Temporal–Spatial Fluctuations of a Phytoplankton Community and Their Association with Environmental Variables Based on Classification and Regression Tree in a Shallow Temperate Mountain River
Abstract
1. Introduction
2. Materials and Methods
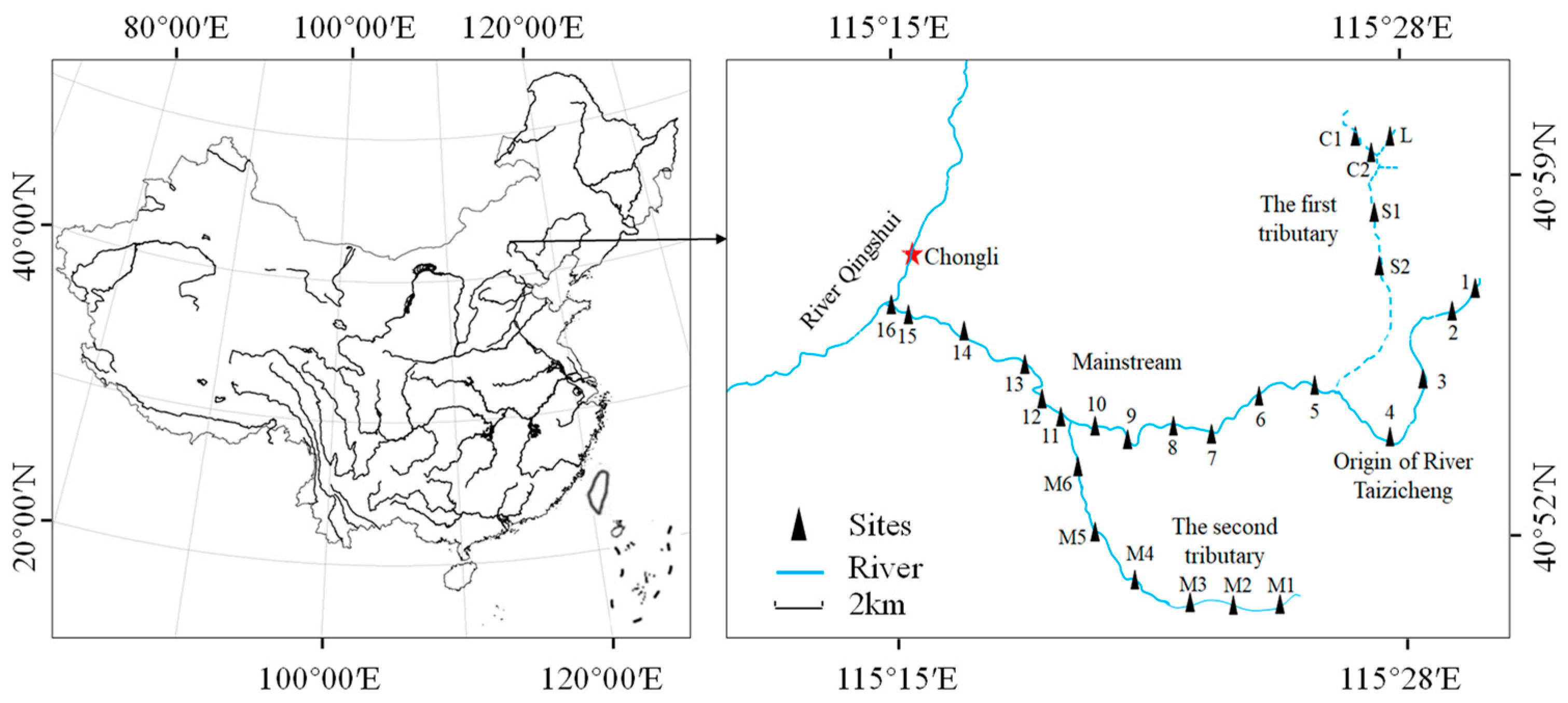
2.1. Study Area
2.2. Sampling and Measurements
2.3. Statistical Analysis
3. Results
3.1. Main Environmental Variables in the River
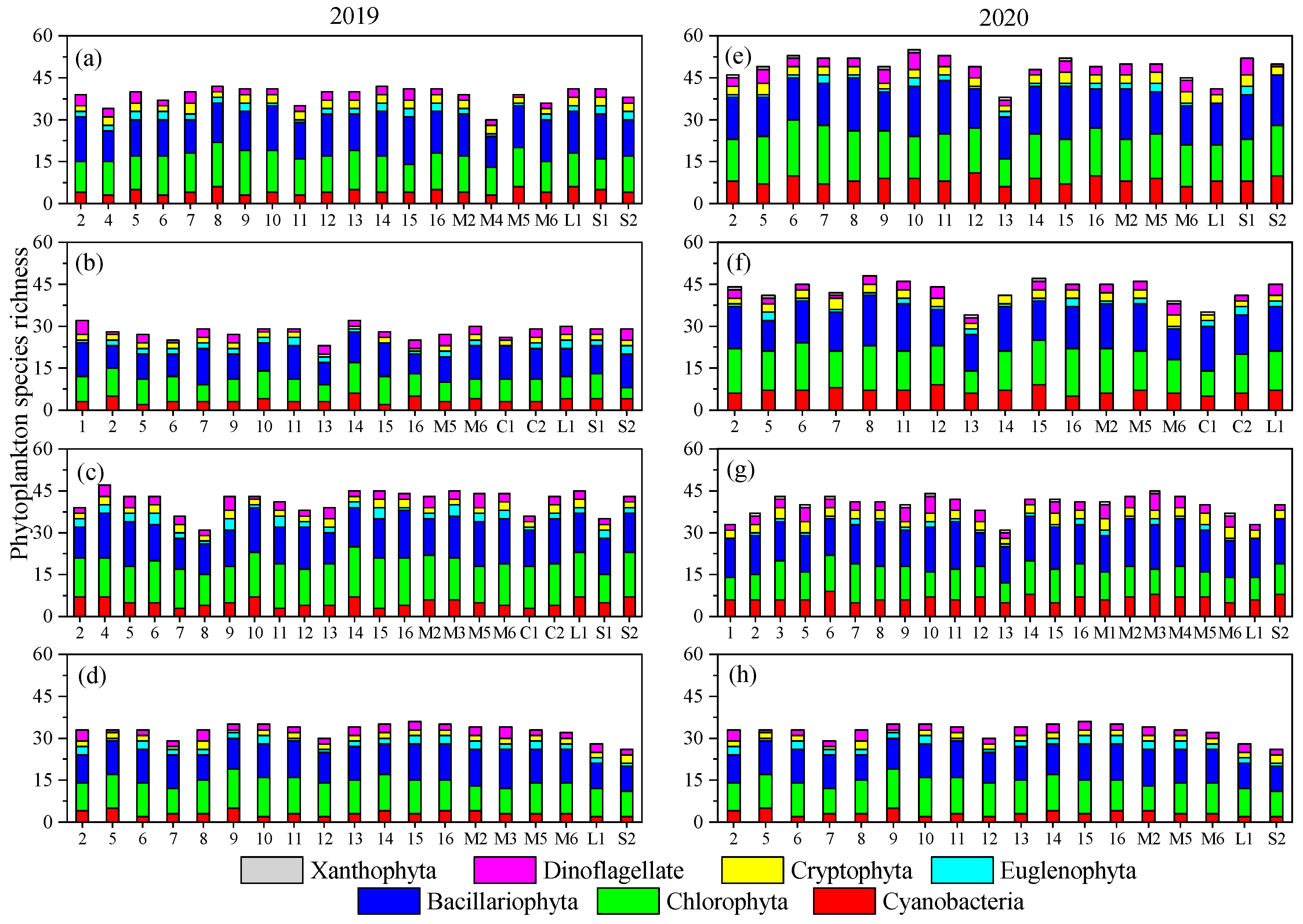
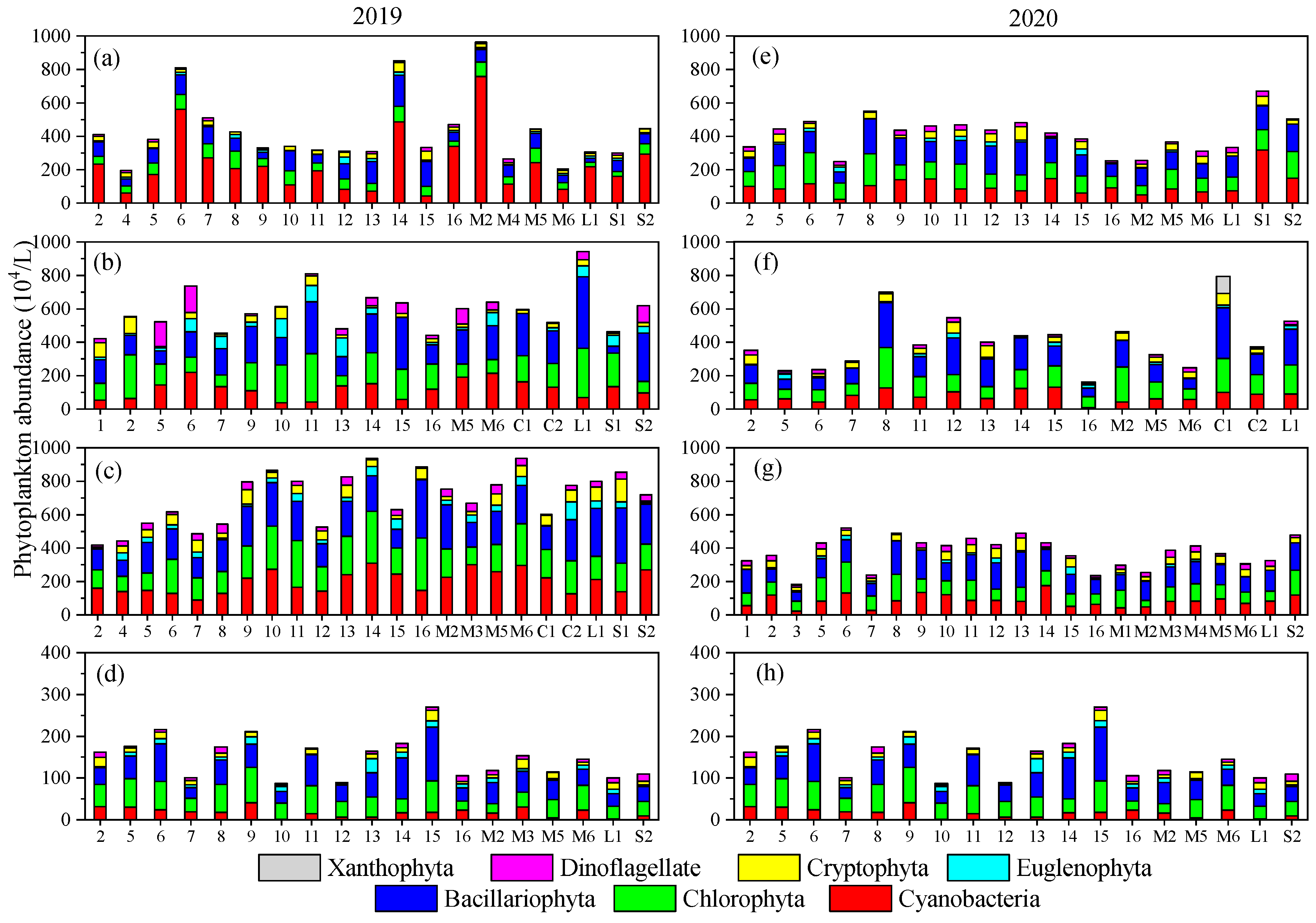
3.2. Temporal–Spatial Variations of Phytoplankton Species Diversity and Abundance
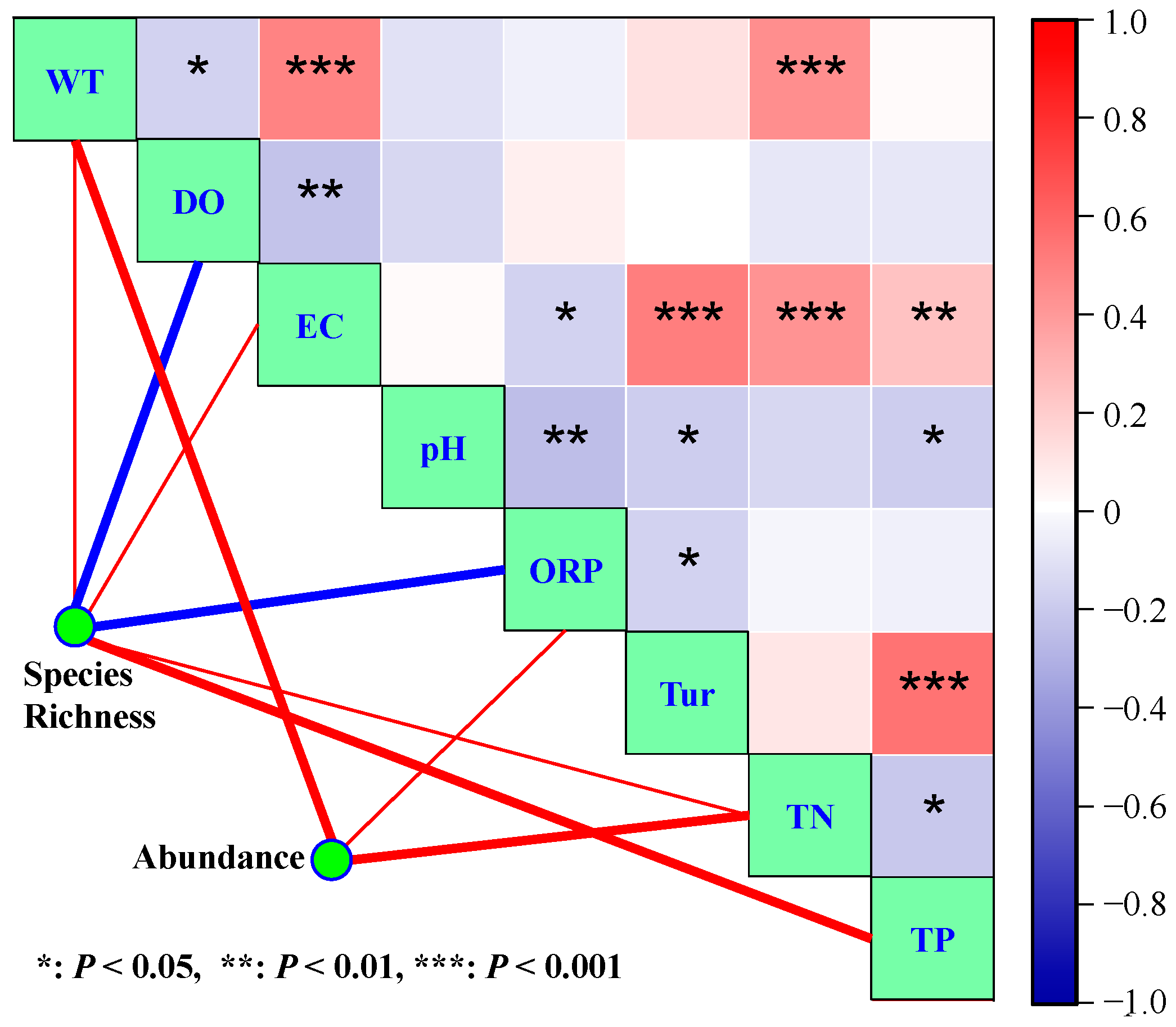
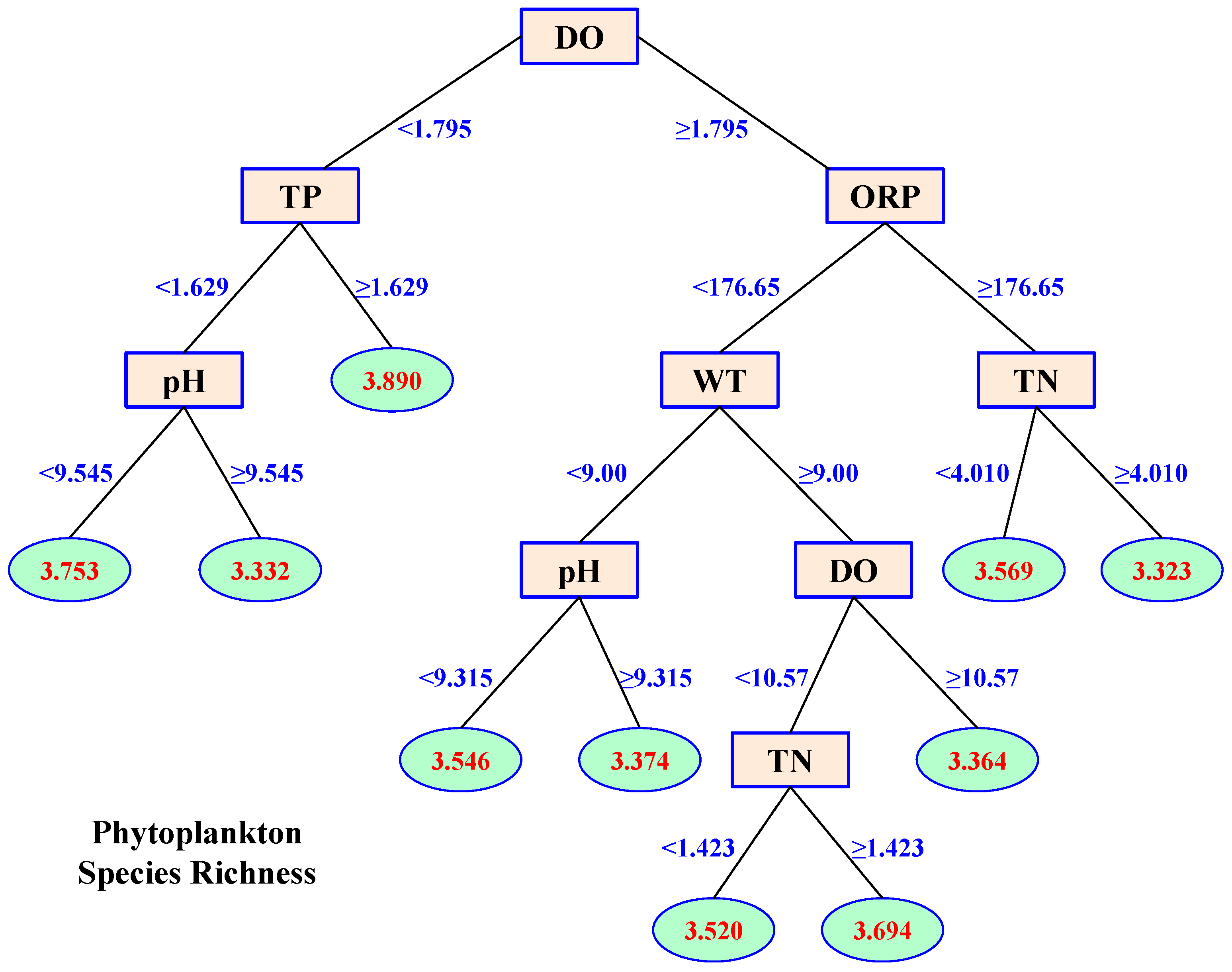
3.3. Effects of Environmental Variables on Phytoplankton Species Diversity
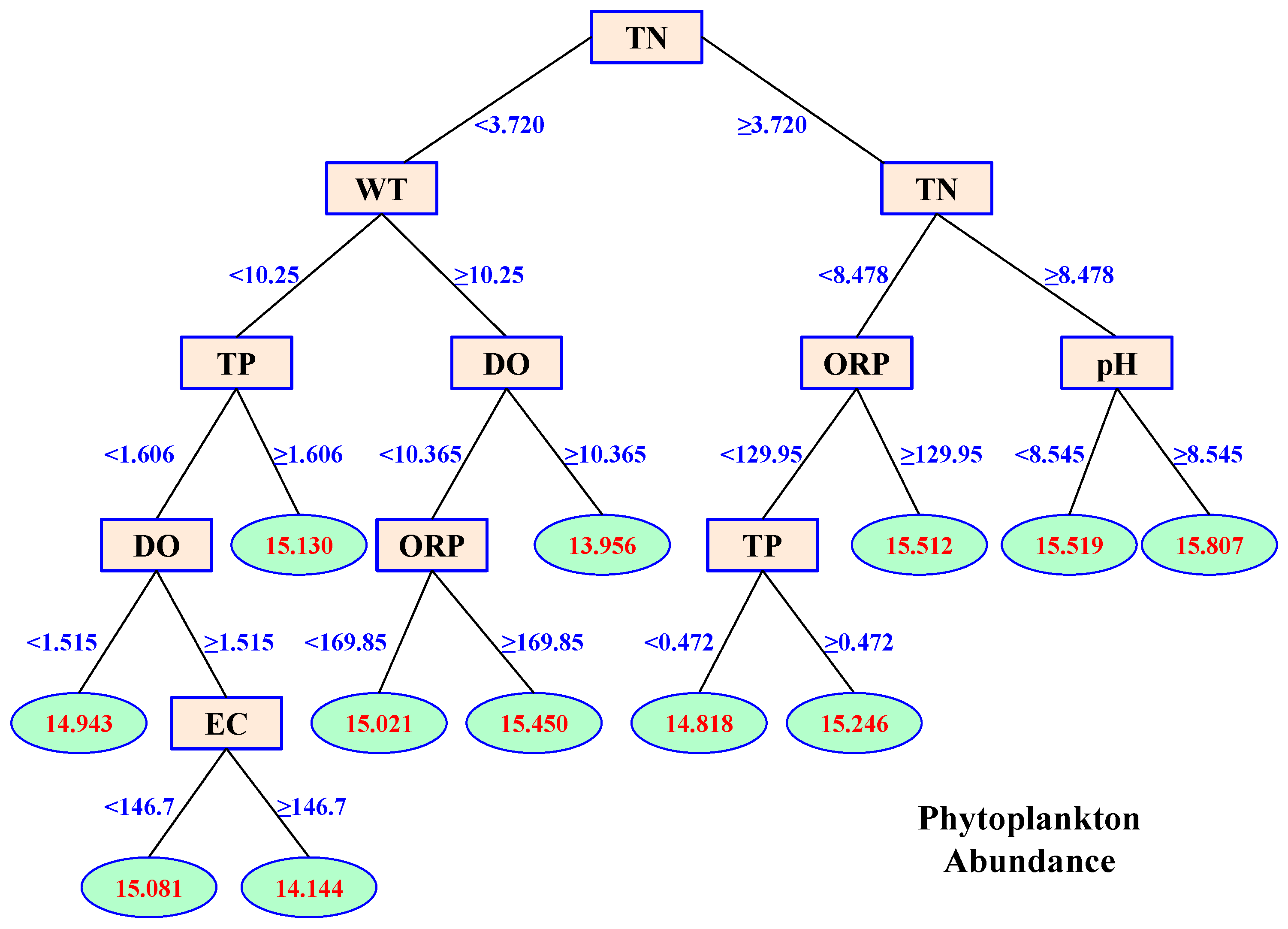
3.4. Effects of Environmental Variables on Phytoplankton Abundance
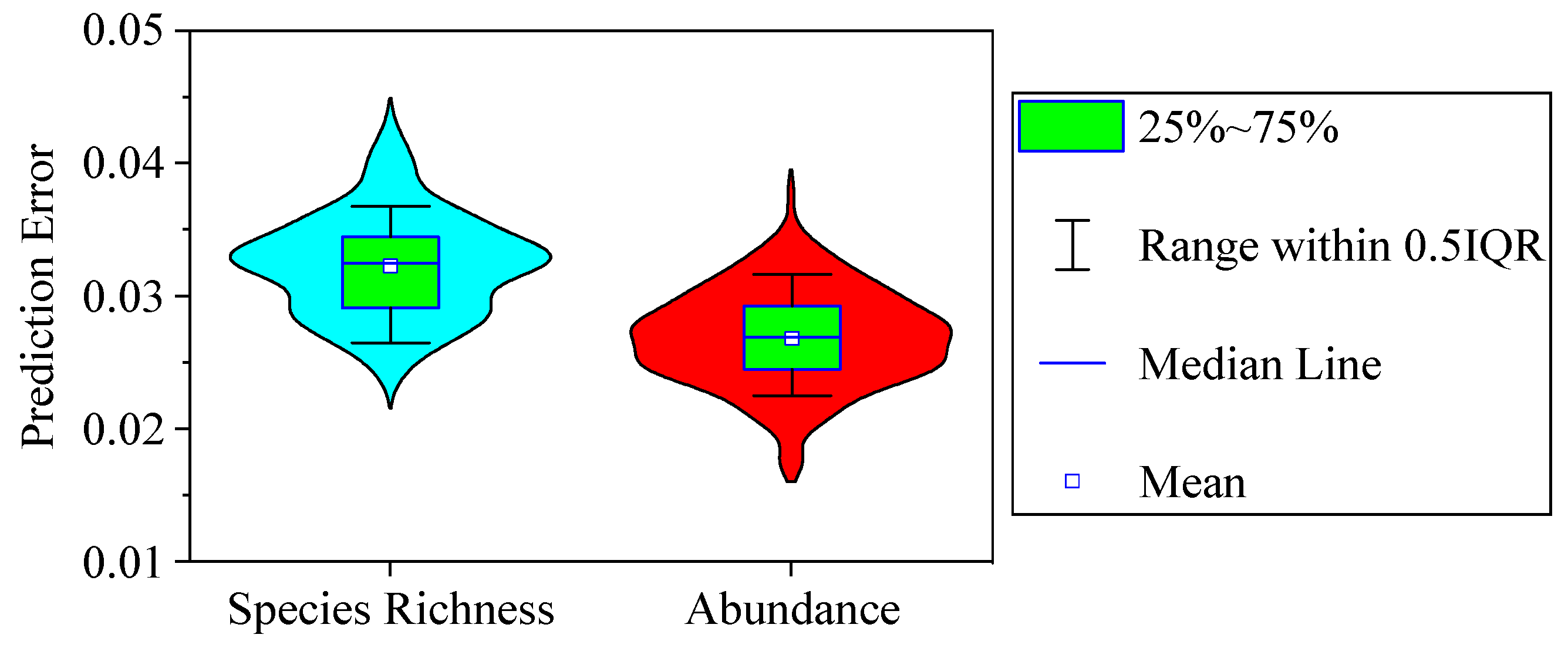
3.5. Model Validation
4. Discussion
5. Conclusions
- (1)
- Both phytoplankton species diversity and abundance varied very substantially in this mountain river due to the fluctuation of the environment. Bacillariophyta, Cyanobacteria, and Chlorophyta were the dominant phyla, and Microcystis aeruginosa was the dominant species. Phytoplankton species diversity was higher while abundance was lower in 2020 than in 2019.
- (2)
- CART analysis indicated that DO, ORP, TN, TP, and WT were the main variables that influenced phytoplankton species diversity, and they explained 36.00%, 13.81%, 11.35%, 9.96%, and 8.80%, respectively, of phytoplankton diversity variance.
- (3)
