Abstract
Crude oil gasification bacteria, including fermenting bacteria, hydrocarbon-oxidizing bacteria, reducing bacteria, and methanogenic bacteria, participate in multi-step reactions involving initial activation, intermediate metabolism, and the methanogenesis of crude oil hydrocarbons. These bacteria degrade crude oil into smaller molecules such as hydrogen, carbon dioxide, acetic acid, and formic acid. Ultimately, they convert it into methane, which can be utilized or stored as a strategic resource. However, the current challenges in crude oil gasification include long production cycles and low efficiency. This paper provides a summary of the microbial flora involved in crude oil gasification, the gasification metabolism pathways within reservoirs, and other relevant information. It specifically focuses on analyzing the factors that affect the efficiency of crude oil gasification metabolism and proposes suggestions for improving this efficiency. These studies deepen our understanding of the potential of reservoir ecosystems and provide valuable insights for future reservoir development and management.
1. Introduction
After experiencing natural energy, hydraulic, and chemical displacement, at least 50% of the crude oil in the reservoir cannot be recovered [1], resulting in a decline in the utilization of oil resources. Since the 1980s and 1990s, researchers such as Zengler [2], Anderson [3], and Cheng [4] have confirmed that the synergistic effect of methanogens and methanotrophs can lead to the degradation and conversion of crude oil into methane, thereby reducing resource loss. Consequently, scientists have proposed “bio-gasification” technology for crude oil [5], which aims to degrade the challenging-to-mine hydrocarbons in the reservoir into small molecular substances such as hydrogen and carbon dioxide through microbial action, ultimately converting them into extractable methane [6], as shown in Figure 1.
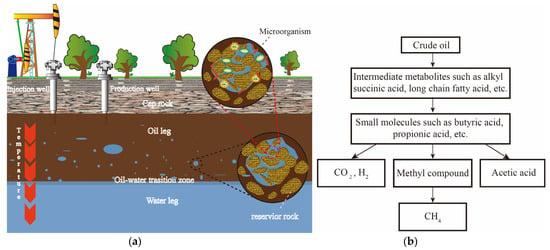
Figure 1.
(a) Reservoir environment diagram; (b) Diagram of the main process of crude oil gasification.
During the biodegradation of crude oil, hydrocarbon substrates are initially activated through pathways involving fumarate addition, hydroxylation, carboxylation, and methylation [7]. Subsequently, these compounds undergo further degradation into intermediate lower-molecular-weight products, including formate, acetate, hydrogen, and carbon dioxide, via pathways involving beta-oxidation and central metabolism. These processes are primarily conducted by hydrocarbon-oxidizing bacteria, fermenting bacteria, and various reducing bacteria, predominantly within the deferribacteres, firmicutes, and proteobacteria phyla [8,9]. Subsequently, the intermediate products formate, acetate, hydrogen, and carbon dioxide are transformed into methane through pathways that include methylotrophic, acetotrophic, and hydrogenotrophic processes [10,11], which involve various methanogenic archaea. The process of crude oil biodegradation engages a diverse range of microorganisms and enzymes that catalyze intricate metabolic reactions using their own synthetases, facilitating adaptation to extreme environments [12]. Consequently, crude oil biodegradation represents a comprehensive and intricate biochemical process, which, to a certain extent, constrains the efficiency of reservoir crude oil gasification due to various factors. To maximize the efficiency of crude oil gasification, Lin et al. [13] systematically researched the impact of temperature, pH, carbon sources, and other factors, elucidating the straightforward correlations between individual variables and the efficiency of crude oil gasification. As artificial intelligence has advanced, machine learning has demonstrated distinct advantages in fitting predictions and evaluating the significance of multiple variables [14,15]. Building on prior research regarding the correlation between individual variables and the efficiency of crude oil gasification, the integration of machine-learning algorithms is anticipated to enable the evaluation of the significance of multiple variables in the crude oil gasification process [16], thus aiding in the precise control of crude oil gasification in engineering applications.
Microorganisms act on the crude oil in the reservoir, leading to its vaporization and subsequent enhancement of the oil’s utilization rate. Additionally, this process yields clean energy, notably methane, thereby advancing the traditional fossil energy sector towards low-carbon practices. This method is anticipated to emerge as a novel solution for global energy requirements in the future [17,18,19]. Despite the significant potential of crude oil gasification, most research on crude oil gasification remains at the laboratory stage, and the widespread implementation of reservoir crude oil gasification technology has yet to be achieved. While existing studies have outlined the primary reaction pathways of the crude oil gasification process, the assessment of the importance and precise regulation of influencing factors has not been achieved due to the gasification efficiency being influenced by various complex factors, including environmental and substrate factors. This paper extensively examined the current state of reservoir crude oil gasification, with a focus on elucidating the reservoir crude oil degradation flora and metabolic pathways. Furthermore, it summarized, analyzed, and evaluated the factors influencing the efficiency of the metabolic process. Based on this analysis, a series of feasible suggestions were proposed to enhance the efficiency of crude oil gasification, and the substantial potential of reservoir crude oil gasification, facilitated by microorganisms, in terms of increased production and carbon reduction, was identified.
2. Microbial Flora Involved in Gasified Degradation of Crude Oil and Their Synergistic Effects
The flora involved in crude oil gasification comprises fermentation bacteria, hydrocarbon-oxidizing bacteria, sulfate-reducing bacteria, nitrate-reducing bacteria, iron-reducing bacteria, and methanogenic archaea [14], as listed in Table 1. As the depth increases, the distribution of crude oil gasification bacteria in the oil reservoir exhibits significant vertical variation, as shown in Figure 2. Aerobic bacteria are primarily distributed in the near-well area, while anaerobic bacteria are mainly found in the far-well end. This distribution pattern arises from the inevitable entry of a small amount of oxygen into the reservoir after a series of oil-displacement methods, creating a relative aerobic zone where aerobic fermentation bacteria and hydrocarbon-oxidizing bacteria are activated to oxidize and degrade hydrocarbons. As hydrocarbon-oxidizing bacteria consume dissolved oxygen in water, an anaerobic environment gradually forms, facilitating the activation of anaerobic and facultative aerobic fermentation bacteria for hydrocarbon degradation. Fermentation bacteria, in conjunction with various reducing bacteria, are responsible for converting hydrocarbons into secondary metabolites such as volatile fatty acids, methyl compounds, acetic acid, carbon dioxide, and hydrogen. Ultimately, methanogenic archaea convert secondary metabolites like methyl compounds, acetic acid, and carbon dioxide into the final product, CH4. Consequently, the crude oil gasification flora can be broadly categorized into two groups: interacting hydrocarbon-degrading flora and methanogenic bacteria, which are responsible for the initial degradation and final methanogenic processes, respectively.

Table 1.
Bacterial flora for crude oil degradation in oil reservoirs.
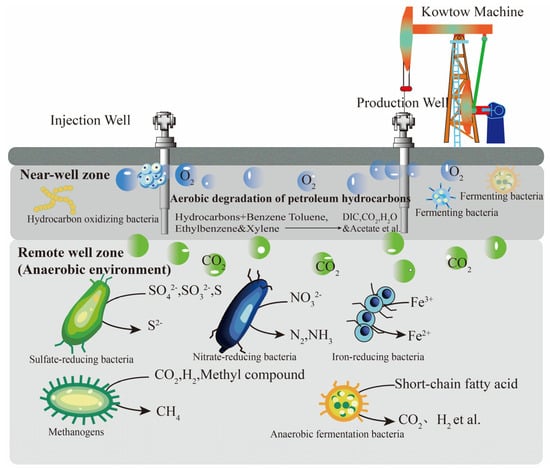
Figure 2.
Longitudinal distribution diagram of crude oil degrading bacteria in the reservoir.
Researchers isolated, screened, and enriched interbacterial communities involved in crude oil hydrocarbon degradation from diverse habitats [36,37,38,39,40], as shown in Table 2. These communities primarily consist of hydrocarbon-oxidizing bacteria, fermentation bacteria, and various reducing bacteria. Hydrocarbon-oxidizing bacteria thrive in shallow aerobic environments, utilizing hydrocarbons as a carbon source and oxygen as an electron acceptor to fuel their growth. Fermentation bacteria break down macromolecules (e.g., long-chain saturated hydrocarbons and aromatic hydrocarbons) in the reservoir, producing small molecules such as short-chain fatty acids, alcohols, methyl groups, and carbon dioxide in both aerobic and anaerobic settings. Reducing bacteria derive energy from electron acceptors (e.g., NO3−, Fe3+, Mn2+, SO42−) present in the reservoir’s in situ environment, metabolizing small molecules like nitrogen, carbon dioxide, and hydrogen sulfide in the process. The strains were identified through high-throughput sequencing, 16S rRNA analysis, metagenomics, and transcriptomic techniques. The analysis revealed that the majority of hydrocarbon-oxidizing bacteria (HOB) belong to genera such as Pseudomonas, Staphylococcus, Streptobacillus, Acinetobacter, and Rhodococcus [41]. These genera have been recognized for their significant role in petroleum hydrocarbon degradation. The isolated fermentation bacteria primarily belong to the genera Thermococcus, Bacteroidetes, Acinetobacter, and Haloanaerobium, among others [15,16,17]. The majority of reduced strains are classified within the genera Smithella, Desulfovibrio, and Desulfobulbus. As biological technology advances, researchers are continuously uncovering new strains. They explore novel metabolic pathways through enriching isolated strains, constructing complex microbial communities and utilizing key metabolic markers and isotope tracers.

Table 2.
Bacteria degraded by crude oil in different reservoirs.
Methanogenic bacteria are widely found in extreme anaerobic environments such as deep oil reservoirs [48], activated sludge, marine sediments, etc., where they are responsible for converting acetic acid, methyl compounds, carbon dioxide, etc., produced by the initial degradation process into CH4. Methanogenic archaea in oil reservoirs are diverse and can be primarily classified into acetoclastic, hydrogenotrophic, and methylotrophic groups based on their nutritional substrates, as listed in Table 3. In addition, Mayumi et al. [54] confirmed that Methermicoccus shengliensis ZC-1 isolated from the Shengli oilfield is capable of degrading methoxylated compounds to produce methane, while Cheng et al. [4] demonstrated that the methanogen Ca. Methanoliparum can directly degrade alkanes to produce methane. The temperature conditions in anaerobic environments can influence the metabolic pathways of methanogenic archaea and the distribution of dominant genera. Psychrophilic methanogens tend to favor acetoclastic metabolism, and the H2/CO2 reduction pathway is no longer present at 6 °C. Mesophilic methanogens mostly have both hydrogenotrophic and acetoclastic metabolic pathways and adjust their dominant pathways according to different environmental conditions. Thermophilic methanogens primarily utilize acetate oxidation and hydrogenotrophic methane-production pathways, and most of them are unable to metabolize formate esters, methanol, or trimethylamine for methylotrophic methane production. Hyperthermophilic methanogens tend to favor the hydrogenotrophic metabolic pathway. Notably, Wang et al. [55] found that the main methane producers in high-temperature hot springs, the order archaeoglobales, have both hydrogenotrophic and methylotrophic pathways for methane production, and they can metabolize methanol and produce methane via the hydrogenotrophic pathway at low temperatures (65 °C), while they use the methylotrophic metabolic pathway at high temperatures (75 °C). Furthermore, there are certain species differences in methane production among different types of culture, with acetoclastic methanogens mostly belonging to the Methanosaeta and Methanococcus genera, while methylotrophic methanogens are mostly classified into the orders Methanococcales, Methanocellales, and Methanomicrobiales.

