Bacterial Lipopeptides Are Effective against Pear Fire Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Isolation of the Bacterium That Causes Fire Blight (E. amylovora C1)
Preparation of a Fire Blight Pathogenic Bacterial Suspension
2.2. Isolation and Identification of Leaves Endophytic Antagonist Bacteria
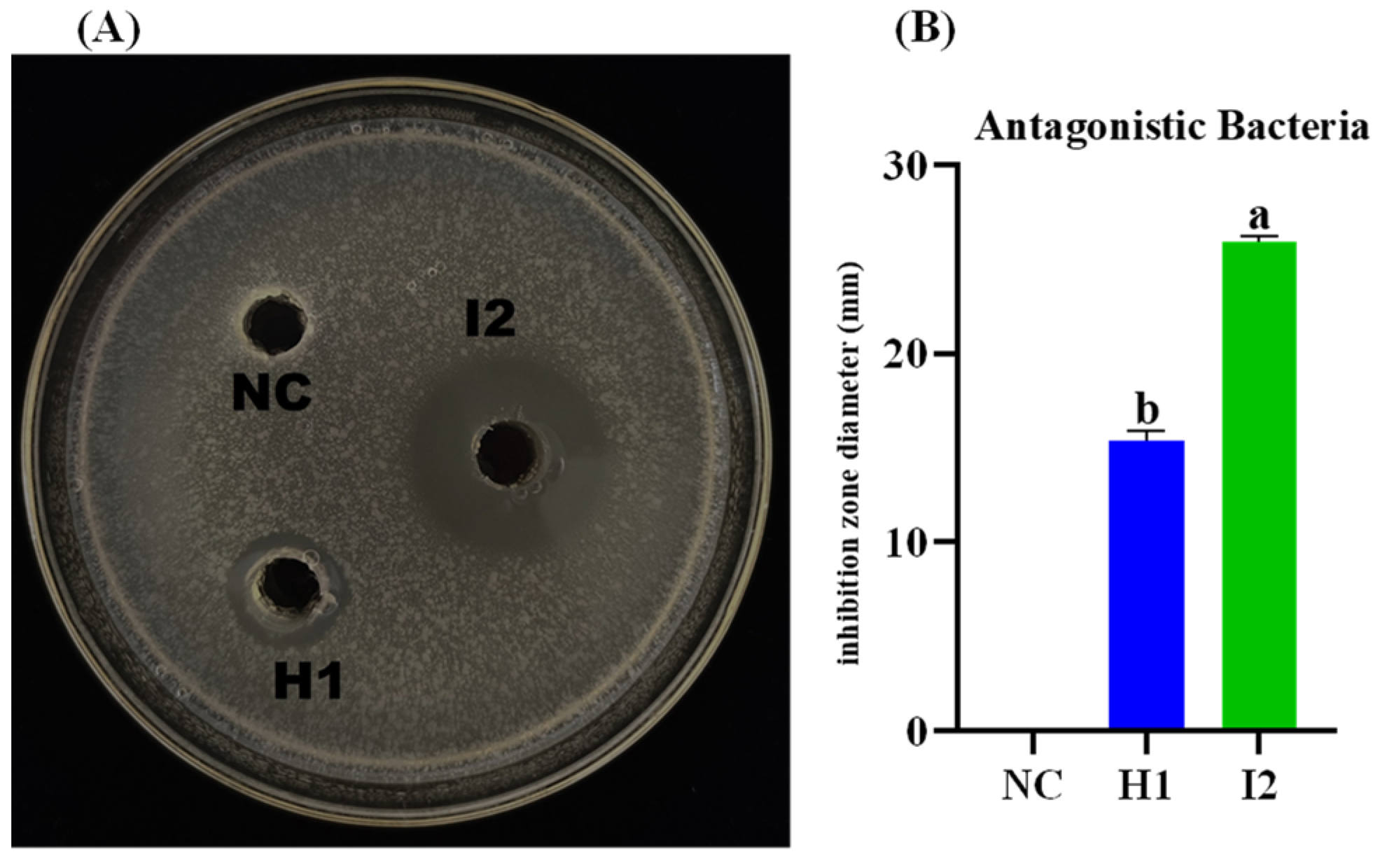
2.3. Antagonist and Pathogenic bacteria (E. amylovora) Screening
2.4. Physiological and Biochemical Characteristics of Antagonistic Bacteria
2.5. Identification of Antagonist Bacterial Strains via Phylogenetic Examination of Their 16S rDNA Sequences
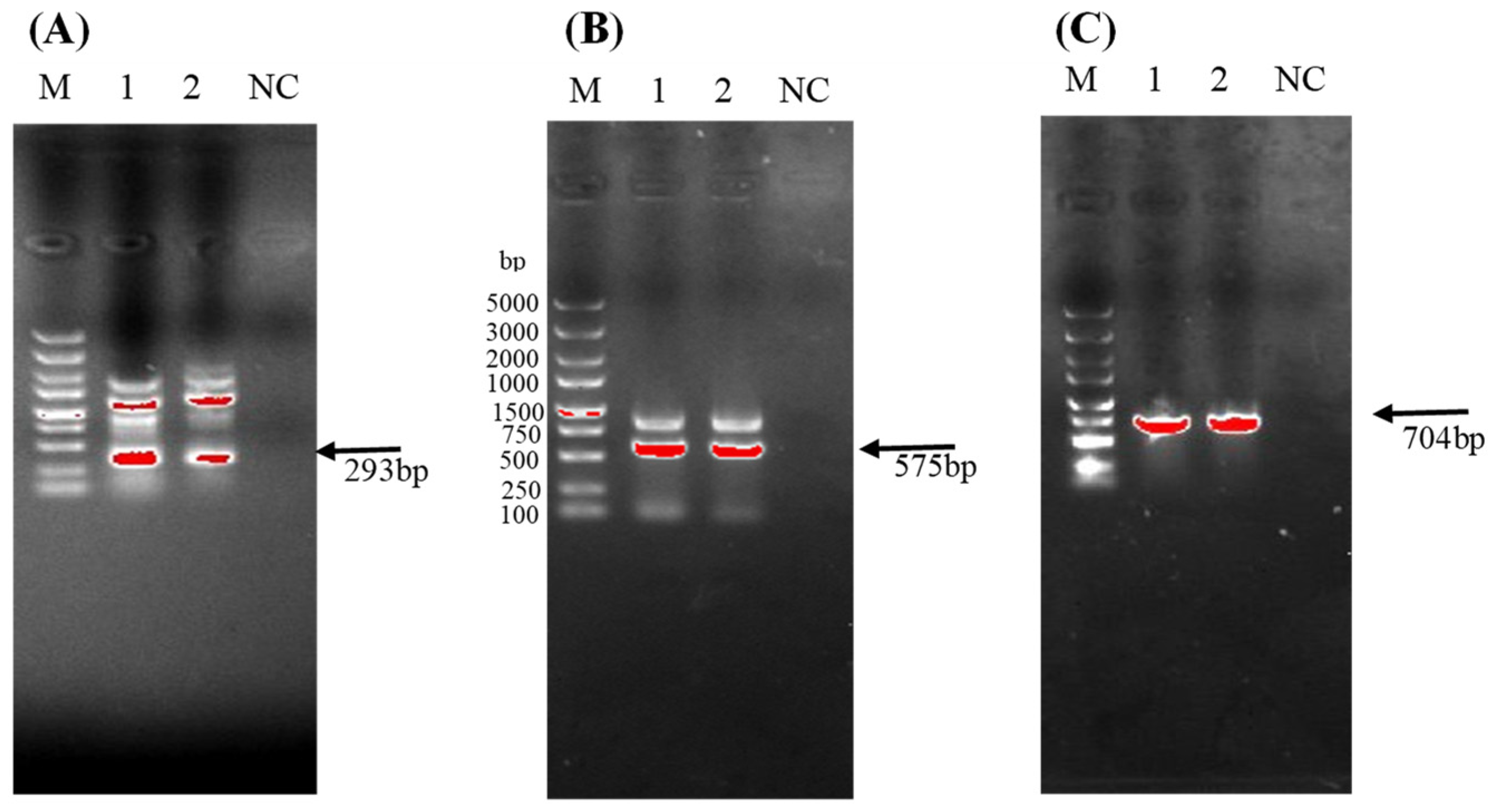
2.6. Identifying the Specific Genes Responsible for Producing Known Antimicrobial Substances
2.7. Antibacterial Activity Observation
2.8. Assessing the Antibacterial Efficacy on Korla Fragrant Pear Leaves and Fruits
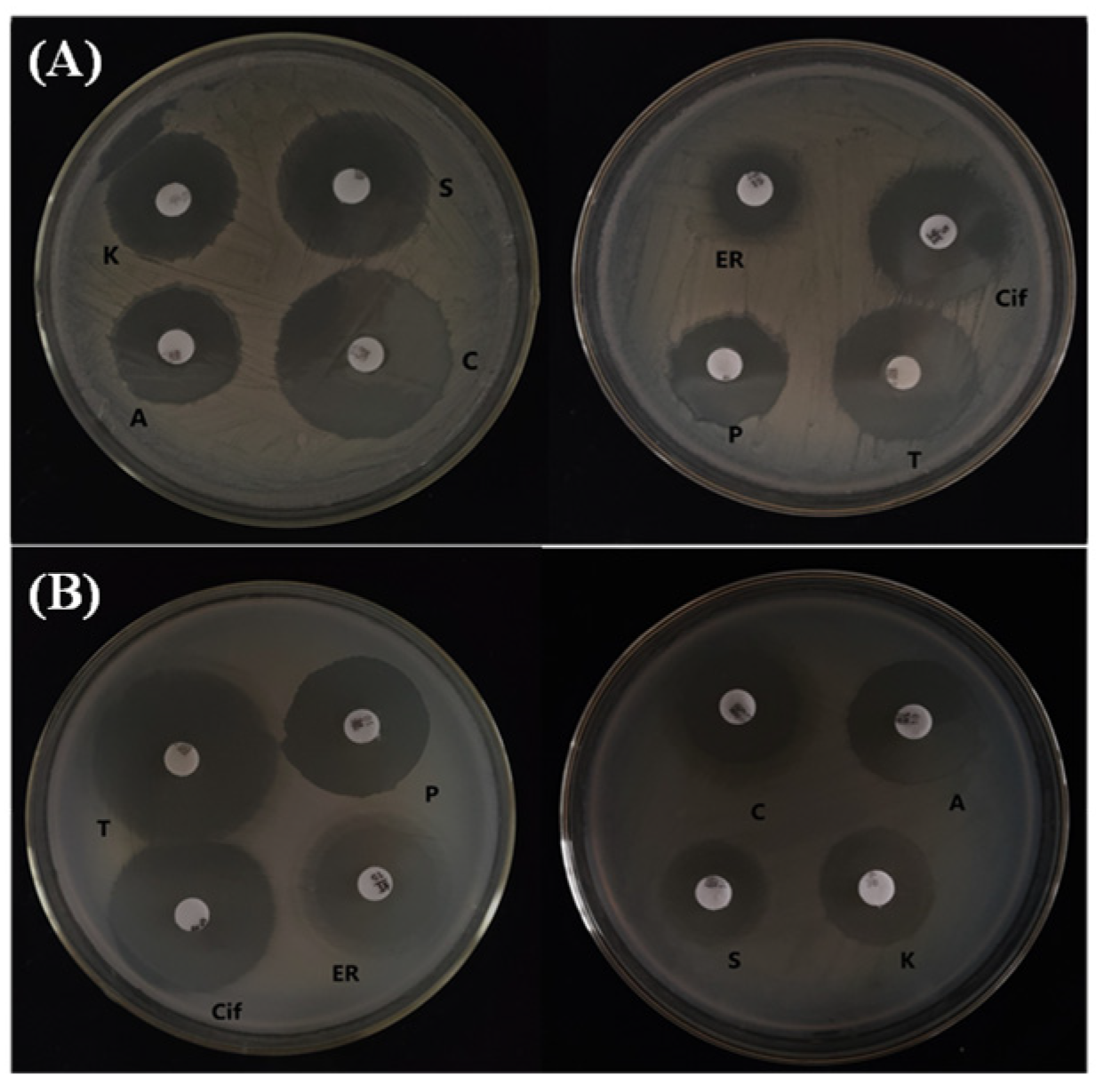
2.9. Antibiotic Sensitivity Test
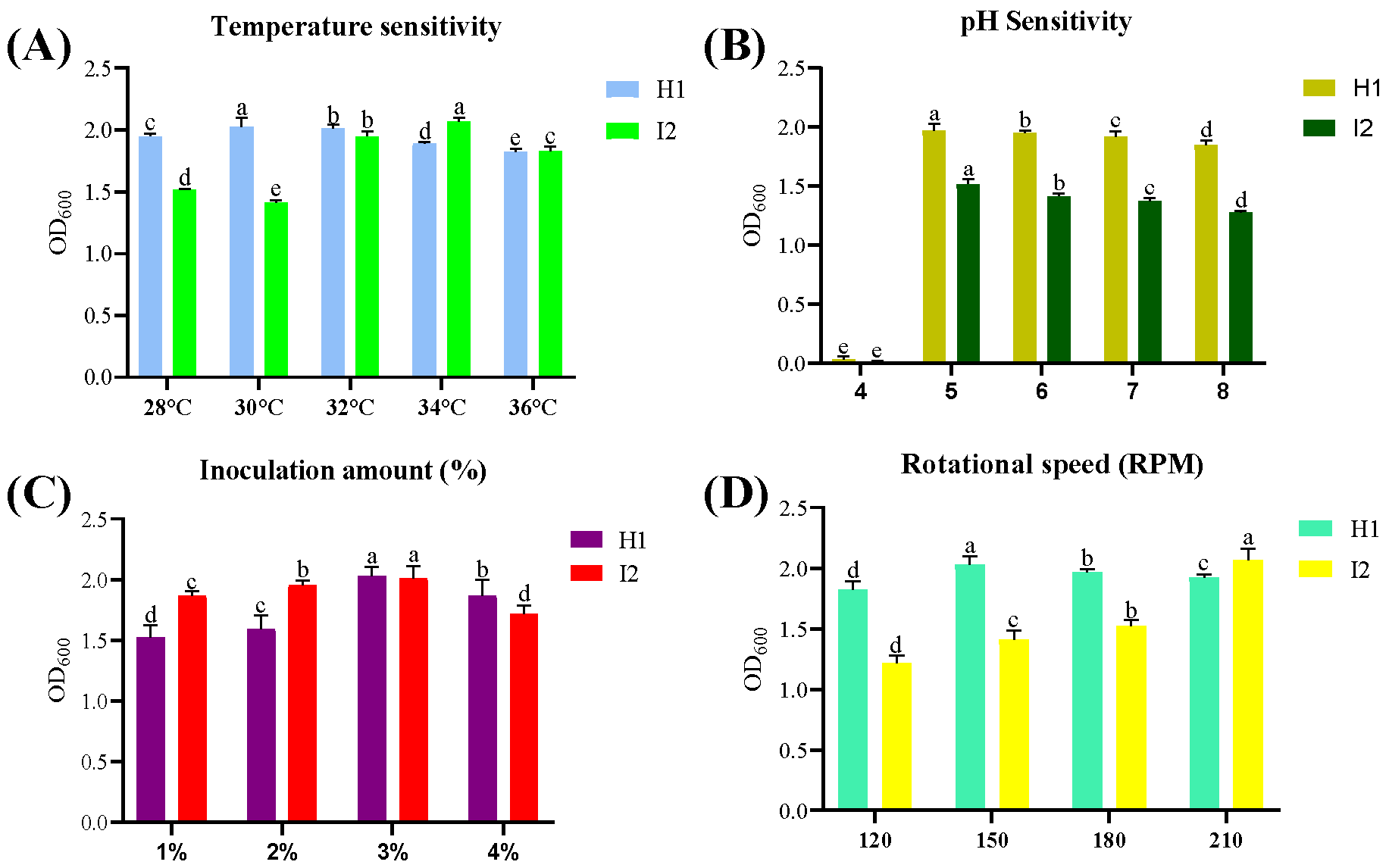
2.10. Growth Optimization of Isolates
2.11. Thin-Layer Chromatography (TLC)
3. Results
3.1. Identification of Bacterial Isolates
3.1.1. DNA-Based Identification
3.1.2. Characterization of Antagonistic Bacterial Species: Morphological, Physiological, and Biochemical Traits
3.2. Antibiotic Sensitivity Test
3.3. Factors Affecting Antagonistic Bacterial Growth Sensitivity Analysis
3.4. Growth Curve of Antagonist Bacteria
3.5. In Vitro Antagonistic Activity of B. subtilis I2 and P. megaterium H1 against E. amylovora
3.6. Identifying the Specific Genes Responsible for Producing Known Antimicrobial Substances
3.7. Antibacterial Filtrate Activity
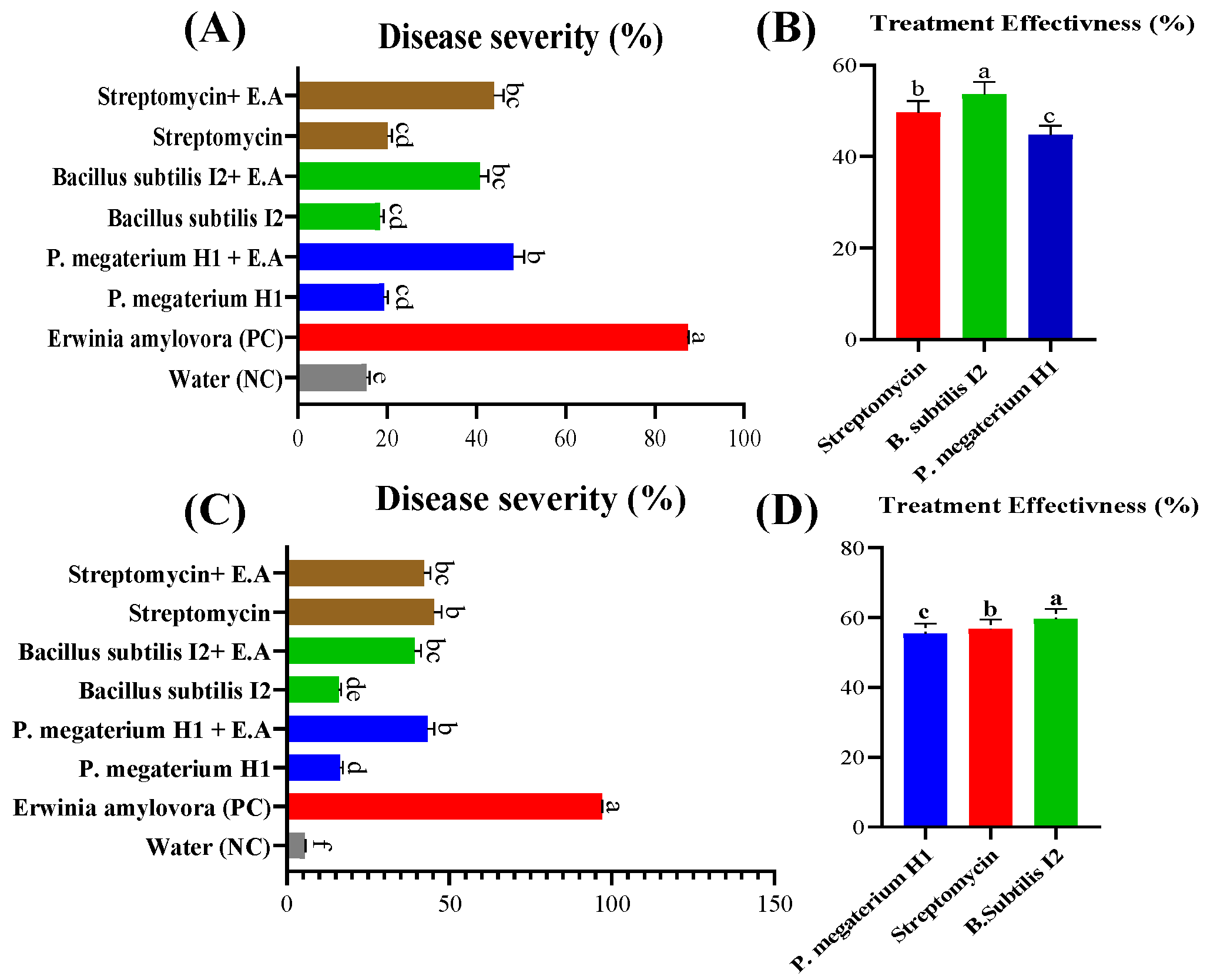
3.8. Efficacy of Bacterial Antagonists on Detached Pear Fruits and Leaves