- Phytoplankton abundance was mainly affected by TN, WT, and TP. Their proportional contributions to the overall explained variance in phytoplankton abundance were 39.40%, 15.70%, and 14.09%, respectively.
- (4)
- The average errors between the empirical and predicted values were 3.23% ± 0.38% (mean ± standard error) for phytoplankton species diversity and 2.69% ± 0.35% for abundance. The phytoplankton community could be predicted precisely by CART analysis.
- (5)
- Most environmental factors had a complex influence on phytoplankton diversity and abundance. Their effects were positive under some conditions but negative under other combinations of concentrations. Therefore, more machine learning methods should be used to explore their complex relationships.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Van Dijken, G.L.; Arrigo, K.R. Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science 2020, 369, 198–202. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Peter, H.; Willen, E. Shifts in phytoplankton species richness and biomass along a latitudinal gradient-consequences for relationships between biodiversity and ecosystem functioning. Freshw. Biol. 2013, 58, 612–623. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhao, L.; Tian, W.; Huang, H. Stability of food webs to biodiversity loss: Comparing the roles of biomass and node degree. Ecol. Indic. 2016, 67, 723–729. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Zimmerman, E.K.; Cardinale, B.J. Is the relationship between algal diversity and biomass in North American lakes consistent with biodiversity experiments? Oikos 2014, 123, 267–278. [Google Scholar] [CrossRef]
