Biogenic Phosphonate Utilization by Globally Distributed Diatom Thalassiosira pseudonana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Experiment Setup
2.2. Determination of Cell Density and Fv/Fm
2.3. Cellular C and N Content
2.4. Determination of DIP and P Content
2.5. Statistical Analysis
3. Results and Discussion
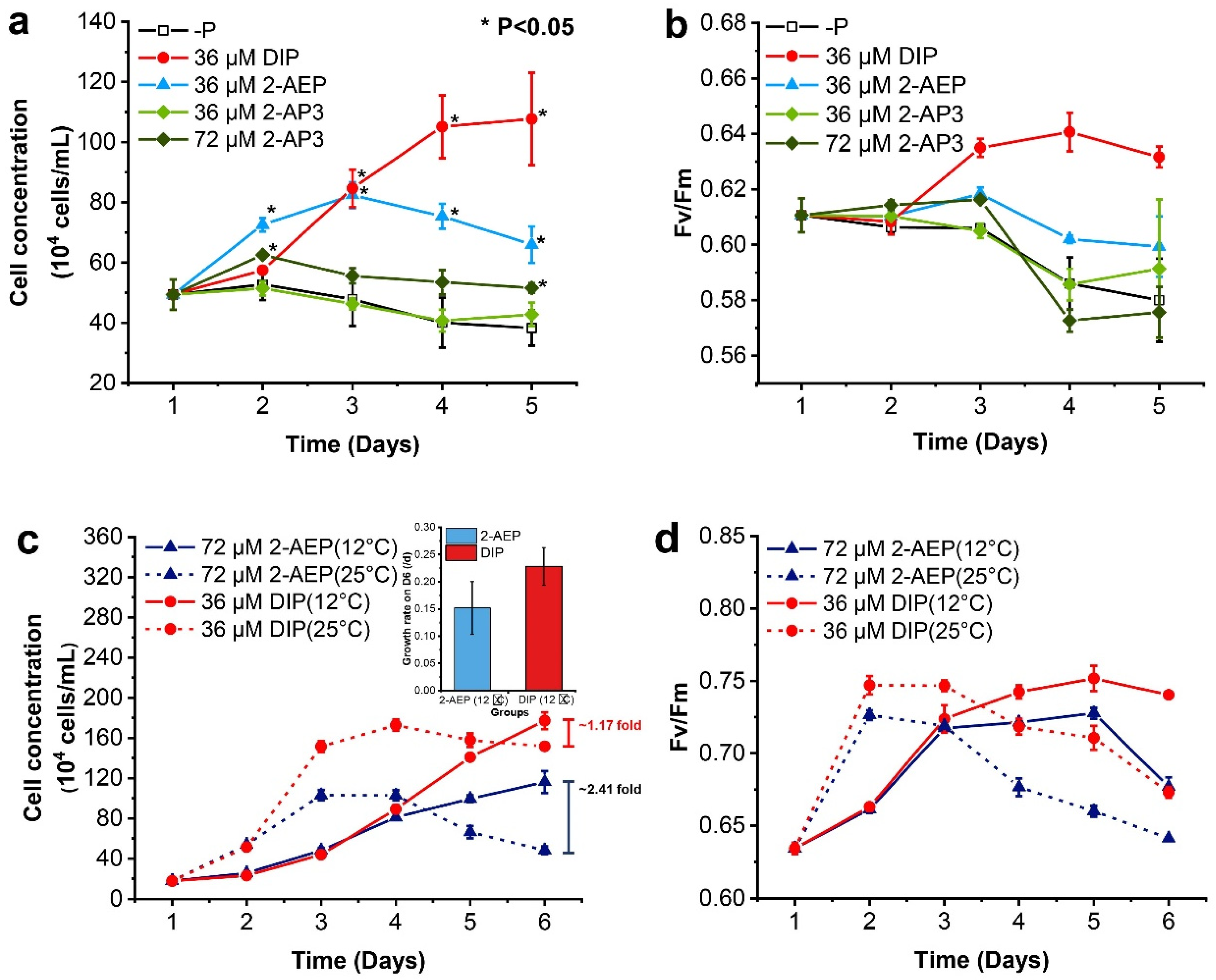
3.1. Differential Growth-Promoting Effects between 2-AEP and 2-AP3
3.2. Different Growth Strategies under Variable P Nutrients and Temperatures
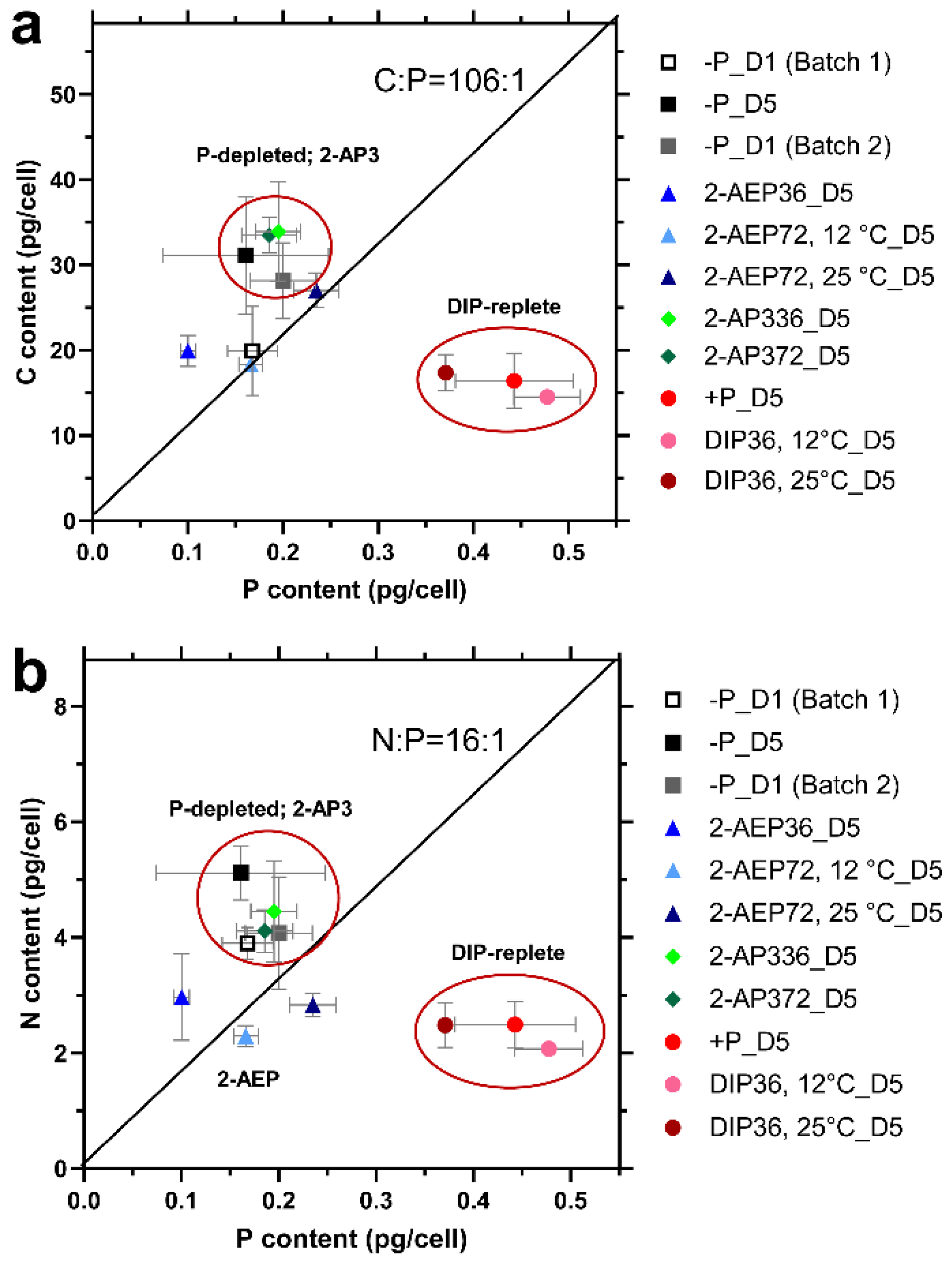
3.3. Changes in Cellular Elemental Stoichiometry of T. pseudonana
3.3.1. Stoichiometry Variation under Different P Conditions
3.3.2. Effect of Temperature on Cellular Stoichiometry
3.4. High Variability of Stoichiometry in Diatom
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karl, D.M.; Tien, G. Temporal variability in dissolved phosphorus concentrations in the subtropical North Pacific Ocean. Mar. Chem. 1997, 56, 77–96. [Google Scholar] [CrossRef]
- Wu, J.F.; Sunda, W.; Boyle, E.A.; Karl, D.M. Phosphate depletion in the western North Atlantic Ocean. Science 2000, 289, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.M.; Ridame, C.; Davey, M.; La Roche, J.; Geider, R.J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 2004, 429, 292–294. [Google Scholar] [CrossRef]
- Lomas, M.W.; Burke, A.L.; Lomas, D.A.; Bell, D.W.; Shen, C.; Dyhrman, S.T.; Ammerman, J.W. Sargasso Sea phosphorus biogeochemistry: An important role for dissolved organic phosphorus (DOP). Biogeosciences 2010, 7, 695–710. [Google Scholar] [CrossRef]
- Torres-Valdes, S.; Roussenov, V.M.; Sanders, R.; Reynolds, S.; Pan, X.; Mather, R.; Landolfi, A.; Wolff, G.A.; Achterberg, E.P.; Williams, R.G. Distribution of dissolved organic nutrients and their effect on export production over the Atlantic Ocean. Glob. Biogeochem. Cycles 2009, 23, GB4019. [Google Scholar] [CrossRef]
- Dawson, R.; Kemp, P. The aminoethylphosphonate-containing lipids of rumen protozoa. Biochem. J. 1967, 105, 837–842. [Google Scholar] [CrossRef]
- Taro, H.; Ikuko, A. Isolation and characterization of new sphingolipids containing N, N-acylmethylaminoethylphosphonic acid and N-acylaminoethylphosphonic acid from the mussel, Corbicula sandai. BBA-Lipids Lipid Metab. 1969, 176, 898–900. [Google Scholar] [CrossRef]