Table 3.
Methanogenic archaea and their metabolic substrates.
Microbial ecological communities in reservoir crude oil gasification exhibit competitive exclusion, mutualistic symbiosis, partial symbiosis, and parasitism among themselves, as depicted in Figure 3. During crude oil gasification, methanogenic archaea and mutualistic hydrocarbon-degrading bacteria engage in a synergistic symbiotic relationship. Specifically, fermenting and methanogenic bacteria within mutualistic hydrocarbon-degrading communities are often closely associated, facilitating electron exchanges through proton flow, formate, or inorganic conductive particles in nanowires [66] and biofilm matrices [67]. Intercalated hydrocarbon-degrading communities break down complex organic compounds like long-chain alkanes and aromatic hydrocarbons into simple molecules such as carbon dioxide, acetate, and formate, providing the necessary carbon source for the growth and metabolism of methanogens [68,69]. Simultaneously, the energy released from methanogen metabolism supports the growth of intercalated hydrocarbon-degrading bacteria. Additionally, sulfate-reducing bacteria, nitrate-reducing bacteria, and hydrogenotrophic methanogens compete for nutrients because of the shared substrate. Due to the higher energy release in the nitrate reduction reaction compared to the sulfate reduction reaction, nitrate-reducing bacteria exhibit greater substrate competitiveness than sulfate-reducing bacteria when utilizing identical substrates. This enables them to competitively inhibit the production of hydrogen sulfide [70,71,72]. Sulfate-reducing bacteria and hydrogenotrophic methanogens can both utilize hydrogen as an electron donor. In sulfate-rich reservoirs, sulfate-reducing bacteria compete with methanogens for hydrogen as a substrate. Hence, to optimize the gasification efficiency of crude oil, it is imperative to judiciously leverage the interaction dynamics among gasification bacteria. This involves maintaining a balanced ratio of nitrate-reducing bacteria to sulfate-reducing bacteria, as well as sulfate-reducing bacteria to hydrogen-trophic methanogens. Such equilibrium ensures the timely and effective decomposition of intermediate metabolites. Additionally, introducing a suitable quantity of hydrogen-producing and acetogenic bacteria is essential to maximize the efficacy of methanogens.
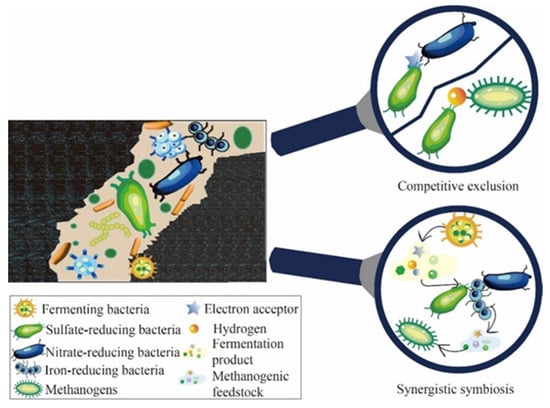
Figure 3.
Microbial interaction during crude oil gasification.
3. Metabolic Mechanisms of Reservoir Crude Oil Gasification
Reservoir crude oil primarily consists of saturated hydrocarbons, aromatic hydrocarbons, asphalt, and other non-hydrocarbons, with over 80% being hydrocarbons [73,74]. Microorganisms degrade the components of crude oil aerobically and anaerobically, gradually transforming them from long-chain complex compounds to small-molecule carbon-containing compounds. Aerobic degradation, involving both facultative anaerobic and aerobic microorganisms, breaks down petroleum hydrocarbons into small molecules like carbon dioxide through the action of oxygenase enzymes, utilizing molecular oxygen as the electron acceptor [75]. In contrast to aerobic degradation, anaerobic degradation is slower and continuous. Facultative aerobic bacteria and anaerobic bacteria use nitrate, sulfate, carbon dioxide, etc. as electron acceptors, ultimately converting petroleum hydrocarbons to CH4 [76,77]. Anaerobic degradation is the predominant process in the gasification of crude oil in reservoirs, typically encompassing multiple reaction steps, including the initiation of activation, fermentation, hydrolysis, anaerobic oxidation, and methanogenesis [78], as depicted in Figure 4. This paper specifically concentrates on the anaerobic degradation of petroleum hydrocarbons and the methanogenic pathway.
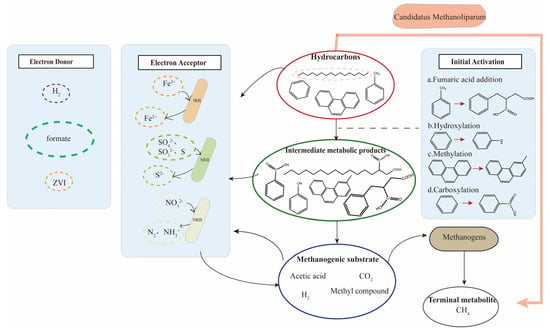
Figure 4.
Overview of crude oil gasification metabolic pathways.
3.1. Mechanism of Anaerobic Degradation Activation in Reservoir Crude Oil
Biegert et al. [79], Rabus et al. [80], and Aitken et al. [35] discovered and proposed the fumaric acid-addition pathway. Kniemeyer et al. [81] clarified that the core of the process involves the addition of fumaric acid to the sub-terminal carbons of alkanes or the alkyl side chain of aromatic compounds through isotopic labeling of intermediate metabolites. Table 4 lists the fumarate addition pathways of different substrates. Mbading et al. [82], Gieg et al. [83], and others have experimentally demonstrated that alkanes can produce 1-methyl alkyl succinic acid through the addition of fumaric acid to the subterminal carbon under various conditions and then undergo carbon backbone rearrangement, isomerization, decarboxylation, and multistep β-oxidation to achieve activated degradation. Herath et al. [84], Yoshikawa et al. [85], Alegbeleye et al. [86], and others found that substituted aromatic hydrocarbons have a fumaric acid-addition pathway similar to that of alkanes. For example, in the case of m-xylene, the methyl group on the benzene ring of m-xylene undergoes an addition reaction with fumaric acid to produce phenylmethyl succinate, which is then oxidatively dehydrogenated through β-oxidation to form benzoyl coenzyme A, entering the central metabolic pathway [84]. Researchers such as Boll [87] and Abu [88] have experimentally confirmed that, despite the high-bond energies making benzene itself difficult to degrade, it can be converted into substituted aromatics under various reducing conditions and subsequently participate in the fumaric acid addition.

Table 4.
Fumaric acid addition pathways of different substrates.
Besides the typical fumaric acid-addition pathway, the initial activation of crude oil components also involves hydroxylation, carboxylation, and methylation pathways. The hydroxylation pathway is relatively underreported, with its main targets being individually substituted aromatics and alkanes, such as ethylbenzene and propylbenzene. For instance, Heider et al. [95] discovered that ethylbenzene dehydrogenase and its encoding gene, ebdABC, are linked to the initiation of anaerobic degradation of ethylbenzene and propylbenzene. This enzyme catalyzes the hydroxylation of the alkyl side chains of ethylbenzene and propylbenzene in anaerobic environments, resulting in the production of 1-phenylethanol and 1-phenylpropanol. Aeckersberg et al. [37] identified the encoding gene, ahy ABC, in the strain Desulfococcusoleovorans Hxd3, which was isolated from oil and water samples due to its analogous function to the encoding gene ebdABC. Table 5 lists the hydroxylation pathways of different substrates.

Table 5.
Hydroxylation pathways of different substrates.
Different from the fumarate-addition pathway and hydroxylation pathway, carboxylation and methylation pathways are primarily suitable for non-substituted aromatic compounds such as benzene, naphthalene, and phenanthrene, as well as a few substituted aromatic compounds like 2-methylnaphthalene and phenol. Abu [88] and Ye [104] et al. have demonstrated that under specific conditions, benzene and naphthalene can be directly carboxylated to form benzoate or naphthalene ester through the assumed carboxylase catalyst (Abc) for degradation. Additionally, Bergmann et al. [105] discovered that sulfate-reducing bacteria N47 could degrade substituted aromatic compounds like 2-methylnaphthalene and phenol via a carboxylation initiation reaction, with the gene sequence of naphthylkylase A reductase (NCR) only found in the anaerobic process of sulfate-reducing naphthalene degradation [106]. Furthermore, Safinowski [107] and Tasi [108] found that in a medium containing carbonate as an activator, methylation reactions led to the production of 2-methylnaphthalene or 2-methyl phenanthrene from naphthalene, phenanthrene, and other aromatic hydrocarbons. This discovery enriched our understanding of the initial activation pathway for aromatic hydrocarbons. Table 6 lists the carboxylation and methylation pathways of different substrates.

Table 6.
Carboxylation and methylation pathways of different substrates.
In conclusion, the initial activation metabolism of crude oil hydrocarbon components under anaerobic conditions primarily involves fumaric acid addition, hydroxylation, carboxylation, and methylation. Subsequently, the initial activation product undergoes a series of changes, such as carbon rearrangement and decarboxylation, transforming into alkyl succinic acid of fatty acids and aromatic hydrocarbons and further enters the central metabolic pathway through β oxidation to metabolize acetic acid, methyl compounds, hydrogen, and carbon dioxide. Using benzene as an example, it undergoes anaerobic activation and degradation to enter the central metabolic process of benzoyl-coA, producing acetic acid, methyl compounds, hydrogen, and carbon dioxide, as illustrated in Figure 5.
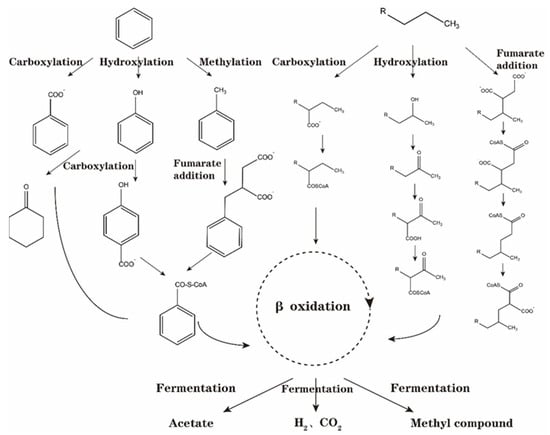
Figure 5.
Metabolic processes of benzene and alkane under different activation pathways of anaerobic degradation initiation.