- Gerhard, M.; Koussoroplis, A.M.; Hillebrand, H.; Striebel, M. Phytoplankton community responses to temperature fluctuations under different nutrient concentrations and stoichiometry. Ecology 2019, 100, e02834. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.I.; Franzè, G.; Kling, J.D.; Wilburn, P.; Kremer, C.T.; Menden-Deuer, S.; Litchman, E.; Hutchins, D.A.; Rynearson, T.A. The interactive effects of temperature and nutrients on a spring phytoplankton community. Limnol. Oceanogr. 2022, 67, 634–645. [Google Scholar] [CrossRef]
- Lauster, G.H.; Hanson, P.C.; Kratz, T.K. Gross primary production and respiration differences among littoral and pelagic habitats in northern Wisconsin lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 1130–1141. [Google Scholar] [CrossRef]
- Staehr, P.A.; Kaj, S.J. Temporal dynamics and regulation of lake metabolism. Limnol. Oceanogr. 2007, 52, 108–120. [Google Scholar] [CrossRef]
- Engel, A. Direct relationship between CO2 uptake and transparent exopolymer particles production in natural phytoplankton. J. Plankton Res. 2002, 24, 49–53. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Yan, T.; Song, J.; Yu, R.; Zhou, M. Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom. J. Oceanol. Limnol. 2021, 39, 500–507. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol-Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Hansen, P.J. Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession. Aquat. Microb. Ecol. 2002, 28, 279–288. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Paradox of enrichment: Destabilization of exploitation ecosystems in ecological time. Science 1971, 171, 385–387. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Boil. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Simões, N.R.; BovoScomparin, V.M.; Jati, S.; Santana, N.F.; Roberto, M.C.; Train, S. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecol. Indic. 2015, 48, 334–341. [Google Scholar] [CrossRef]
- Sañudo-Wilhelmy, S.A.; Tovar-Sanchez, A.; Fu, F.X.; Capone, D.G.; Carpenter, E.J.; Hutchins, D.A. The impact of surface-adsorbed phosphorus on phytoplankton Redfield stoichiometry. Nature 2004, 432, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.M.; Arrigo, K.R. Magnitude of oceanic nitrogen fixation influenced by the nutrient uptake ratio of phytoplankton. Nat. Geosci. 2010, 3, 412–416. [Google Scholar] [CrossRef]
- Aubry, F.B.; Berton, A.; Bastianini, M.; Socal, G.; Acri, F. Phytoplankton succession in a coastal area of the NW Adriatic, over a 10-year sampling period (1990–1999). Cont. Shelf Res. 2004, 24, 97–115. [Google Scholar] [CrossRef]
- Tedesco, L.; Socal, G.; Bianchi, F.; Acri, F.; Veneri, D.; Vichi, M. NW Adriatic Sea biogeochemical variability in the last 20 years (1986–2005). Biogeosciences 2007, 4, 673–687. [Google Scholar] [CrossRef]
- Huang, J.; Gao, J.; Xu, Y.; Liu, J. Towards better environmental software for spatio-temporal ecological models: Lessons from developing an intelligent system supporting phytoplankton prediction in lakes. Ecol. Inform. 2015, 25, 49–56. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Overview of the model types available for development of ecological models. Ecol. Model. 2008, 215, 3–9. [Google Scholar] [CrossRef]