- Quin, L.D.; Quin, G.S. Screening for carbon-bound phosphorus in marine animals by high-resolution 31P-NMR spectroscopy: Coastal and hydrothermal vent invertebrates. Comp. Biochem. Phys. B 2001, 128, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, R.L. The Role of Phosphonates in Living Systems; CRC Press Inc: Boca Raton, FL, USA, 1983. [Google Scholar]
- Acker, M.; Hogle, S.L.; Berube, P.M.; Hackl, T.; Coe, A.; Stepanauskas, R.; Chisholm, S.W.; Repeta, D.J. Phosphonate production by marine microbes: Exploring new sources and potential function. Proc. Natl. Acad. Sci. USA 2022, 119, e2113386119. [Google Scholar] [CrossRef]
- Shoguchi, E.; Tanaka, M.; Takeuchi, T.; Shinzato, C.; Satoh, N. Probing a Coral Genome for Components of the Photoprotective Scytonemin Biosynthetic Pathway and the 2-Aminoethylphosphonate Pathway. Mar. Drugs 2013, 11, 559–570. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Chappell, P.D.; Haley, S.T.; Moffett, J.W.; Orchard, E.D.; Waterbury, J.B.; Webb, E.A. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.P.; Quinn, J.P.; McGrath, J.W. Phosphate insensitive aminophosphonate mineralisation within oceanic nutrient cycles. ISME J. 2018, 12, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, X.; Li, L.; Lin, S. Differential growth responses of marine phytoplankton to herbicide glyphosate. PLoS ONE 2016, 11, e0151633. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.L.; You, Y.C.; Wang, H.W.; Wang, J.T.; Li, L.; Ma, J.; Lin, X. Transcriptomic-guided phosphonate utilization analysis unveils evidence of clathrin-mediated endocytosis and phospholipid synthesis in the model diatom, Phaeodactylum tricornutum. Msystems 2022, 7, e00563. [Google Scholar] [CrossRef] [PubMed]
- Whitney, L.P.; Lomas, M.W. Phosphonate utilization by eukaryotic phytoplankton. Limnol. Oceanogr. Lett. 2019, 4, 18–24. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J.; Jang, T.Y.; Jang, S.H.; Franks, P.J. Inhibition of growth rate and swimming speed of the harmful dinoflagellate Cochlodinium polykrikoides by diatoms: Implications for red tide formation. Harmful Algae 2014, 37, 53–61. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Liang, J.R.; Gao, Y.H.; Lin, R.C.; Gao, H.; Xing, X.L.; Ma, J.; Luo, Q.Q. Influence of the concentration ratio of nitrogen to phosphorus on the growth and interspecies competition of two red tide algae. Acta Oceanol. Sin. 2007, 26, 107–115. [Google Scholar]