3.2. Mechanism of Methanogenesis
Petroleum hydrocarbons undergo a series of anaerobic degradation reactions, gradually transforming into volatile fatty acids, soluble degradants, acetic acid, carbon dioxide, and hydrogen. Ultimately, under methanogenic conditions, the anaerobic degradation metabolites, including acetic acid, methyl compounds, hydrogen, and carbon dioxide, are converted into methane by methanogenic archaea, as shown in Figure 6. The methanogenic metabolic pathways can be broadly categorized into two groups: the reduction of carbon dioxide to produce methane, and the decomposition of small molecules such as acetic acid and methyl compounds into methane. These pathways are further subdivided into three core methanogenic pathways: the hydrogen–trophic pathway, the acetotrophic pathway, and the methyl–trophic pathway. Table 7 illustrates the major methanogenic metabolic pathways and the corresponding Gibbs energy of the reaction. Additionally, other metabolic pathways may be activated under specific conditions. For instance, in environments with high temperatures and low pH, an inter-interacting acetic acid oxidation pathway is stimulated, wherein acetic acid is oxidized by inter-interacting acetic acid oxidizing bacteria to produce hydrogen and carbon dioxide, ultimately leading to methane production by hydrotrophic methanogens. The direct acetic acid oxidation pathway is activated at high concentrations of CO2.
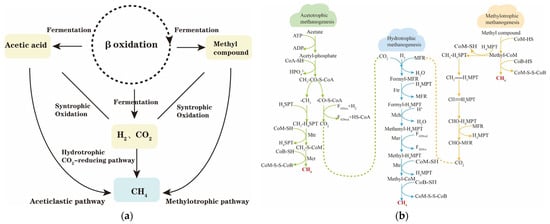
Figure 6.
(a) Terminal metabolism process of crude oil gasification; (b) overview of the main methanogenic metabolic pathways.

Table 7.
Overview of different methane production pathways.
In the hydrogenotrophic methanogenesis reaction, H2 reduces CO2 to methane. This process involves the transfer of carbon units between coenzymes and dehydration condensation reactions, as depicted in Figure 7. Electron transfer is mediated by the deazaflavin coenzyme F420 or ferredoxin (Fd), while various hydrogenases and/or dehydrogenases conduct the reduction of these electron carriers. The realization of the hydrotropic pathway heavily relies on electron transfer, and the current widely recognized electron bifurcation theory based on flavin and the Eha hypothesis of energy conversion hydrogenase play crucial roles. Herrmann et al. [116] proposed the electron bifurcation theory based on flavin, in which flavin protein splits electron pairs into a low-potential electron and a high-potential electron. This process achieves low-potential electron-reducing Fd under the mediation of ferredoxin (Fd) and simultaneously releases energy through the high-potential electron reduction of NADH. Lie et al. [117] verified the electron bifurcation hypothesis based on flavin through experiments and found that the energy-converting hydrogenase Eha, as a means to provide electrons to formylmethanofuran dehydrogenase, can supplement the electron bifurcation in a non-hybrid manner.
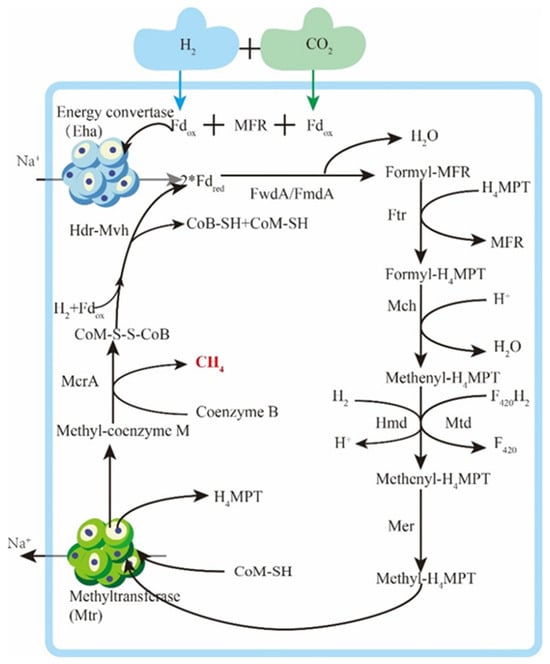
Figure 7.
Hydrotrophication methanogenic metabolic pathway.
There are two metabolic pathways of methanogenesis using acetic acid as a substrate. One is the direct reduction pathway of acetic acid, involving the direct reduction of methyl in acetic acid to methane, as shown in Figure 8. The second is the interacting acetic acid-oxidation pathway, where acetic acid is oxidized by inter-interacting acetic acid-oxidizing bacteria to produce hydrogen and carbon dioxide, ultimately leading to methane production by hydrogenotrophic methanogens. Both pathways compete for acetic acid as the substrate. Dolfing et al. [118], based on physicochemical theory, analyzed that under high-temperature and low-pH conditions, the reaction of acetic acid mutual oxidation, combined with the h hydrotrophic methanogenesis reaction, is more thermodynamically favorable. High concentrations of carbon dioxide may stimulate the acetic acid-reduction pathway. Studies involving animals or humans, and other research requiring ethical approval, must specify the approving authority and the corresponding ethical-approval code. The two-step reaction involved in the reciprocal oxidation of acetic acid is shown in Equations (1) and (2). The overall reaction for the production of methane from acetic acid is shown in Equation (3).
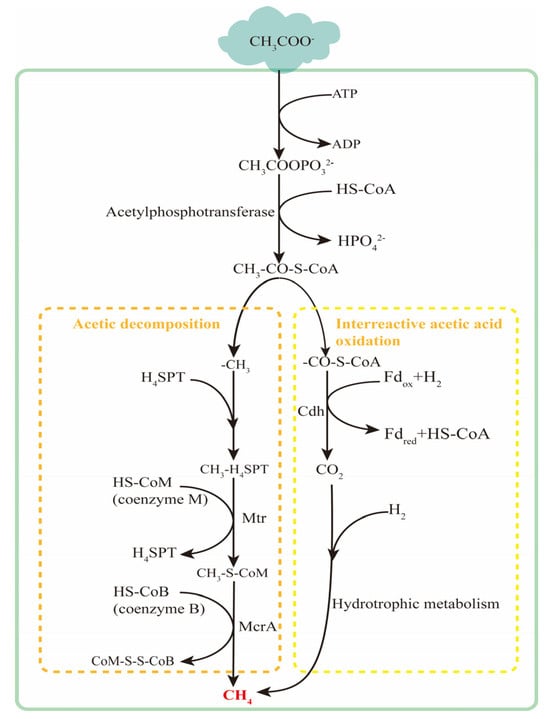
Figure 8.
Acetotrophic methanogenic metabolic pathway.
The methylotrophic pathway mainly utilizes methyl compounds such as methanol and trimethylamine as substrates, as depicted in Figure 9. Since methyl species are not as widely distributed in nature as acetic acid and carbon dioxide, the methylotrophic pathway is primarily found in highly mineralized environments such as salt lakes. The methylotrophic pathway can be further categorized into the methyl lyase type and the H2-reduced methyl compound type. The term “methyl cleavage type” refers to the production of methane through the self-disproportionation reaction of methyl compounds. Duszenko et al. [119] discovered that extreme halophilic methanogens, such as methananotronarchaea, utilize C1 methyl compounds as electron acceptors and formate or hydrogen as electron donors. Through the action of methyltransferase, one out of every four methyl compounds is oxidized to CO2 via the reverse hydrotrophic pathway, while the remaining three are reduced to methane, with the loss of electrons being internally resolved. The distinction between H2 reduction of methyl compounds lies in the fact that, during H2 reduction of methyl compounds, methyl compounds solely function as electron acceptors and are directly reduced to methane, making the process more efficient and straightforward. Yang et al. [120] demonstrated that in the anaerobic culture system of carbon dioxide and formic acid in reservoir production fluid, both the symbiotic oxidation of formic acid linked to hydrotrophic methanogenesis and the direct cracking of formic acid methanogenesis occur.
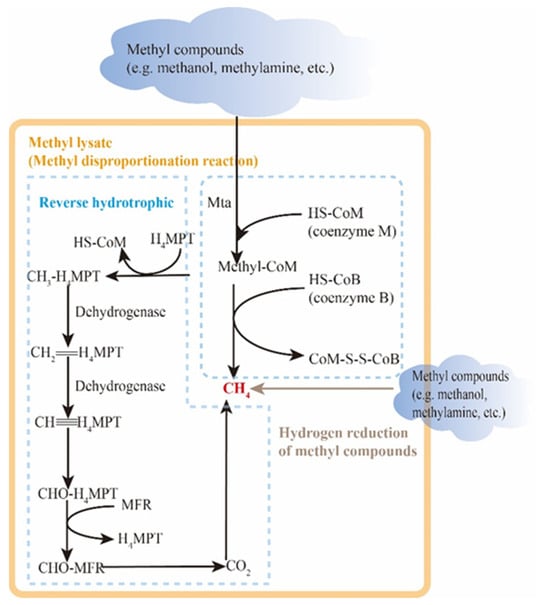
Figure 9.
Methylotrophic methanogenesis pathway.
In addition to the aforementioned approaches, Zhou et al. [121] comprehensively utilized carbon isotope labeling, metagenomic (transcriptomic) analysis, and other technologies to investigate the novel methanogenic archaea Candidatus Methanoliparum. Their research confirmed that this archaeon has the capability to autonomously degrade long-chain alkanes and produce methane, challenging the conventional understanding of methanogenic metabolism within bacterial groups. Furthermore, they identified a new methane-producing pathway, the long-chain alkane-degradation pathway.
4. Assessment of the Significance of Various Factors Influencing the Efficiency of the Reservoir Crude Oil Gasification Process
Currently, research on crude oil gasification is predominantly focused on the laboratory stage, and the limited gasification efficiency hinders its widespread application in engineering practice. Therefore, there is a need for a comprehensive understanding of the key factors influencing the efficiency of crude oil gasification. Building upon prior research, this paper provides a summary and analysis of the factors affecting crude oil gasification efficiency and proposes potential methods for evaluating their significance by integrating statistical analysis and machine-learning algorithms.
4.1. Factors Affecting the Efficiency of Crude Oil Gasification
The microbial oil gasification process primarily occurs at the oil–water interface of formations or in the micro-water environment of water-in-oil emulsion droplets. The findings indicate that the physical properties of the two phases involved at the oil–water interface and the contact area of the two phases somewhat influence gasification efficiency. The physical properties of the water phase, including the pH value, salinity, trace-element content, ammonia content, total nitrogen content, ammonia-to-alkalinity ratio, and concentration of inhibitors that predominantly impact the gasification process through microbial activity and metabolic pathways. Under normal conditions, the optimal pH for in-situ methanogenic bacteria in oil reservoirs is approximately 7. Chen et al. [122] observed that the appropriate initial pH value can enhance microbial activity and reduce the residence period, thereby increasing methane production. Salinity, as a significant measure of the salt content of formation water, to some extent affects the selection of methane-producing pathways. Waldron et al. [123] found that low salinity mainly favors hydrogen and acetic acid nutrient types, while high salinity favors methyl nutrient types. Additionally, trace elements such as Fe, Co, and Ni are closely associated with the enzymatic reaction rate of key enzymes in the gasification process, indirectly affecting the gasification rate. The physical properties of oil reservoirs, including porosity, permeability, saturation, capillary force, and wettability, also influence the growth and metabolism of microorganisms, thereby affecting crude oil gasification. In general, higher rock porosity provides more space for microbial growth, facilitating aggregation and growth. Furthermore, the greater the hydrophilicity of the rock surface, the stronger the adsorption of nutrients, including organic acids, which promote microbial metabolism and indirectly impact gasification. Additionally, the contact area of the oil–water phase directly affects the uptake and utilization efficiency of oil hydrocarbon components and the formation of water nutrients by bacteria.