- Huang, J.; Gao, J.; Hörmann, G. Hydrodynamic-phytoplankton model for short-term forecasts of phytoplankton in Lake Taihu, China. Limnologica 2012, 42, 7–18. [Google Scholar] [CrossRef]
- Pathak, D.; Hutchins, M.; Brown, L.; Loewenthal, M.; Scarlett, P.; Armstrong, L.; Nicholls, D.; Bowes, M.; Edwards, F. Hourly prediction of phytoplankton biomass and its environmental controls in lowland rivers. Water Resour. Res. 2021, 57, e2020WR028773. [Google Scholar] [CrossRef]
- Bourel, M.; Crisci, C.; Martínez, A. Consensus methods based on machine learning techniques for marine phytoplankton presence–absence prediction. Ecol. Inform. 2017, 42, 46–54. [Google Scholar] [CrossRef]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. River phytoplankton prediction model by Artificial Neural Network: Model performance and selection of input variables to predict time-series phytoplankton proliferations in a regulated river system. Ecol. Inform. 2006, 1, 235–245. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Cao, M.; Sun, X.; Du, Z.; Liu, R.; Ye, X. Deep-learning-based approach for prediction of algal blooms. Sustainability 2016, 8, 1060. [Google Scholar] [CrossRef]
- Volf, G.; Atanasova, N.; Kompare, B.; Precali, R.; Ožanić, N. Descriptive and prediction models of phytoplankton in the northern Adriatic. Ecol. Model. 2011, 222, 2502–2511. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman & Hall (Wadsworth, Inc.): New York, NY, USA, 1984. [Google Scholar]
