; Nudler, E.; Szabo, C. H2S, a bacterial defense mechanism against the host immune response. Infect. Immun. 2018, 87, e00272-18. [Google Scholar] [CrossRef]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Smirnova, G.V.; Tyulenev, A.; Sutormina, L.; Kalashnikova, T.; Muzyka, N.; Ushakov, V.; Samoilova, Z.; Oktyabrsky, O. Regulation of cysteine homeostasis and its effect on Escherichia coli sensitivity to ciprofloxacin in LB medium. Int. J. Mol. Sci. 2024, 25, 4424. [Google Scholar] [CrossRef] [PubMed]
- Visick, J.E.; Clarke, S. RpoS- and OxyR-independent induction of HPI catalase of stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J. Bacteriol. 1997, 179, 4158–4163. [Google Scholar] [CrossRef]
- Ransmeier, J.C.; Stekol, J.A. Production of hydrogen sulfide from sulfur-containing compounds by various bacteria. II. Experiments with synthetic medium. Proc. Soc. Exp. Biol. Med. 1942, 51, 92–94. [Google Scholar] [CrossRef]
- Korshunov, S.; Imlay, K.R.C.; Imlay, J.A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016, 101, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, I.; Wiriyathanawudhiwong, N.; Morigasaki, S.; Nakatani, T.; Kadokura, H.; Takagi, H. The L-cysteine/L-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J. Biol. Chem. 2010, 285, 17479–17487. [Google Scholar] [CrossRef]
- Imlay, K.R.C.; Korshunov, S.; Imlay, J.A. Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J. Bacteriol. 2015, 19, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Auger, S.; Danchin, A.; Martin-Verstraete, I. Global expression profile of Bacillus subtilis grown in the presence of sulfate or methionine. J. Bacteriol. 2000, 184, 5179–5186. [Google Scholar] [CrossRef]
- Burguière, P.; Auger, S.; Hullo, M.F.; Danchin, A.; Martin-Verstraete, I. Three different systems participate in L-cystine uptake in Bacillus subtilis. J. Bacteriol. 2004, 186, 4875–4884. [Google Scholar] [CrossRef]
- Even, S.; Burguière, P.; Auger, S.; Soutourina, O.; Danchin, A.; Martin-Verstraete, I. Global control of cysteine metabolism by CymR in Bacillus subtilis. J. Bacteriol. 2006, 188, 2184–2197. [Google Scholar] [CrossRef] [PubMed]
- Tanous, C.; Soutourina, O.; Raynal, B.; Hullo, M.-F.; Mervelet, P.; Gilles, A.M.; Noirot, P.; Danchin, A.; England, P.; Martin-Verstraete, I. The CymR regulator in complex with the enzyme CysK controls cysteine metabolism in Bacillus subtilis. J. Biol. Chem. 2008, 283, 35551–35560. [Google Scholar] [CrossRef] [PubMed]
- Fahey, R.C.; Brown, W.C.; Adams, W.B.; Worsham, M.B. Occurrence of glutathione in bacteria. J. Bacteriol. 1978, 133, 1126–1129. [Google Scholar] [CrossRef]
- Loewen, P.C. Levels of glutathione in Escherichia coli. Can. J. Biochem. 1979, 57, 107–111. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Oktyabrsky, O.N. Glutathione in bacteria. Biochemistry 2005, 70, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.L.; Rawat, M.; La Clair, J.J.; Jothivasan, V.K.; Budiarto, T.; Hamilton, C.J.; Claiborne, A.; Helmann, J.D.; Fahey, R.C. Bacillithiol is an antioxidant thiol produced in Bacilli. Nat. Chem. Biol. 2009, 5, 625–627. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Antelmann, H.; Loi, V.V.; Helmann, J.D. The role of bacillithiol in gram-positive firmicute. Antioxid. Redox Signal. 2018, 28, 445–462. [Google Scholar] [CrossRef]
- Newton, L.; Arnold, K.; Price, M.S.; Sherrill, C.; Delcardayre, S.B.; Aharonovitz, Y.; Cohen, G.; Davies, J.; Fahey, R.C.; Davis, C. Distribution of thiols in microorganisms: Mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 1996, 178, 1990–1995. [Google Scholar] [CrossRef]
- Fahey, R.C. Glutathione analogs in prokaryotes. Biochim. Biophys. Acta 2013, 1830, 3182–3198. [Google Scholar] [CrossRef]
- Schaeffer, P.; Millet, J.; Aubert, J.P. Catabolic repression of bacterial sporulation. Proc. Nat. Acad. Sci. USA 1965, 54, 704–711. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Fauque, G.D. Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 2009, 68, 41–98. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodríguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus megaterium Rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Allah, E.F.A. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Hyder, S.; Rizvi, Z.F.; Santos-Villalobos, S.; Santoyo, G.; Gondal, A.S.; Khalid, N.; Fatima, S.N.; Nadeem, M.; Rafique, K.; Rani, A. Applications of plant growth-promoting rhizobacteria for increasing crop production and resilience. J. Plant Nutr. 2023, 46, 2551–2580. [Google Scholar] [CrossRef]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide 2014, 41, 72–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).