The efficiency of crude oil gasification heavily relies on the substrate supply and microbial utilization rate. The primary substrates for crude oil gasification encompass the carbon source, electron acceptor, and electron donor. Some of the carbon originates from the hydrocarbon components inside the reservoirs, while the rest comes from external sources such as carbon dioxide or bicarbonate. The reservoir’s hydrocarbon components include short-chain hydrocarbons, long-chain normal alkanes, long-chain isomeric alkanes, aromatic hydrocarbons, cycloalkanes, and asphaltenes, among others. According to Table 8, the methane production efficiency of different hydrocarbon mechanisms indicates that the gasification efficiency of alkanes is markedly greater than that of asphaltenes. Furthermore, Mayumi and others observed that the injection of carbon dioxide as an exogenous carbon source accelerated the methane-production time [114]. Approximately 50% of the methane generated by gasification in the presence of hexadecane came from exogenous bicarbonate, leading to a rise in methane yield from 78.36 μmol to 147 μmol, and the methane-generation rate rose from 0.10 μmol/d to 0.16 μmol/d [124], which promoted methane formation to a certain extent.

Table 8.
Effects of hydrocarbon substrate, electron acceptor, temperature, and other major factors on methanogenesis rate.
Additionally, the gasification of crude oil involves a variety of electron acceptors, including Mn4+, NO3−, Fe3+, SO42−, and CO2, in response to the decrease in REDOX potential. The participation of electron acceptors aids in consuming the organic acids produced in the degradation process of crude oil and maintaining a dynamic equilibrium between the organic acids produced by the degradation of crude oil components and those broken down through subsequent oxidation. Simultaneously, due to the competition for substrates among sulfate-reducing bacteria, nitrate-reducing bacteria, and methane-producing bacteria, excessive NO3− and SO42− directly inhibit the formation of methane. Therefore, maintaining an appropriate electron acceptor ratio is crucial for the continuation of the initial degradation and methanogenesis process, as well as for the impact of methanogenesis. In comparison to abundant electron acceptors, the types of electron donors in oil reservoirs are scarce, with hydrogen and formate being the main contributors to the process of crude oil gasification. The environmental concentration of hydrogen is crucial as the direct electron donor for methane production, influencing the proportion of various pathways in the methane production process. Transcriptomic [129,130] and proteomic [131,132] studies have shown that the methanogenic pathway is heavily influenced by H2. Dolfing et al. [118] found that the partial pressure of H2, in most oil reservoirs, is typically maintained at a low level (<10−2 atm) or is sometimes undetectable, so it is necessary to introduce or stimulate H2-producing bacteria when constructing the crude oil degradation methanogenic bacteria in simulated in situ gasification experiments. Additionally, there are limited studies on alternative electron donors. For instance, ZVI (zero-valent iron) can be utilized as an alternative electron donor. Ma et al. [128] used a ZVI-modified substrate as an alternative electron donor. Scanning Electron Microscopy–Energy-Dispersive X-ray Spectroscopy (SEM-EDS) and hydrogen mass balance results proved that ZVI was feasible, and the rate of reduction was improved. An adequate amount of electron acceptors and donors is crucial to achieve efficient electron transport of microorganisms, ensuring the continuous and stable process of initial degradation and methanogenesis.
Besides the environmental and substrate factors mentioned above, the oil gasification process encompasses a variety of enzymes, which determine the reaction rate and direction to some extent. The primary activation process of crude oil anaerobic degradation primarily involves fumarate addition and exhibits specific enzymes and coding genes that catalyze different substrates. The most common ones are alkyl succinate synthetase and methyl succinate synthetase, which catalyze alkanes. Alkyl succinate synthetase is mainly used to catalyze the degradation of long-chain normal alkanes, while the methyl–alkyl succinate synthetase is primarily used for anaerobic degradation of normal alkanes in the C6-to-C8 range. The associated genes for their α-subunits are ass ABC [133] and masD [134], respectively. Meanwhile, with the development of whole-gene technology, Zhu et al. [135] hypothesized that the coding genes pfl D and pfl C also have functions similar to ass ABC. Additionally, phenyl methyl succinic synthetase is responsible for catalyzing the degradation of aromatic substituents such as toluene, xylene, and ethylbenzene, whose encoding gene is bssABC, while the anaerobic degradation of naphthalene and methylnaphthalene of PAHs depends on naphthalene methyl succinic synthetase, whose encoding gene is nms [106].
Methyl-coenzyme M is involved in the final step of methanogenesis, catalyzing the reaction of Coenzyme B (CoB-SH) to produce the final product CH4. Grabarse and colleagues compared the crystal structure and gene sequence of the methyl-coenzyme M reductase protein of different methanogenic organisms and found that they were closely related [136]. Meanwhile, through an analysis of the crystal structure of the protein, it was found that they contained two forms, MCR-Ⅰ and MCR-Ⅱ. MCR-Ⅰ is encoded by mcrBDCGA, while the proteins in Methanococcales and Methanobacteriales are different from MCR-Ⅰ and encoded by mrtBDGA [137]. To further clarify the relationship between coding genes and methane-production efficiency, scientists found that the copy number of the mcrA gene in the hydrogen–trophic pathway was significantly positively correlated with the methane-production efficiency through genetic library and quantitative PCR amplification, while the correlation between the transcription number of mcrA and the activity of specific methanogenic bacteria was not obvious [138].
In addition to methyl-coenzyme M, there are also key enzymes and coding gene clusters in the three major methanogenic core pathways. A variety of hydrogenases and dehydrogenases are involved in the electron gain and loss of carbon dioxide reduction through the hydrogenotrophic pathway, including energy-converting hydrogenase (Ech), F420-reducing hydrogenase (Frh), tungsten-containing methanofuran dehydrogenase (Fwd), and molybdenum-containing methanofuran dehydrogenase (Fmd). Among them, the structure of molybdenum formylmethanofuran dehydrogenase (Fmd) and its related gene cluster FmdABC are very similar to FwdABCD in function and coding sequence [139]. The central enzyme of the acetoclastic pathway is carbon monoxide dehydrogenase, which, by structural analysis, is found to exist in the form of a dimer composed of L, M, and S triacyl groups, and its core metabolic gene is CdhABGDE. A comparison of the abundance of all key genes shows that the cdhD gene has a low abundance and exists in all acetogenic methanogenic processes. In the methylotrophic pathway, coenzyme M methyltransferase (Mtr) plays a key role that is responsible for transferring methyl from methanol and methylamine to coenzyme M to generate methyl-Coenzyme M (CH3-S-CoM). Sequencing found that its main component, subunit 8, was encoded by MtrECDBAFGH [140]. Additionally, Hedderich et al. [141] and Zhou et al. [33] found that mta, mtb, mts, mtq, mtt, mtm, and other coding genes exist in the genome of methanogenic archaea. In short, with the development of cryo-electron microscopy and metagenomic technology, researchers have gained insights into the functional genes of key enzymes based on the analysis of the structural characteristics of key enzyme proteins, providing new ideas for discovering new metabolic pathways in the future and improving methane production from oil reservoirs through protein-transformation engineering.
4.2. Multivariate Importance Evaluation Methods for Enhancing Oil Reservoir Gasification Efficiency
Oil reservoir gasification efficiency is influenced by various factors, including environmental, substrate, and biological variables, as shown in Table 9. A precise evaluation of the relative significance of each factor aids researchers in identifying key regulatory targets, streamlining experimental design, and enhancing time efficiency. To quantitatively assess the relative importance of these factors, Darlingto [142], Johnson [143], and their colleagues introduced evaluation metrics such as the correlation coefficient, partial correlation coefficient, standardized regression coefficient, covariance, dominance analysis, and relative weight. Currently, simple correlation analyses such as the Pearson [144], Spearman [145], and Kendall correlation analyses [146], as well as complex nonlinear analysis methods, including multiple correspondence analysis, classified nonlinear principal component analysis, nonlinear typical analysis, and gray correlation analysis [147], are predominantly utilized for experimental results with relatively small sample sizes. These methodologies have found application in engineering-problem domains, such as the prediction of initial productivity in oil wells. Song Xuanyi and colleagues employed Pearson correlation analysis to construct a correlation matrix for 10 characteristic parameters, including porosity and permeability and subsequently assessed the correlation of each parameter. Their findings revealed a strong linear relationship between porosity and permeability, as well as a correlation between oil-layer thickness, perforation-section thickness, and fracturing light. This suggests the potential for simplified grouping of multiple variables [148]. In this study, utilizing experimental data from Cheng et al. [4] regarding the degradation of various crude oil components at different temperatures and durations, a correlation analysis heat map was generated based on the Spearman correlation coefficient, as depicted in Figure 10. The figure illustrates that saturated hydrocarbons and non-hydrocarbon substances exhibit a significantly higher time dependency compared to aromatic hydrocarbons. Furthermore, a notable correlation between saturated hydrocarbons and non-hydrocarbon substances suggests that saturated hydrocarbons play a pivotal role in enhancing crude oil degradation efficiency.

Table 9.
Factors affecting the efficiency of crude oil gasification.
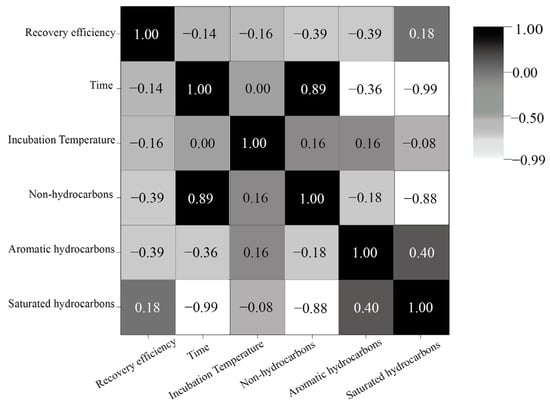
Figure 10.
Spearman correlation analysis heat map of different crude oil components at different temperatures and times.