- De’Ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Zhang, Z.S.; Huang, X.F. Research Methods of Freshwater Plankton; Science Press: Beijing, China, 1991. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematic, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Kaufman, M.H.; Cardenas, M.B.; Buttles, J.; Kessler, A.J.; Cook, P.L. Hyporheic hot moments: Dissolved oxygen dynamics in the hyporheic zone in response to surface flow perturbations. Water Resour. Res. 2017, 53, 6642–6662. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xu, L.; Xie, S.; Feng, J. Interactive effects of temperature and nutrients on the phytoplankton community in an urban river in China. Environ. Monit. Assess. 2019, 191, 688. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.P.; Chrzanowski, T.H. Seasonal dynamics of phytoplankton in two warm temperate reservoirs: Association of taxonomic composition with temperature. J. Plankton Res. 2006, 28, 1–17. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Chen, C.; Wu, H.; Lin, L.; Li, M. Seasonal succession of phytoplankton in two temperate artificial lakes with different water sources. Environ. Sci. Pollut. R. 2020, 27, 42324–42334. [Google Scholar] [CrossRef]
- Bourel, M.; Segura, A.M. Multiclass classification methods in ecology. Ecol. Indic. 2018, 85, 1012–1021. [Google Scholar] [CrossRef]
- Crisci, C.; Terra, R.; Pacheco, J.P.; Ghattas, B.; Bidegain, M.; Goyenola, G.; Lagomarsino, J.J.; Méndez, G.; Mazzeo, N. Multi-model approach to predict phytoplankton biomass and composition dynamics in a eutrophic shallow lake governed by extreme meteorological events. Ecol. Model. 2017, 360, 80–93. [Google Scholar] [CrossRef]

| Variables | WT | DO (mg/L) | EC (μS/cm) | pH | ORP | Tur (NTU) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|---|---|---|