- Zheng, J.W.; Mao, X.T.; Ye, M.H.; Li, H.Y.; Liu, J.S.; Yang, W.D. Allelopathy and underlying mechanism of Karenia mikimotoi on the diatom Thalassiosira pseudonana under laboratory condition. Algal Res. 2021, 54, 102229. [Google Scholar] [CrossRef]
- Sheehan, C.E.; Baker, K.G.; Nielsen, D.A.; Petrou, K. Temperatures above thermal optimum reduce cell growth and silica production while increasing cell volume and protein content in the diatom Thalassiosira pseudonana. Hydrobiologia 2020, 847, 4233–4248. [Google Scholar] [CrossRef]
- Li, M.Z.; Shi, X.G.; Guo, C.T.; Lin, S.J. Phosphorus deficiency inhibits cell division but not growth in the dinoflagellate Amphidinium carterae. Front. Microbiol. 2016, 7, 826. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.H. Improved analysis for “particulate” organic carbon and nitrogen from seawater 1. Limnol. Oceanogr. 1974, 19, 984–989. [Google Scholar] [CrossRef]
- Jeffries, D.S.; Dieken, F.; Jones, D. Performance of the autoclave digestion method for total phosphorus analysis. Water Res. 1979, 13, 275–279. [Google Scholar] [CrossRef]
- Tamari, M. Digestion, absorption and excretion of free-form-ciliatine in rats. J. Agric. Chem. Soc. Jpn. 1971, 45, 433–440. [Google Scholar]
- Curley, J.M.; Henderson, T.O. The incorporation of 2-aminoethylphosphonic acid into rat liver diacylglyceroaminoethylphosphonate. Lipids 1972, 7, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Horigane, A.; Horiguchi, M.; Matsumoto, T. Metabolism of 2-amino-3-phosphonopropionic acid in rats. BBA-Lipids Lipid Metab. 1979, 572, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Akira, H.; Masaaki, H.; Tatsuro, M. Metabolism of 2-amino-3-phosphono [3-14C] propionic acid in cell-free preparations of Tetrahymena. BBA-Lipids Lipid Metab. 1980, 618, 383–388. [Google Scholar] [CrossRef]