Moreover, the gasification process of crude oil relies on the involvement of a microbial community and associated biological enzymes. In their study, Liu et al. [149] analyzed the microbial community using non-metric multidimensional scaling (NMDS) and subsequently subjected it to similarity analysis (ANOSIM). Their findings revealed that various factors, particularly temperature and the initial community, were the primary driving forces influencing the microbial community, as depicted in Figure 11. Notably, oils and nutrients were found to play a secondary role. However, this analysis solely pertains to the structure of the microbial community. To accurately evaluate the variables of oil gasification, it becomes imperative to consider the enzymes and multiple functional genes involved in the intricate nature of biological structures and genetic polymorphism results in a comprehensive crude oil gasification dataset comprising hundreds or thousands of feature parameters. Depending solely on traditional feature correlation-evaluation methods for analysis becomes time-consuming and laborious. Faced with extensive datasets, machine-learning algorithm modeling demonstrates a superior performance compared to traditional correlation analysis methods. Common machine-learning algorithms encompass linear regression, logistic regression, linear discriminant analysis, naive Bayes, KNN, artificial neural networks, and random forest, among others, as listed in Table 10. Selecting the suitable algorithm based on the dataset’s characteristics and the intended implementation conditions is crucial. Table 5 provides a summary of common machine-learning algorithms, along with their respective advantages and disadvantages. By randomly selecting different variables, assigning random values to each predictor, and calculating the error rate, one can select a feature variable and introduce random noise to the sample’s feature variable. This enables the comparison of the variance change predicted by the model after the value is randomly replaced [150]. The proportion of the model’s variance increase serves as a criterion for assessing the importance of variables, revealing the relationship between each variable and the oil gasification efficiency in the reservoir. A higher error in the overall model after replacement indicates a stronger importance of the predictor variable. Kacalla and colleagues constructed a database comprising 32 variables from 93 reservoirs on the Norwegian continental shelf. They employed the random forest algorithm to develop three prediction models and investigated the impact of various input variables on recovery rate and production. Their analysis revealed that when predicting the oil storage rate, the most crucial variables were associated with the size of the oilfield, including cumulative oil production, number of wells, oil production (OIP), and rock volume [151]. In a separate study, Yu et al. [152] utilized the random forest (RF) machine-learning algorithm to explore the factors influencing the high and stable production of a single well within a specific well group in the Junggar Basin. Their findings indicated that reservoir quality and oil saturation are the primary controlling factors, establishing the fundamental basis for achieving high and stable oil production [153]. These studies demonstrate the significant advantages of the random forest model in evaluating the importance of multiple variables in large datasets. Consequently, its application in assessing the multivariate importance of the crude oil gasification process is anticipated to enable the precise regulation of the gasification process.
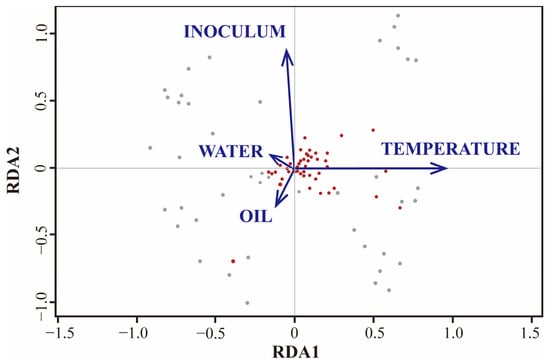
Figure 11.
Two-line plot of microbial community redundancy analysis (RDA) under oil, temperature, nutrients, and initial community constraints [149]. The red and gray dots represent the two treatments.

Table 10.
Comparison of advantages and disadvantages of various machine-learning algorithms.
5. Opportunities and Challenges of Microbial Assisted Crude Oil Gasification in Practical Applications
The utilization of microbial assistance in the gasification of crude oil presents both opportunities and challenges in practical applications. This pioneering approach involves microbial assistance to enhance the gasification process of refractory crude oil in reservoirs, thereby enhancing the utilization of petroleum resources and promoting the sustainable development of the petroleum industry. In addition, under either anaerobic or aerobic conditions microorganisms reveal their large potential in biodegradation of contaminants [148], especially crude oil-contaminated wastewater [156], crude oil-polluted soil [157], sewage sludge, composition [158], etc.
The successful implementation of the technology encounters numerous challenges. To address the practical requirements of engineering applications, the technical feasibility of microbial-assisted crude oil gasification under reservoir geothermal conditions must be prioritized. This necessitates researchers to comprehend the distinctions between simulated laboratory operating conditions and the genuine engineering temperature and pressure conditions. Additionally, to fulfill the demands of large-scale production, continuous testing and optimization of the reaction are essential to enhance the efficiency of crude oil gasification and achieve cost-effective process control. Therefore, while microbial-assisted crude oil gasification is expected to drive sustainable development in the oil industry, these complexities also need to be carefully considered to effectively integrate it into practical situations.
Laboratory simulation experiments have validated the gasification of crude oil by microorganisms. Thiagarajan et al. have further corroborated the microbial-assisted crude oil gasification process, yielding methane in deepwater oil reservoirs in the Gulf of Mexico [151]. This confirmation underscores the technical viability of microbial-assisted crude oil gasification initiatives. Currently, most projects in this realm remain at the laboratory stage, constrained to some extent by the intricate reservoir conditions. Bridging the gap between real geological conditions (<200 °C) and laboratory settings (30–110 °C) is paramount for industrial implementation [153]. While existing experiments predominantly employ single-factor variable methods, focusing on simplified temperature, pressure, and crude oil compositions, the actual reservoir conditions exhibit significant temperature and pressure variations due to depth, coupled with more complex crude oil compositions. These factors influence the anaerobic degradation rate of crude oil and impede the reliable extrapolation of experimental outcomes to practical engineering applications. Due to the complexity of practical applications, the multi-variable coupling relationship should be fully considered before engineering practice, and computational fluid mechanics and quantum chemistry calculation methods [153] should be combined to simulate the microbial assisted crude oil gasification process in real reservoirs.
Currently, the primary challenge in crude oil gasification is the low efficiency of the process. To meet the demands of large-scale production, the continual enhancement of expertise in microbial-assisted metabolic for crude oil gasification is essential. This involves seeking more efficient and direct metabolic pathways, optimizing the microbial flora combination to maximize the synergistic effect of crude oil-degrading bacteria. Furthermore, it is crucial to pinpoint the key factors influencing gasification efficiency and enhance it by introducing exogenous nutrients and alternative electron donors.
Additionally, engineering practices face a range of risks, including environmental hazards and quality and safety risks linked to diverse integrated technologies. The primary focus of crude oil gasification lies in abandoned well groups within depleted reservoirs. Setting up a strong oil and gas field infrastructure along with a thorough risk-assessment system lays the groundwork for repurposing abandoned reservoirs as natural gas-storage sites [159]. Furthermore, refurbishing old wells using methods like gravel packing can notably improve well-safety performance and reduce the risk of leaks.
6. Conclusions and Prospect
In light of the “2030” carbon peak and “2060” carbon neutrality commitment, carbon capture, utilization, and storage (CCUS) technology holds significant potential for application and development [160]. The China Carbon Dioxide Capture, Utilization, and Storage (CCUS) Annual Report 2021 underscores that CCUS technology stands as the sole option for decarbonizing fossil energy. The biodegradation of crude oil components by in situ microorganisms can effectively enhance resource utilization and simultaneously convert exogenous carbon dioxide into methane, which can be utilized as natural gas or stored as a strategic resource, holding substantial implications for economic development and carbon emission reduction.
This study provides a comprehensive overview of the primary metabolic pathways and microbial communities involved in oil reservoir gasification, along with an exploration of the potential biological prospects of oil gasification in diverse environments. Numerous environmental factors, including the temperature, contact area, substrate, nutrients, and electron acceptor, directly impact the efficiency of crude oil gasification. However, among these factors, the assessment of importance indicates that the proportion of crude oil components and the contact area between microorganisms and petroleum hydrocarbons are more manageable. Thus, under suitable conditions, implementing substrate regulation and developing new biocompatible surfactants to enhance the contact surface between petroleum hydrocarbons and microorganisms may serve as a potential approach to effectively improve the efficiency of crude oil gasification. Further research is essential to understand the steps and limitations of the speed of crude oil gasification in the future, and the utilization of big data and the random forest model to assess the importance of multiple variables and introduce evaluation indicators for quantitatively measuring the relative significance of various factors in the gasification process. Unraveling the mechanisms behind these correlations may pave the way for addressing the challenge of crude oil gasification efficiency and realizing the large-scale application of crude oil gasification facilitated by microorganisms in the future.
In addition, 16S rRNA and metagenomic technologies were utilized to investigate the microbial community involved in crude oil gasification. It was discovered that addressing the challenge of crude oil gasification efficiency may necessitate the cumulative synergistic symbiosis among different types of hydrocarbon-degrading bacteria, including fermentation bacteria, sulfate-reducing bacteria, and methanogens. Consequently, the development of appropriate microbial consortia can enhance the utilization rate of recalcitrant crude oil components and facilitate the timely and efficient conversion of intermediate metabolites into end products. In the future, there is a need to expand and enrich the repertoire of functional bacterial resources through high-throughput screening methods, uncover untapped reservoirs of petroleum hydrocarbon-degrading bacteria, and further refine the strategy for constructing artificial microbial communities.
Based on this foundation, potential undiscovered pathways for the degradation of crude oil components by unknown bacterial populations may exist, warranting the urgent advancement and enhancement of microbial-isolation technologies. This involves integrating meta-transcriptomic, metagenomic technologies, and low-throughput biomarker monitoring to analyze and identify potentially more efficient pathways for crude oil metabolism.