3.9. Analyzing Lipopeptides with TLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreau, M.; Degrave, A.; Vedel, R.; Bitton, F.; Patrit, O.; Renou, J.-P.; Barny, M.-A.; Fagard, M. EDS1 contributes to nonhost resistance of Arabidopsis thaliana against Erwinia amylovora. Mol. Plant-Microbe Interact. 2012, 25, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Oberhuber, C.; Loncaric, I.; Heissenberger, B.; Keck, M.; Scheiner, O.; Hoffmann-Sommergruber, K. Fireblight (Erwinia amylovora) affects Mal d 1-related allergenicity in apple. Eur. J. Plant Pathol. 2011, 131, 1–7. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Martens, S.; Norelli, J.L.; Barny, M.-A.; Sundin, G.W.; Smits, T.H.; Duffy, B. Fire blight: Applied genomic insights of the pathogen and host. Annu. Rev. Phytopathol. 2012, 50, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front. Microbiol. 2019, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Koczan, J.M.; Lenneman, B.R.; McGrath, M.J.; Sundin, G.W. Cell surface attachment structures contribute to biofilm formation and xylem colonization by Erwinia amylovora. Appl. Environ. Microbiol. 2011, 77, 7031–7039. [Google Scholar] [CrossRef] [PubMed]
- Persen, U.; Gottsberger, R.; Reisenzein, H. Spread of Erwinia amylovora in apple and pear trees of different cultivars after artificial inoculation. In Proceedings of the XII International Workshop on Fire Blight 896, Warsaw, Poland, 20 August 2010; pp. 319–330. [Google Scholar]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Pandey, S.; Giri, K.; Kumar, R.; Mishra, G.; Raja Rishi, R. Nanopesticides: Opportunities in crop protection and associated environmental risks. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1287–1308. [Google Scholar] [CrossRef]