- Berges, J.A.; Varela, D.E.; Harrison, P.J. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2002, 225, 139–146. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Chien, C.T.; Pahlow, M.; Schartau, M.; Li, N.; Oschlies, A. Effects of phytoplankton physiology on global ocean biogeochemistry and climate. Sci. Adv. 2023, 9, eadg1725. [Google Scholar] [CrossRef]
- Sharoni, S.; Halevy, I. Nutrient ratios in marine particulate organic matter are predicted by the population structure of well-adapted phytoplankton. Sci. Adv. 2020, 6, eaaw9371. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Alexander, H.; Whitney, L.P.; Drzewianowski, A.; Bulygin, V.V.; Bertrand, E.M. The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS ONE 2012, 7, e33768. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, M.H.; Sun, H.X.; Bowler, C.; Chua, N.H. Noncoding and coding transcriptome responses of a marine diatom to phosphate fluctuations. New Phytol. 2016, 210, 497–510. [Google Scholar] [CrossRef]
- Quigg, A.; Finkel, Z.V.; Irwin, A.J.; Rosenthal, Y.; Ho, T.Y.; Reinfelder, J.R.; Schofield, O.; Morel, F.M.M.; Falkowski, P.G. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 2003, 425, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.R.; Martiny, A.C. Ecological stoichiometry of ocean plankton. Ann. Rev. Mar. Sci. 2018, 10, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.S.; Sexton, J.; Riggins, T.; Brown, J.; Lomas, M.W.; Martiny, A.C. High variability in cellular stoichiometry of carbon, nitrogen, and phosphorus within classes of marine eukaryotic phytoplankton under sufficient nutrient conditions. Front. Microbiol. 2018, 9, 241843. [Google Scholar] [CrossRef] [PubMed]
- Martiny, A.C.; Pham, C.T.; Primeau, F.W.; Vrugt, J.A.; Moore, J.K.; Levin, S.A.; Lomas, M.W. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 2013, 6, 279–283. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Dossena, M.; Trimmer, M.; Woodward, G.; Allen, A.P. Temperature and the biogeography of algal stoichiometry. Glob. Ecol. Biogeogr. 2015, 24, 562–570. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Robinson, D.H.; Worthen, D.L.; Dunbar, R.B.; DiTullio, G.R.; VanWoert, M.; Lizotte, M.P. Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Dunbar, R.B.; Lizotte, M.P.; Robinson, D.H. Taxon-specific differences in C/P and N/P drawdown for phytoplankton in the Ross Sea, Antarctica. Geophys. Res. Lett. 2002, 29, 44-1–44-4. [Google Scholar] [CrossRef]