Author Contributions
S.N.: investigation, writing—original draft, formal analysis, data curation; W.L.: writing—review and editing, validation, formal analysis, resources; Z.J.: formal analysis, validation, data curation, writing—review and editing; K.W.: investigation, formal analysis, software; Y.M.: investigation, formal analysis; Y.L.: investigation, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Science and Technology project of the CNPC in China (grant No. 2021ZZ05).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank two anonymous reviewers for their constructive feedback.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems, and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [PubMed]
- Zengler, K.; Richnow, H.H.; Rosselló-Mora, R.; Michaelis, W.; Widdel, F. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 1999, 401, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.T.; Lovley, D.R. Naphthalene and benzene degradation under Fe (III)-reducing conditions in petroleum-contaminated aquifers. Bioremediat. J. 1999, 3, 121–135. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, S.; Li, Q.; Chen, J.; Zhang, H.; Lu, Y. Progressive degradation of crude oil n-alkanes coupled to methane production under mesophilic and thermophilic conditions. PLoS ONE 2014, 9, e113253. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Xiong, Y.; Hou, Z.; Xie, H.; Zhao, J.; Tan, X.; Luo, J. Microbial-mediated CO2 methanation and renewable natural gas storage in depleted petroleum reservoirs: A review of biogeochemical mechanism and perspective. Gondwana Res. 2022, 22, 184–198. [Google Scholar] [CrossRef]
- Bian, X.-Y.; Maurice Mbadinga, S.; Liu, Y.-F.; Yang, S.-Z.; Liu, J.-F.; Ye, R.-Q.; Gu, J.-D.; Mu, B.-Z. Insights into the anaerobic biodegradation pathway of n-alkanes in oil reservoirs by detection of signature metabolites. Sci. Rep. 2015, 5, 9801. [Google Scholar] [CrossRef]
- Xia, W.; Shen, W.; Yu, L.; Zheng, C.; Yu, W.; Tang, Y. Conversion of petroleum to methane by the indigenous methanogenic consortia for oil recovery in heavy oil reservoir. Appl. Energy 2016, 171, 646–655. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, K.; Li, B.-G.; Mbadinga, S.M.; Zhou, L.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Simulation of in situ oil reservoir conditions in a laboratory bioreactor testing for methanogenic conversion of crude oil and analysis of the microbial community. Int. Biodeterior. Biodegrad. 2019, 136, 24–33. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Wu, D.; Tringe, S.G. Methyl-based methanogenesis: An ecological and genomic review. Microbiol. Mol. Biol. Rev. 2023, 87, e00024-22. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 1987, 56, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; De Vrieze, J.; He, G.; Li, X.; Li, J. Temperature regulates methane production through the function centralization of microbial community in anaerobic digestion. Bioresour. Technol. 2016, 216, 150–158. [Google Scholar] [CrossRef]
- Murdoch, W.J.; Singh, C.; Kumbier, K.; Abbasi-Asl, R.; Yu, B. Definitions, methods, and applications in interpretable machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 22071–22080. [Google Scholar] [CrossRef] [PubMed]
- Conard, A.M.; DenAdel, A.; Crawford, L. A spectrum of explainable and interpretable machine learning approaches for genomic studies. Wiley Interdiscip. Rev.-Comput. Stat. 2023, 15, e1617. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Chen, C.; Yan, B.; Tao, J.; Chen, G. Interpretable machine learning for predicting and evaluating hydrogen production via supercritical water gasification of biomass. J. Clean. Prod. 2021, 316, 128244. [Google Scholar] [CrossRef]
- Soeder, D.J. Greenhouse gas sources and mitigation strategies from a geosciences perspective. Adv. Geo-Energy Res. 2021, 5, 274–285. [Google Scholar] [CrossRef]
- Dou, L.; Sun, L.; Lyu, W.; Wang, M.; Gao, F.; Gao, M.; Jiang, H. Trend of global carbon dioxide capture, utilization and storage industry and challenges and countermeasures in China. Pet. Explor. Dev. 2023, 50, 1246–1260. [Google Scholar] [CrossRef]
- Tyne, R.; Barry, P.; Lawson, M.; Byrne, D.; Warr, O.; Xie, H.; Hillegonds, D.; Formolo, M.; Summers, Z.; Skinner, B. Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs. Nature 2021, 600, 670–674. [Google Scholar] [CrossRef]
- Duncan, K.E.; Gieg, L.M.; Parisi, V.A.; Tanner, R.S.; Tringe, S.G.; Bristow, J.; Suflita, J.M. Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ. Sci. Technol. 2009, 43, 7977–7984. [Google Scholar] [CrossRef]
- Orphan, V.; Goffredi, S.; Delong, E.; Boles, J. Geochemical influence on diversity and microbial processes in high temperature oil reservoirs. Geomicrobiol. J. 2003, 20, 295–311. [Google Scholar] [CrossRef]
- Stetter, K.O.; Huber, R.; Blöchl, E.; Kurr, M.; Eden, R.; Fielder, M.; Cash, H.; Vance, I. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 1993, 365, 743–745. [Google Scholar] [CrossRef]
- Nazina, T.; Sokolova, D.; Grouzdev, D.; Semenova, E.; Babich, T.; Bidzhieva, S.; Serdukov, D.; Volkov, D.; Bugaev, K.; Ershov, A.; et al. The Potential Application of Microorganisms for Sustainable Petroleum Recovery from Heavy Oil Reservoirs. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef]
- Bogatyrenko, E.A.; Kim, A.V.; Polonik, N.S.; Dunkai, T.I.; Ponomareva, A.L.; Dashkov, D.V. Psychrotrophic Hydrocarbon-Oxidizing Bacteria Isolated from Bottom Sediments of Peter the Great Bay, Sea of Japan. Oceanology 2022, 62, 379–389. [Google Scholar] [CrossRef]
- Grouzdev, D.S.; Sokolova, D.S.; Poltaraus, A.B.; Nazina, T.N. Draft Genome Sequence of Halomonas titanicae Strain TAT1, a Hydrocarbon-Oxidizing Halophilic Bacterium Isolated from a Petroleum Reservoir in Russia. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.M.; Ershov, A.P.; Sokolova, D.S.; Tourova, T.P.; Nazina, T.N. Diversity and Biotechnological Potential of Nitrate-Reducing Bacteria from Heavy-Oil Reservoirs (Russia). Microbiology 2020, 89, 685–696. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wei, F.D.; Xu, R.; Cheng, T.; Ma, Y.L. Characterization and genomic analysis of a nitrate reducing bacterium from shale oil in the Ordos Basin and the associated biosurfactant production. J. Environ. Chem. Eng. 2022, 10, 108776. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Ramos, L.; Abreu, F.; Lins, U.; de Sousa, M.P.; dos Santos, V.; Penna, M.; Seldin, L. Long-term souring treatment using nitrate and biocides in high-temperature oil reservoirs. Fuel 2021, 288, 119731. [Google Scholar] [CrossRef]
- Marietou, A. Sulfate reducing microorganisms in high temperature oil reservoirs. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 116, pp. 99–131. [Google Scholar]
- Gao, P.K.; Fan, K.Y. Sulfur-oxidizing bacteria (SOB) and sulfate-reducing bacteria (SRB) in oil reservoir and biological control of SRB: A review. Arch. Microbiol. 2023, 205, 162. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, F.; Xu, T.; Liu, Y.L.; Du, Y.; Wang, C.; Liu, T.S.; Gao, J.; He, Y.L.; Wang, X.T.; et al. Culture-dependent and culture-independent methods reveal microbe-clay mineral interactions by dissimilatory iron-reducing bacteria in an integral oilfield. Sci. Total Environ. 2022, 840, 156577. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.Q.; Lu, Y.W.; Mbadinga, S.M.; Liu, Y.F.; Li, X.X.; Wang, B.; Lv, H.M.; Liu, J.F.; Yang, S.Z.; et al. Dominant and Active Methanogens in the Production Waters From a High-Temperature Petroleum Reservoir by DNA- and RNA-Based Analysis. Geomicrobiol. J. 2021, 38, 191–198. [Google Scholar] [CrossRef]
- Christman, G.D.; León-Zayas, R.I.; Summers, Z.M.; Biddle, J.F. Methanogens Within a High Salinity Oil Reservoir From the Gulf of Mexico. Front. Microbiol. 2020, 11, 570714. [Google Scholar] [CrossRef] [PubMed]
- Aitken, C.M.; Jones, D.M.; Maguire, M.J.; Gray, N.D.; Sherry, A.; Bowler, B.F.J.; Ditchfield, A.K.; Larter, S.R.; Head, I.M. Evidence that crude oil alkane activation proceeds by different mechanisms under sulfate-reducing and methanogenic conditions. Geochim. Cosmochim. Acta 2013, 109, 162–174. [Google Scholar] [CrossRef]
- Wentzel, A.; Ellingsen, T.E.; Kotlar, H.-K.; Zotchev, S.B.; Throne-Holst, M. Bacterial metabolism of long-chain n-alkanes. Appl. Microbiol. Biotechnol. 2007, 76, 1209–1221. [Google Scholar] [CrossRef]
- Aeckersberg, F.; Bak, F.; Widdel, F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 1991, 156, 5–14. [Google Scholar] [CrossRef]
- Liu, C.; Gu, R.; Zhu, L. Modified Biological Carbon Immobilized Polycyclic Aromatic Hydrocarbon Degradation Mixed Microbial Inoculum for Degrading Polycyclic Aromatic Hydrocarbon and Repairing Polycyclic Aromatic Hydrocarbon Contaminated Soil, Comprises Manganese Dioxide Modified Biological Carbon and Microorganisms Flora. CN115369108-A, 22 August 2022. [Google Scholar]
- Radice, R.P.; De Fabrizio, V.; Donadoni, A.; Scopa, A.; Martelli, G. Crude Oil Bioremediation: From Bacteria to Microalgae. Processes 2023, 11, 442. [Google Scholar] [CrossRef]
- Laczi, K.; Kis, A.E.; Szilagyi, A.; Bounedjoum, N.; Bodor, A.; Vincze, G.E.; Kovacs, T.; Rakhely, G.; Perei, K. New Frontiers of Anaerobic Hydrocarbon Biodegradation in the Multi-Omics Era. Front. Microbiol. 2020, 11, 590049. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 425106. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Li, K.-P.; Zhou, L.; Wang, L.-Y.; Yang, S.-Z.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Analysis of alkane-dependent methanogenic community derived from production water of a high-temperature petroleum reservoir. Appl. Microbiol. Biotechnol. 2012, 96, 531–542. [Google Scholar] [CrossRef]
- Ping, H.; Li, Z.; Zhi-Song, C.; Xiu-Chun, G.; Li, T. Isolation, identification and diversity analysis of petroleum-degrading bacteria in Shengli Oil Field wetland soil. Yingyong Shengtai Xuebao 2009, 20, 1202–1208. [Google Scholar]
- Zhou, L.; Li, K.-P.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Analyses of n-alkanes degrading community dynamics of a high-temperature methanogenic consortium enriched from production water of a petroleum reservoir by a combination of molecular techniques. Ecotoxicology 2012, 21, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, J.; Bai, Z.; Hecker, M.; Giesy, J.P. Distribution of petroleum degrading genes and factor analysis of petroleum contaminated soil from the Dagang Oilfield, China. Sci. Rep. 2015, 5, 11068. [Google Scholar] [CrossRef]
- Liu, B.; Ju, M.; Liu, J.; Wu, W.; Li, X. Isolation, identification, and crude oil degradation characteristics of a high-temperature, hydrocarbon-degrading strain. Mar. Pollut. Bull. 2016, 106, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Yu, C.; Wang, R.; Si, Y.; Masakorala, K.; Yuan, H.; Yao, J.; Zhang, J. Effects of oxygen injection on oil biodegradation and biodiversity of reservoir microorganisms in Dagang oil field, China. Int. Biodeterior. Biodegrad. 2015, 98, 59–65. [Google Scholar] [CrossRef]
- Kryachko, Y.; Dong, X.; Sensen, C.W.; Voordouw, G. Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie Van Leeuwenhoek 2012, 101, 493–506. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Caffrey, S.M.; Soh, J.; Agrawal, A.; Brown, D.; Budwill, K.; Dong, X.; Dunfield, P.F.; Foght, J.; Gieg, L.M. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ. Sci. Technol. 2013, 47, 10708–10717. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Clavijo, C.; Gieg, L.M. Conversion of crude oil to methane by a microbial consortium enriched from oil reservoir production waters. Front. Microbiol. 2014, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Penner, T.J.; Foght, J.M. Mature fine tailings from oil sands processing harbour diverse methanogenic communities. Can. J. Microbiol. 2010, 56, 459–470. [Google Scholar] [CrossRef]