- Vanneste, J.; Cornish, D.; Spinelli, F.; Yu, J. Colonisation of apple and pear leaves by different strains of biological control agents of fire blight. N. Z. Plant Prot. 2004, 57, 49–53. [Google Scholar] [CrossRef]
- Chen, X.-H.; Scholz, R.; Borriss, M.; Junge, H.; Mögel, G.; Kunz, S.; Borriss, R. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J. Biotechnol. 2009, 140, 38–44. [Google Scholar] [CrossRef]
- Broggini, G.A.; Duffy, B.; Holliger, E.; Schärer, H.-J.; Gessler, C.; Patocchi, A. Detection of the fire blight biocontrol agent Bacillus subtilis BD170 (Biopro®) in a Swiss apple orchard. Eur. J. Plant Pathol. 2005, 111, 93–100. [Google Scholar] [CrossRef]
- Roselló, M.; Peñalver, J.; Llop, P.; Gorris, M.T.; Cambra, M.; López, M.; Chartier, R.; García, F.; Montón, C. Identification of an Erwinia sp. different from Erwinia amylovora and responsible for necrosis on pear blossoms. Can. J. Plant Pathol. 2006, 28, 30–41. [Google Scholar] [CrossRef]
- Sharifazizi, M.; Harighi, B.; Sadeghi, A. Evaluation of biological control of Erwinia amylovora, causal agent of fire blight disease of pear by antagonistic bacteria. Biol. Control 2017, 104, 28–34. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Montesinos, E. Lysozyme enhances the bactericidal effect of BP100 peptide against Erwinia amylovora, the causal agent of fire blight of rosaceous plants. BMC Microbiol. 2017, 17, 39. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Bonaterra, A.; Montesinos, E. Mechanisms of antagonism of Pseudomonas fluorescens EPS62e against Erwinia amylovora, the causal agent of fire blight. Int. Microbiol. 2007, 10, 123. [Google Scholar] [CrossRef]
- Tancos, K.; Villani, S.; Kuehne, S.; Borejsza-Wysocka, E.; Breth, D.; Carol, J.; Aldwinckle, H.; Cox, K. Prevalence of streptomycin-resistant Erwinia amylovora in New York apple orchards. Plant Dis. 2016, 100, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Lindow, S. Field performance of antagonistic bacteria identified in a novel laboratory assay for biological control of fire blight of pear. Biol. Control 2001, 22, 66–71. [Google Scholar] [CrossRef]