| April 2019 | 8.82 ± 4.15 (0.2, 13.5) | 9.97 ± 1.05 (8.17, 12.92) | 228.9 ± 57.9 (102.1, 326.6) | 8.40 ± 0.21 (8.20, 8.88) | 150.6 ± 32.0 (78.5, 183.2) | 310.9 ± 215.0 (3.54, 602.9) | 2.04 ± 1.92 (0.01, 5.20) | 2.83 ± 2.31 (0.08, 9.70) |
| June 2019 | 15.34 ± 2.91 (10.5, 19.9) | 10.42 ± 2.29 (6.62, 14.80) | 265.9 ± 75.1 (166.9, 380.3) | 8.38 ± 0.23 (7.95, 8.76) | 187.96 ± 21.3 (135.7, 214.3) | 102.4 ± 93.8 (1.08, 304.3) | 7.65 ± 2.56 (0.62, 10.59) | 0.35 ± 0.31 (0.02, 2.14) |
| August 2019 | 11.62 ± 2.23 (6.6, 14.7) | 5.46 ± 2.24 (2.71, 9.81) | 288.4 ± 74.8 (154.2, 371.6) | 8.67 ± 0.54 (7.90, 10.81) | 111.9 ± 28.4 (63.6, 173.5) | 292.9 ± 251.8 (2.24, 600.0) | 10.0 ± 1.12 (6.26, 11.38) | 0.14 ± 0.13 (0.01, 0.47) |
| October 2019 | 5.19 ± 2.28 (0.6, 9.9) | 3.62 ± 1.78 (0.02, 7.38) | 255.2 ± 61.0 (142.8, 329.9) | 8.63 ± 0.39 (8.27, 9.65) | 139.2 ± 23.18 (67.9, 181.6) | 352.8 ± 258.0 (7.93, 600.0) | 2.18 ± 0.63 (1.03, 3.19) | 0.57 ± 0.51 (0.07, 1.56) |
| April 2020 | 11.37 ± 3.63 (5.1, 17.4) | 0.38 ± 0.30 (0.01, 1.65) | 313.4 ± 131.4 (152.0, 782.0) | 8.44 ± 2.07 (0.49, 11.56) | 115.5 ± 35.1 (24.4, 182.9) | 181.0 ± 156.6 (3.41, 452.9) | 4.45 ± 1.54 (0.30, 6.41) | 3.04 ± 0.30 (2.77, 3.70) |
| June 2020 | 14.02 ± 3.53 (7.0, 20.3) | 0.61 ± 0.49 (0.01, 1.31) | 320.5 ± 94.9 (147.9, 433.8) | 8.41 ± 1.24 (3.69, 9.44) | 117.2 ± 31.2 (71.7, 199.3) | 88.7 ± 113.7 (1.56, 427.8) | 4.28 ± 2.13 (0.01, 8.25) | 0.19 ± 0.12 (0.06, 0.49) |
| August 2020 | 15.40 ± 2.66 (10.4, 19.8) | 7.99 ± 0.44 (7.03, 8.97) | 292.0 ± 89.7 (137.1, 417.4) | 8.59 ± 0.31 (8.11, 9.46) | 47.65 ± 18.58 (4.7, 72.9) | 370.4 ± 266.2 (8.17, 629.0) | 3.96 ± 1.77 (0.98, 7.62) | 0.92 ± 0.65 (0.11, 1.97) |