- Siddique, T.; Penner, T.; Semple, K.; Foght, J.M. Anaerobic biodegradation of longer-chain n-alkanes coupled to methane production in oil sands tailings. Environ. Sci. Technol. 2011, 45, 5892–5899. [Google Scholar] [CrossRef]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Mochimaru, H.; Tamaki, H.; Yamamoto, K.; Yoshioka, H.; Suzuki, Y.; Kamagata, Y.; Sakata, S. Methane production from coal by a single methanogen. Science 2016, 354, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, Y.-N.; Evans, P.N.; Guo, Q.; Zhou, F.; Nie, M.; Jin, Q.; Zhang, Y.; Zhai, X.; Zhou, M. Evidence for nontraditional mcr-containing archaea contributing to biological methanogenesis in geothermal springs. Sci. Adv. 2023, 9, eadg6004. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, Y.Y.; Yao, H.Y.; Chapman, S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022, 823, 153636. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.Q.H.; Enault, F.; Midoux, C.; Mariadassou, M.; Chapleur, O.; Mazéas, L.; Loux, V.; Bouchez, T.; Krupovic, M.; Bize, A. Diversity of novel archaeal viruses infecting methanogens discovered through coupling of stable isotope probing and metagenomics. Environ. Microbiol. 2022, 24, 4853–4868. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Groussin, M.; Gouy, M.; Brochier-Armanet, C. The Molecular Determinants of Thermoadaptation: Methanococcales as a Case Study. Mol. Biol. Evol. 2021, 38, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.Z.; Huang, X.Y.; Ma, R.J.; Li, J.T.; Wang, F.P.; Jiao, N.Z.; Zhang, R. Potential metabolic and genetic interaction among viruses, methanogen and methanotrophic archaea, and their syntrophic partners. Isme Commun. 2022, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.F.A.; Day, L.A.; Costa, K.C. Formate-Dependent Heterodisulfide Reduction in a Methanomicrobiales Archaeon. Appl. Environ. Microbiol. 2021, 87, e02698-20. [Google Scholar] [CrossRef] [PubMed]
- Weidenbach, K.; Wolf, S.; Kupczok, A.; Kern, T.; Fischer, M.A.; Reetz, J.; Urbanska, N.; Künzel, S.; Schmitz, R.A.; Rother, M. Characterization of Blf4, an Archaeal Lytic Virus Targeting a Member of the Methanomicrobiales. Viruses 2021, 13, 1934. [Google Scholar] [CrossRef]
- Thomas, C.M.; Taib, N.; Gribaldo, S.; Borrel, G. Comparative genomic analysis of Methanimicrococcus blatticola provides insights into host adaptation in archaea and the evolution of methanogenesis. Isme Commun. 2021, 1, 47. [Google Scholar] [CrossRef]
- García-Maldonado, J.Q.; Latisnere-Barragán, H.; Escobar-Zepeda, A.; Cadena, S.; Ramírez-Arenas, P.J.; Vázquez-Juárez, R.; Rojas-Contreras, M.; López-Cortés, A. Revisiting Microbial Diversity in Hypersaline Microbial Mats from Guerrero Negro for a Better Understanding of Methanogenic Archaeal Communities. Microorganisms 2023, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Dridi, B.; Raoult, D.; Drancourt, M. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of Archaea: Towards the universal identification of living organisms. Apmis 2012, 120, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Brugère, J.F.; Borrel, G.; Gaci, N.; Tottey, W.; O’Toole, P.W.; Malpuech-Brugère, C. Archaebiotics Proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 2014, 5, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [PubMed]
- Gieg, L.M.; Davidova, I.A.; Duncan, K.E.; Suflita, J.M. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ. Microbiol. 2010, 12, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.; Hubert, C. Mechanisms and monitoring of oil reservoir souring control by nitrate or perchlorate injection. Microb. Communities Util. Hydrocarb. Lipids Memb. Metagenomics Ecophysiol. 2019, 225, 249. [Google Scholar]
- Fan, F.; Zhang, B.; Liu, J.; Cai, Q.; Lin, W.; Chen, B. Towards sulfide removal and sulfate reducing bacteria inhibition: Function of biosurfactants produced by indigenous isolated nitrate reducing bacteria. Chemosphere 2020, 238, 124655. [Google Scholar] [CrossRef]
- Stucki, G.; Hanselmann, K.W.; Hürzeler, R.A. Biological sulfuric acid transformation: Reactor design and process optimization. Biotechnol. Bioeng. 1993, 41, 303–315. [Google Scholar] [CrossRef]
- Overton, E.B.; Wade, T.L.; Radović, J.R.; Meyer, B.M.; Miles, M.S.; Larter, S.R. Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 2016, 29, 50–63. [Google Scholar] [CrossRef]
- Difan, H.; Jinchao, L.; Dajiang, Z. Maturation sequence of continental crude oils in hydrocarbon basins in China and its significance. Org. Geochem. 1990, 16, 521–529. [Google Scholar] [CrossRef]
- Olajire, A.; Essien, J. Aerobic degradation of petroleum components by microbial consortia. J. Pet. Environ. Biotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Wang, B.; Kuang, S.; Shao, H.; Wang, L.; Wang, H. Anaerobic-petroleum degrading bacteria: Diversity and biotechnological applications for improving coastal soil. Ecotoxicol. Environ. Saf. 2021, 224, 112646. [Google Scholar] [CrossRef]
- Rajbongshi, A.; Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World J. Microbiol. Biotechnol. 2021, 37, 111. [Google Scholar] [CrossRef]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2021, 157, 105156. [Google Scholar] [CrossRef]
- Biegert, T.; Fuchs, G.; Heider, J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 1996, 238, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Widdel, F. Utilization of alkylbenzenes during anaerobic growth of pure cultures of denitrifying bacteria on crude oil. Appl. Environ. Microbiol. 1996, 62, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Kniemeyer, O.; Fischer, T.; Wilkes, H.; Glöckner, F.O.; Widdel, F. Anaerobic degradation of ethylbenzene by a new type of marine sulfate-reducing bacterium. Appl. Environ. Microbiol. 2003, 69, 760–768. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Wang, L.-Y.; Zhou, L.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeterior. Biodegrad. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Agrawal, A.; Gieg, L.M. In situ detection of anaerobic alkane metabolites in subsurface environments. Front. Microbiol. 2013, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Herath, A.; Wawrik, B.; Qin, Y.; Zhou, J.; Callaghan, A.V. Transcriptional response of Desulfatibacillum alkenivorans AK-01 to growth on alkanes: Insights from RT-qPCR and microarray analyses. FEMS Microbiol. Ecol. 2016, 92, fiw062. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of volatile organic compounds and their effects on biodegradability under co-existing conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic aromatic hydrocarbons: A critical review of environmental occurrence and bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Boll, M.; Estelmann, S.; Heider, J. Anaerobic degradation of hydrocarbons: Mechanisms of hydrocarbon activation in the absence of oxygen. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 3–29. [Google Scholar]
- Abu Laban, N.; Selesi, D.; Rattei, T.; Tischler, P.; Meckenstock, R.U. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 2010, 12, 2783–2796. [Google Scholar] [CrossRef]
- Ji, J.-H.; Liu, Y.-F.; Zhou, L.; Irfan, M.; Mbadinga, S.M.; Pan, P.; Chen, J.; Liu, J.-F.; Yang, S.-Z.; Sand, W. Methanogenic biodegradation of C13 and C14 n-alkanes activated by addition to fumarate. Int. Biodeterior. Biodegrad. 2020, 153, 104994. [Google Scholar] [CrossRef]
- Gieg, L.M.; Toth, C.R. Signature metabolite analysis to determine in situ anaerobic hydrocarbon biodegradation. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 361–390. [Google Scholar]
- Tremblay, P.-L.; Zhang, T. Functional genomics of metal-reducing microbes degrading hydrocarbons. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2020; pp. 233–253. [Google Scholar]
- Bozinovski, D.; Herrmann, S.; Richnow, H.-H.; von Bergen, M.; Seifert, J.; Vogt, C. Functional analysis of an anaerobic m-xylene-degrading enrichment culture using protein-based stable isotope probing. FEMS Microbiol. Ecol. 2012, 81, 134–144. [Google Scholar] [CrossRef]
- Godin, S.; Kubica, P.; Ranchou-Peyruse, A.; Le Hecho, I.; Patriarche, D.; Caumette, G.; Szpunar, J.; Lobinski, R. An LC-MS/MS method for a comprehensive determination of metabolites of BTEX anaerobic degradation in bacterial cultures and groundwater. Water 2020, 12, 1869. [Google Scholar] [CrossRef]
- Marozava, S.; Mouttaki, H.; Müller, H.; Laban, N.A.; Probst, A.J.; Meckenstock, R.U. Anaerobic degradation of 1-methylnaphthalene by a member of the Thermoanaerobacteraceae contained in an iron-reducing enrichment culture. Biodegradation 2018, 29, 23–39. [Google Scholar] [CrossRef]
- Heider, J.; Szaleniec, M.; Suenwoldt, K.; Boll, M. Ethylbenzene dehydrogenase and related molybdenum enzymes involved in oxygen-independent alkyl chain hydroxylation. J. Mol. Microbiol. Biotechnol. 2016, 26, 45–62. [Google Scholar] [CrossRef]
- Aeckersberg, F.; Rainey, F.A.; Widdel, F. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 1998, 170, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, A.V.; Tierney, M.; Phelps, C.D.; Young, L. Anaerobic biodegradation of n-hexadecane by a nitrate-reducing consortium. Appl. Environ. Microbiol. 2009, 75, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, A.-K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Boll, M.; Heider, J.; Meckenstock, R.U.; Buckel, W.; Einsle, O.; Ermler, U.; Golding, B.T.; Gunsalus, R.P.; Kroneck, P.M. Anaerobic microbial degradation of hydrocarbons: From enzymatic reactions to the environment. Microb. Physiol. 2016, 26, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.M.; Rajendran, A.; Thangavelu, V. Recent advances in the biodegradation of phenol: A review. Asian J. Exp. Biol. Sci. 2010, 1, 219–234. [Google Scholar]
- Toth, C.R.; Berdugo-Clavijo, C.; O’Farrell, C.M.; Jones, G.M.; Sheremet, A.; Dunfield, P.F.; Gieg, L.M. Stable isotope and metagenomic profiling of a methanogenic naphthalene-degrading enrichment culture. Microorganisms 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Li, L.; Lin, C.; Dong, W.; Shen, W.; Yong, X.; Jia, H.; Wu, X.; Zhou, J. Anaerobic biodegradation of pyrene by Klebsiella sp. LZ6 and its proposed metabolic pathway. Environ. Technol. 2018, 41, 2130–2139. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, Y.; Fan, F.; Wang, Y.; Liu, X.; Ding, A.; Dou, J. Biodegradation of benzo (a) pyrene by Microbacterium sp. strain under denitrification: Degradation pathway and effects of limiting electron acceptors or carbon source. Biochem. Eng. J. 2017, 121, 131–138. [Google Scholar] [CrossRef]
- Ye, Q.; Liang, C.; Chen, X.; Fang, T.; Wang, Y.; Wang, H. Molecular characterization of methanogenic microbial communities for degrading various types of polycyclic aromatic hydrocarbon. J. Environ. Sci. 2019, 86, 97–106. [Google Scholar] [CrossRef]
- Bergmann, F.D.; Selesi, D.; Meckenstock, R.U. Identification of new enzymes potentially involved in anaerobic naphthalene degradation by the sulfate-reducing enrichment culture N47. Arch. Microbiol. 2011, 193, 241–250. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. Microb. Physiol. 2016, 26, 92–118. [Google Scholar] [CrossRef]