- McGhee, G.C.; Sundin, G.W. Evaluation of kasugamycin for fire blight management, effect on nontarget bacteria, and assessment of kasugamycin resistance potential in Erwinia amylovora. Phytopathology 2011, 101, 192–204. [Google Scholar] [CrossRef]
- Kamber, T.; Buchmann, J.P.; Pothier, J.F.; Smits, T.H.; Wicker, T.; Duffy, B. Fire blight disease reactome: RNA-seq transcriptional profile of apple host plant defense responses to Erwinia amylovora pathogen infection. Sci. Rep. 2016, 6, 21600. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Proceedings of the Nineteenth International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; pp. 255–261. [Google Scholar]
- Stockwell, V.; Johnson, K.; Sugar, D.; Loper, J. Control of fire blight by Pseudomonas fluorescens A506 and Pantoea vagans C9-1 applied as single strains and mixed inocula. Phytopathology 2010, 100, 1330–1339. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.M. Development of a Bacteriophage-Based Biopesticide for Fire Blight. Ph.D. Thesis, Brock University, St. Catharines, ON, Canada, 2007. [Google Scholar]
- Cui, Z.; Sun, L. Isolation and characterization of Priestia megaterium KD7 for the biological control of pear fire blight. Front. Microbiol. 2023, 14, 1099664. [Google Scholar] [CrossRef] [PubMed]
- Gromyko, O.; Tistechok, S.; Roman, I.; Aravitska, O.; Luzhetskyy, A.; Parnikoza, I.; Fedorenko, V. Isolation and characterization of culturable actinobacteria associated with Polytrichum strictum (Galindez Island, the maritime Antarctic). Ukr. Antarct. J. 2021, 1, 82–97. [Google Scholar] [CrossRef]