| October 2020 | 6.71 ± 2.99 (0.0, 11.1) | 10.94 ± 3.00 (8.80, 22.71) | 282.8 ± 67.1 (151.9, 346.0) | 9.03 ± 0.51 (8.35, 10.03) | 82.99 ± 33.64 (7.3, 141.8) | 141.5 ± 169.1 (0.90, 600.0) | 3.22 ± 1.60 (0.54, 5.50) | 0.40 ± 0.35 (0.01, 2.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Wang, Z.; Kong, H.; Tian, Y.; Huang, T. Temporal–Spatial Fluctuations of a Phytoplankton Community and Their Association with Environmental Variables Based on Classification and Regression Tree in a Shallow Temperate Mountain River. Microorganisms 2024, 12, 1612. https://doi.org/10.3390/microorganisms12081612
Tian W, Wang Z, Kong H, Tian Y, Huang T. Temporal–Spatial Fluctuations of a Phytoplankton Community and Their Association with Environmental Variables Based on Classification and Regression Tree in a Shallow Temperate Mountain River. Microorganisms. 2024; 12(8):1612. https://doi.org/10.3390/microorganisms12081612
Chicago/Turabian StyleTian, Wang, Zhongyu Wang, Haifei Kong, Yonglan Tian, and Tousheng Huang. 2024. "Temporal–Spatial Fluctuations of a Phytoplankton Community and Their Association with Environmental Variables Based on Classification and Regression Tree in a Shallow Temperate Mountain River" Microorganisms 12, no. 8: 1612. https://doi.org/10.3390/microorganisms12081612
APA StyleTian, W., Wang, Z., Kong, H., Tian, Y., & Huang, T. (2024). Temporal–Spatial Fluctuations of a Phytoplankton Community and Their Association with Environmental Variables Based on Classification and Regression Tree in a Shallow Temperate Mountain River. Microorganisms, 12(8), 1612. https://doi.org/10.3390/microorganisms12081612