- Safinowski, M.; Meckenstock, R.U. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ. Microbiol. 2006, 8, 347–352. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Kumar, M.; Lin, J.-G. Anaerobic biotransformation of fluorene and phenanthrene by sulfate-reducing bacteria and identification of biotransformation pathway. J. Hazard. Mater. 2009, 164, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Risso, C.; Smith, J.A.; Lovley, D.R. Genome-scale analysis of anaerobic benzoate and phenol metabolism in the hyperthermophilic archaeon Ferroglobus placidus. ISME J. 2012, 6, 146–157. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Mouttaki, H. Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr. Opin. Biotechnol. 2011, 22, 406–414. [Google Scholar] [CrossRef]
- Selesi, D.; Meckenstock, R.U. Anaerobic degradation of the aromatic hydrocarbon biphenyl by a sulfate-reducing enrichment culture. FEMS Microbiol. Ecol. 2009, 68, 86–93. [Google Scholar] [CrossRef][Green Version]
- Kraiselburd, I.; Brüls, T.; Heilmann, G.; Kaschani, F.; Kaiser, M.; Meckenstock, R.U. Metabolic reconstruction of the genome of candidate Desulfatiglans TRIP_1 and identification of key candidate enzymes for anaerobic phenanthrene degradation. Environ. Microbiol. 2019, 21, 1267–1286. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, S.; Herbst, F.-A.; Bahr, A.; Duarte, M.; Pieper, D.H.; Jehmlich, N.; Seifert, J.; von Bergen, M.; Bombach, P.; Richnow, H.H. Anaerobic naphthalene degradation by sulfate-reducing Desulfobacteraceae from various anoxic aquifers. FEMS Microbiol. Ecol. 2015, 91, fiv006. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 2013, 4, 1998. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Rui, J.; Li, X. Research progress in biochemical metabolic pathways of methanogenesis. J. Appl. Environ. Biol. 2015, 21, 1–9. [Google Scholar]
- Herrmann, G.; Jayamani, E.; Mai, G.; Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 2008, 190, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Lie, T.J.; Costa, K.C.; Lupa, B.; Korpole, S.; Whitman, W.B.; Leigh, J.A. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 15473–15478. [Google Scholar] [CrossRef] [PubMed]
- Dolfing, J.; Larter, S.R.; Head, I.M. Thermodynamic constraints on methanogenic crude oil biodegradation. ISME J. 2008, 2, 442–452. [Google Scholar] [CrossRef]
- Duszenko, N.; Buan, N.R. Physiological evidence for isopotential tunneling in the electron transport chain of methane-producing archaea. Appl. Environ. Microbiol. 2017, 83, e00950-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-C.; Zhou, L.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Formate-dependent microbial conversion of CO2 and the dominant pathways of methanogenesis in production water of high-temperature oil reservoirs amended with bicarbonate. Front. Microbiol. 2016, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.-j.; Liu, P.-f.; Fu, L.; Laso-Pérez, R.; Yang, L.; Bai, L.-p.; Li, J.; Yang, M.; Lin, J.-z. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, S.; Xie, L. Effect of pH on anaerobic hydrogen consumption, methane production and microbial community at high temperature. Chem. Ind. Eng. Progress 2019, 38, 3816–3822. [Google Scholar]
- Waldron, P.J.; Petsch, S.T.; Martini, A.M.; Nüsslein, K. Salinity constraints on subsurface archaeal diversity and methanogenesis in sedimentary rock rich in organic matter. Appl. Environ. Microbiol. 2007, 73, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liang, B.; Wang, L.-Y.; Zhou, L.; Mbadinga, S.M.; Gu, J.-D.; Mu, B.-Z. Microbial reduction of CO2 from injected NaH13CO3 with degradation of n-hexadecane in the enrichment culture derived from a petroleum reservoir. Int. Biodeterior. Biodegrad. 2018, 127, 192–200. [Google Scholar] [CrossRef]
- Jiménez, N.; Richnow, H.H.; Vogt, C.; Treude, T.; Krüger, M. Methanogenic hydrocarbon degradation: Evidence from field and laboratory studies. Microb. Physiol. 2016, 26, 227–242. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]
- De Rezende, J.R.; Oldenburg, T.B.; Korin, T.; Richardson, W.D.; Fustic, M.; Aitken, C.M.; Bowler, B.F.; Sherry, A.; Grigoryan, A.; Voordouw, G. Anaerobic microbial communities and their potential for bioenergy production in heavily biodegraded petroleum reservoirs. Environ. Microbiol. 2020, 22, 3049–3065. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, L.; Mbadinga, S.M.; Gu, J.-D.; Mu, B.-Z. Accelerated CO2 reduction to methane for energy by zero valent iron in oil reservoir production waters. Energy 2018, 147, 663–671. [Google Scholar] [CrossRef]
- Hendrickson, E.L.; Haydock, A.K.; Moore, B.C.; Whitman, W.B.; Leigh, J.A. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. USA 2007, 104, 8930–8934. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, E.L.; Leigh, J.A. Roles of coenzyme F420-reducing hydrogenases and hydrogen-and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J. Bacteriol. 2008, 190, 4818–4821. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Hendrickson, E.L.; Zhang, Y.; Wang, T.; Taub, F.; Moore, B.C.; Porat, I.; Whitman, W.B.; Hackett, M.; Leigh, J.A. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol. Cell. Proteom. 2006, 5, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, T.; Hendrickson, E.L.; Lie, T.J.; Hackett, M.; Leigh, J.A. Quantitative proteomics of nutrient limitation in the hydrogenotrophic methanogen Methanococcus maripaludis. BMC Microbiol. 2009, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, A.V.; Wawrik, B.; Chadhain, S.M.N.; Young, L.Y.; Zylstra, G.J. Anaerobic alkane-degrading strain AK-01 contains two alkylsuccinate synthase genes. Biochem. Biophys. Res. Commun. 2008, 366, 142–148. [Google Scholar] [CrossRef]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of hydrocarbons in n-alkane-utilizing anaerobic bacteria. J. Mol. Microbiol. Biotechnol. 2016, 26, 138–151. [Google Scholar] [CrossRef]
- Zhu, J.; Shimizu, K. The effect of pfl gene knockout on the metabolism for optically pure D-lactate production by Escherichia coli. Appl. Microbiol. Biotechnol. 2004, 64, 367–375. [Google Scholar] [CrossRef]
- Grabarse, W.; Mahlert, F.; Shima, S.; Thauer, R.K.; Ermler, U. Comparison of three methyl-coenzyme M reductases from phylogenetically distant organisms: Unusual amino acid modification, conservation and adaptation. J. Mol. Biol. 2000, 303, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Lueders, T.; Chin, K.J.; Conrad, R.; Friedrich, M. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 2001, 3, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Schauer-Gimenez, A.; Bhattad, U.; Kearney, C.; Struble, C.A.; Zitomer, D.; Maki, J.S. Methyl coenzyme M reductase (mcrA) gene abundance correlates with activity measurements of methanogenic H2/CO2-enriched anaerobic biomass. Microb. Biotechnol. 2014, 7, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hochheimer, A.; Linder, D.; Thauer, R.K.; Hedderich, R. The molybdenum formylmethanofuran dehydrogenase operon and the tungsten formylmethanofuran dehydrogenase operon from Methanobacterium thermoautotrophicum: Structures and transcriptional regulation. Eur. J. Biochem. 1996, 242, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.; Thauer, R.K. The Na+-translocating methyltransferase complex from methanogenic archaea. Biochim. Biophys. Acta (BBA)-Bioenerg. 2001, 1505, 28–36. [Google Scholar] [CrossRef]
- Hedderich, R.; Whitman, W.B. Physiology and biochemistry of the methane-producing Archaea. Prokaryotes 2006, 2, 1050–1079. [Google Scholar]
- Darlington, R.B. Multiple regression in psychological research and practice. Psychol. Bull. 1968, 69, 161. [Google Scholar] [CrossRef]
- Johnson, J.W. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivar. Behav. Res. 2000, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Munandar, T.A.; Sumiati, S.; Rosalina, V. Pattern of symptom correlation on type of heart disease using approach of pearson correlation coefficient. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 022086. [Google Scholar] [CrossRef]
- Kumar, A.; Abirami, S. Aspect-based opinion ranking framework for product reviews using a Spearman’s rank correlation coefficient method. Inf. Sci. 2018, 460, 23–41. [Google Scholar]
- Yang, S.; Li, X.; Gong, Z.; Li, D.; Cheng, X.; Cai, W. New Principle of Pilot Protection Based on Kendall’s Rank Correlation Coefficient. IOP Conf. Ser. Mater. Sci. Eng. 2021, 827, 012016. [Google Scholar]
- Zhao, B.; Zhang, H.; Wang, H.; Wang, Z. Factors influencing gas well productivity in fractured tight sandstone reservoir. J. Pet. Explor. Prod. Technol. 2022, 12, 183–190. [Google Scholar] [CrossRef]
- Narayanan, C.; Narayan, V. Biological wastewater treatment and bioreactor design: A review. Sustain. Environ. Res. 2019, 29, 33. [Google Scholar] [CrossRef]
- Liu, J.; Bacosa, H.P.; Liu, Z. Potential Environmental Factors Affecting Oil-Degrading Bacterial Populations in Deep and Surface Waters of the Northern Gulf of Mexico. Front. Microbiol. 2017, 7, 2131. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Ali, S.A.; Calka, B.; Bielecka, E.; Linh, N.T.T.; Pham, Q.B. Urban flood vulnerability assessment in a densely urbanized city using multi-factor analysis and machine learning algorithms. Theor. Appl. Climatol. 2022, 149, 639–659. [Google Scholar] [CrossRef]
- Thiagarajan, N.; Kitchen, N.; Xie, H.; Ponton, C.; Lawson, M.; Formolo, M.; Eiler, J. Identifying thermogenic and microbial methane in deep water Gulf of Mexico Reservoirs. Geochim. Cosmochim. Acta 2020, 275, 188–208. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Z.; Jiang, Q.; Wang, J.; Zheng, J.; Zhang, T. Analysis of factors of productivity of tight conglomerate reservoirs based on random forest algorithm. ACS Omega 2022, 7, 20390–20404. [Google Scholar] [CrossRef]
- Qi, Y.; Cai, C.-F.; Sun, P.; Wang, D.-W.; Zhu, H.-J. Crude oil cracking in deep reservoirs: A review of the controlling factors and estimation methods. Pet. Sci. 2023, 20, 1978–1997. [Google Scholar] [CrossRef]
- Wu, Y.-c.; Feng, J.-w. Development and application of artificial neural network. Wirel. Pers. Commun. 2018, 102, 1645–1656. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Kumar, L.; Chugh, M.; Kumar, S.; Kumar, K.; Sharma, J.; Bharadvaja, N. Remediation of petrorefinery wastewater contaminants: A review on physicochemical and bioremediation strategies. Process Saf. Environ. Prot. 2022, 159, 362–375. [Google Scholar] [CrossRef]
- Haghsheno, H.; Arabani, M. Geotechnical properties of oil-polluted soil: A review. Environ. Sci. Pollut. Res. 2022, 29, 32670–32701. [Google Scholar] [CrossRef] [PubMed]
- De Bertoldi, M.d.; Vallini, G.; Pera, A. The biology of composting: A review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Bai, M.; Shen, A.; Meng, L.; Zhu, J.; Song, K. Well completion issues for underground gas storage in oil and gas reservoirs in China. J. Pet. Sci. Eng. 2018, 171, 584–591. [Google Scholar] [CrossRef]
- Zou, C.; Wu, S.; Yang, Z.; Pan, S.; Wang, G.; Jiang, X.; Guan, M.; Yu, C.; Yu, Z.; Shen, Y. Progress, challenge and significance of building a carbon industry system in the context of carbon neutrality strategy. Pet. Explor. Dev. 2023, 50, 210–228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).