- Avelar Ferreira, P.A.; Bomfeti, C.A.; Lima Soares, B.; de Souza Moreira, F.M. Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World J. Microbiol. Biotechnol. 2012, 28, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Mondal, A.K.; Mukhopadhyay, A. Unique level of Multi-Drug Resistance (MDR) in Pseudomonas aeruginosa strain DEB1. Int. J. Sci Res 2014, 3, 455–460. [Google Scholar] [CrossRef]
- Chauhan, A.; Jindal, T.; Chauhan, A.; Jindal, T. Biochemical and molecular methods for bacterial identification. In Microbiological Methods for Environment, Food and Pharmaceutical Analysis; Springer: Cham, Switzerland, 2020; pp. 425–468. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis 1. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rohlf, F.J.; Sokal, R.R. The accuracy of phylogenetic estimation using the neighbor-joining method. Evolution 1993, 47, 471–486. [Google Scholar] [CrossRef]
- Joshi, R.; McSpadden Gardener, B.B. Identification and characterization of novel genetic markers associated with biological control activities in Bacillus subtilis. Phytopathology 2006, 96, 145–154. [Google Scholar] [CrossRef]
- Hu, X.; Fang, Q.; Li, S.; Wu, J.; Chen, J. Isolation and characterization of endophytic and rhizosphere bacterial antagonists of soft rot pathogen from Pinellia ternata. FEMS Microbiol. Lett. 2009, 295, 10–16. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Zeng, Q.; Sundin, G.W. Genome-wide identification of Hfq-regulated small RNAs in the fire blight pathogen Erwinia amylovora discovered small RNAs with virulence regulatory function. BMC Genom. 2014, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Gür, A.; Baştaş, K.K. Effectiveness of Phosphorous acid, Bacillus subtilis and Copper Compounds on Apple cv. Gala with M9 Rootstock in the Control of Fire Blight. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 2595–2600. [Google Scholar] [CrossRef]