- Toseland, A.; Daines, S.J.; Clark, J.R.; Kirkham, A.; Strauss, J.; Uhlig, C.; Lenton, T.M.; Valentin, K.; Pearson, G.A.; Moulton, V. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 2013, 3, 979–984. [Google Scholar] [CrossRef]
- Tanioka, T.; Garcia, C.A.; Larkin, A.A.; Garcia, N.S.; Fagan, A.J.; Martiny, A.C. Global patterns and predictors of C:N:P in marine ecosystems. Commun. Earth Environ. 2022, 3, 271. [Google Scholar] [CrossRef] [PubMed]
- Sanudo-Wilhelmy, S.A.; Tovar-Sanchez, A.; Fu, F.X.; Capone, D.G.; Carpenter, E.J.; Hutchins, D.A. The impact of surface-adsorbed phosphorus on phytoplankton Redfield stoichiometry. Nature 2004, 432, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Longo, A.F.; Chambers, L.R.; Huang, R.; Benitez-Nelson, C.; Dyhrman, S.T.; Diaz, J.M.; Tang, Y.; Ingall, E.D. Understanding marine dissolved organic matter production: Compositional insights from axenic cultures of Thalassiosira pseudonana. Limnol. Oceanogr. 2016, 61, 2222–2233. [Google Scholar] [CrossRef]
- Thompson, P. The response of growth and biochemical composition to variations in daylength, temperature, and irradiance in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). J. Phycol. 1999, 35, 1215–1223. [Google Scholar] [CrossRef]
- O'Donnell, D.R.; Beery, S.M.; Litchman, E. Temperature-dependent evolution of cell morphology and carbon and nutrient content in a marine diatom. Limnol. Oceanogr. 2021, 66, 4334–4346. [Google Scholar] [CrossRef]
- Perry, M. Phosphate utilization by an oceanic diatom in phosphorus-limited chemostat culture and in the oligotrophic waters of the central North Pacific 1. Limnol. Oceanogr. 1976, 21, 88–107. [Google Scholar] [CrossRef]
- Peters, F.; Arin, L.; Marrasé, C.; Berdalet, E.; Sala, M.M. Effects of small-scale turbulence on the growth of two diatoms of different size in a phosphorus-limited medium. J. Mar. Syst. 2006, 61, 134–148. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Sreeush, M.G.; Timmermann, A.; Karl, D.M.; Church, M.J.; Lee, S.S.; Yamaguchi, R. Nutrient uptake plasticity in phytoplankton sustains future ocean net primary production. Sci. Adv. 2022, 8, eadd2475. [Google Scholar] [CrossRef]