- Wayne, P. Performance Standards for Antimicrobial Disc Susceptibility Testing; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002; Volume 12, pp. 1–53. [Google Scholar] [CrossRef]
- Ross, T.; Zhang, D.; McQuestin, O.J. Temperature governs the inactivation rate of vegetative bacteria under growth-preventing conditions. Int. J. Food Microbiol. 2008, 128, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Bu, H.; Liao, L.; Li, J.; Zhang, N.; Shi, F. Isolation, Identification and Biocontrol Potential of Antagonistic Fungi against Zanthoxylum bungeanum Root Rot. Chin. J. Biol. Control 2023, 39, 176. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.R.; Tandon, T.; Sharma, A.; Kanwar, S.S. Lipopeptide antibiotic production by Bacillus velezensis KLP2016. J. Appl. Pharm. Sci. 2018, 8, 091–098. [Google Scholar] [CrossRef]
- Soussi, S.; Essid, R.; Hardouin, J.; Gharbi, D.; Elkahoui, S.; Tabbene, O.; Cosette, P.; Jouenne, T.; Limam, F. Utilization of grape seed flour for antimicrobial lipopeptide production by Bacillus amyloliquefaciens C5 strain. Appl. Biochem. Biotechnol. 2019, 187, 1460–1474. [Google Scholar] [CrossRef] [PubMed]
- Klee, S.M.; Sinn, J.P.; McNellis, T.W. The apple fruitlet model system for fire blight disease. In Plant Innate Immunity: Methods and Protocols; Humana: New York, NY, USA, 2019; pp. 187–198. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar]
- Mannaa, M.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: Study II. Mycobiology 2018, 46, 52–63. [Google Scholar] [CrossRef]
- Brewer, L.; Palmer, J. Global pear breeding programmes: Goals, trends and progress for new cultivars and new rootstocks. In Proceedings of the XI International Pear Symposium 909, Patagonia, Argentina, 23–26 November 2010; pp. 105–119. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Iqbal, S.; Begum, F.; Rabaan, A.A.; Aljeldah, M.; Al Shammari, B.R.; Alawfi, A.; Alshengeti, A.; Sulaiman, T.; Khan, A. Classification and multifaceted potential of secondary metabolites produced by Bacillus subtilis group: A comprehensive review. Molecules 2023, 28, 927. [Google Scholar] [CrossRef] [PubMed]
- Théatre, A.; Hoste, A.; Rigolet, A.; Benneceur, I.; Bechet, M.; Ongena, M.; Deleu, M.; Jacques, P. Bacillus sp.: A remarkable source of bioactive lipopeptides. In Biosurfactants for the Biobased Economy; Springer: Cham, Switzerland, 2022; pp. 123–179. [Google Scholar]
- Villegas-Escobar, V.; González-Jaramillo, L.M.; Ramírez, M.; Moncada, R.N.; Sierra-Zapata, L.; Orduz, S.; Romero-Tabarez, M. Lipopeptides from Bacillus sp. EA-CB0959: Active metabolites responsible for in vitro and in vivo control of Ralstonia solanacearum. Biol. Control 2018, 125, 20–28. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Z.; Li, Y.; Zhang, X.; Duan, Y.; Wang, Q. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Front. Microbiol. 2017, 8, 1973. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.T.; Bloch, C.; Carmona-Ribeiro, A.M.; Di Mascio, P. Lipopeptides produced by a soil Bacillus megaterium strain. Microb. Ecol. 2009, 57, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural products from Bacillus subtilis with antimicrobial properties. Chin. J. Chem. Eng. 2015, 23, 744–754. [Google Scholar] [CrossRef]
- Tunio, A.A.; Naqvi, S.H.; REHMAN, T.; Bhutto, M.A.; MUGHERİ, M.H. Determination of Antioxidant, Antimicrobial Properties with Evaluation of Biochemicals and Phytochemicals Present in Oscillatoria limosa of District Jamshoro, Pakistan. Yuz. Yıl Univ. J. Agric. Sci. 2022, 32, 538–547. [Google Scholar] [CrossRef]