- Goldman, J.C.; McCarthy, J.J.; Peavey, D.G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 1979, 279, 210–215. [Google Scholar] [CrossRef]
- Liefer, J.D.; Garg, A.; Fyfe, M.H.; Irwin, A.J.; Benner, I.; Brown, C.M.; Follows, M.J.; Omta, A.W.; Finkel, Z.V. The macromolecular basis of phytoplankton C:N:P under nitrogen starvation. Front. Microbiol. 2019, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Liu, H.B.; Ke, Y.; Li, B. Effect of the silica content of diatoms on protozoan grazing. Front. Mar. Sci. 2017, 4, 202. [Google Scholar] [CrossRef]
- Thomas, S.; Burdett, H.; Temperton, B.; Wick, R.; Snelling, D.; McGrath, J.W.; Quinn, J.P.; Munn, C.; Gilbert, J.A. Evidence for phosphonate usage in the coral holobiont. ISME J. 2010, 4, 459–461. [Google Scholar] [CrossRef] [PubMed]

| Culture | T (°C) | P Nutrient Concentration | Culture Condition |
|---|---|---|---|
| Seed | Starvation treated 8–10 days (<0.3 μM) |
| |
| −P a | No addition | ||
| +P b | DIP (36 μM) | ||
| Batch 1 | 20 | 2-AEP (36 μM) | |
| 2-AP3 (36, 72 μM) | |||
| Batch 2 | 12/25 | 2-AEP (72 μM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Shen, Y.; Wang, H.; Sun, X.; Ma, J.; Lin, X. Biogenic Phosphonate Utilization by Globally Distributed Diatom Thalassiosira pseudonana. Microorganisms 2024, 12, 761. https://doi.org/10.3390/microorganisms12040761
Shu H, Shen Y, Wang H, Sun X, Ma J, Lin X. Biogenic Phosphonate Utilization by Globally Distributed Diatom Thalassiosira pseudonana. Microorganisms. 2024; 12(4):761. https://doi.org/10.3390/microorganisms12040761
Chicago/Turabian StyleShu, Huilin, Yuan Shen, Hongwei Wang, Xueqiong Sun, Jian Ma, and Xin Lin. 2024. "Biogenic Phosphonate Utilization by Globally Distributed Diatom Thalassiosira pseudonana" Microorganisms 12, no. 4: 761. https://doi.org/10.3390/microorganisms12040761
APA StyleShu, H., Shen, Y., Wang, H., Sun, X., Ma, J., & Lin, X. (2024). Biogenic Phosphonate Utilization by Globally Distributed Diatom Thalassiosira pseudonana. Microorganisms, 12(4), 761. https://doi.org/10.3390/microorganisms12040761