| S/No | Extract | RF Values (HI) | RF Values (I2) | Standard RF Values | Lipopeptides |
|---|---|---|---|---|---|
| 1 | Methanol | 0.23 | 0.25 | -- | unknown |
| 2 | Methanol | -- | 0.35 | 0.35 ± 0.04 | Bacillomycin D |
| 3 | Methanol | 0.43 | -- | 0.42 ± 0.04 | Fengycins |
| 4 | Methanol | 0.56 | 0.54 | 0.50 ± 0.04 | Iturins |
| 5 | Methanol | 0.64 | 0.63 | 0.62 ± 0.04 | Surfactins |
| S/n | Biochemical Test | Result for B. subtilis I2 | Result for P. megaterium H1 |
|---|---|---|---|
| 1 | Gram stain | + | + |
| 2 | Shape | Rod | Rod |
| 3 | Capsule | + | − |
| 4 | Motility | + | + |
| 5 | Colony color | White | White |
| 6 | Catalase test | + | + |
| 6 | Methyl Red test | − | − |
| 8 | Voges–Proskauer test | + | + |
| 9 | Simon citrate | + | + |
| 10 | Starch hydrolysis | + | + |
| 11 | Gelatin hydrolysis | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, I.u.; Hu, L.; Jiang, Y.; Wei, J.; Afzal, M.; Sun, L. Bacterial Lipopeptides Are Effective against Pear Fire Blight. Microorganisms 2024, 12, 896. https://doi.org/10.3390/microorganisms12050896
Din Iu, Hu L, Jiang Y, Wei J, Afzal M, Sun L. Bacterial Lipopeptides Are Effective against Pear Fire Blight. Microorganisms. 2024; 12(5):896. https://doi.org/10.3390/microorganisms12050896
Chicago/Turabian StyleDin, Ihsan ud, Lina Hu, Yuan Jiang, Jie Wei, Muhammad Afzal, and Li Sun. 2024. "Bacterial Lipopeptides Are Effective against Pear Fire Blight" Microorganisms 12, no. 5: 896. https://doi.org/10.3390/microorganisms12050896
APA StyleDin, I. u., Hu, L., Jiang, Y., Wei, J., Afzal, M., & Sun, L. (2024). Bacterial Lipopeptides Are Effective against Pear Fire Blight. Microorganisms, 12(5), 896. https://doi.org/10.3390/microorganisms12050896







