An Untargeted Metabolomic Analysis of Lacticaseibacillus (L.) rhamnosus, Lactobacillus (L.) acidophilus, Lactiplantibacillus (L.) plantarum and Limosilactobacillus (L.) reuteri Reveals an Upregulated Production of Inosine from L. rhamnosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacterial Strains and Growth Conditions
2.2. Preparation of Cell-Free Supernatants (CFS) from Lactic Acid Bacterial Strains
2.3. Liquid Chromatography–Electrospray/High-Resolution Mass Spectrometry (HPLC-ESI/HRMS)
2.4. Compounds Discoverer Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Z. Health Promoting Activities of Probiotics. J. Food Biochem. 2019, 43, e12944. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and Intestinal Diseases: Mechanisms of Action and Clinical Applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of Lactic Acid Bacteria: Extending the Family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Murphy, E.F.; Clarke, S.F.; Marques, T.M.; Hill, C.; Stanton, C.; Ross, R.P.; O’Doherty, R.M.; Shanahan, F.; Cotter, P.D. Antimicrobials: Strategies for Targeting Obesity and Metabolic Health? Gut Microbes 2013, 4, 48–53. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu Y Abreu, A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The Clinical Evidence for Postbiotics as Microbial Therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Roman, R.; Last, A.; Mirhakkak, M.H.; Sprague, J.L.; Möller, L.; Großmann, P.; Graf, K.; Gratz, R.; Mogavero, S.; Vylkova, S.; et al. Lactobacillus rhamnosus Colonisation Antagonizes Candida albicans by Forcing Metabolic Adaptations That Compromise Pathogenicity. Nat. Commun. 2022, 13, 3192. [Google Scholar] [CrossRef]
- Thoda, C.; Touraki, M. Immunomodulatory Properties of Probiotics and Their Derived Bioactive Compounds. Appl. Sci. 2023, 13, 4726. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, L.; Sala, A.; Ardizzoni, A.; De Seta, F.; Singh, D.K.; Gacser, A.; Blasi, E.; Pericolini, E. Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro. Microbiol. Spectr. 2022, 10, e02696-21. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Cristofaro, V.; Sullivan, M.P.; Adam, R.M. Inosine—A Multifunctional Treatment for Complications of Neurologic Injury. Cell Physiol. Biochem. 2018, 49, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.H.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Züllig, T.; Zandl-Lang, M.; Trötzmüller, M.; Hartler, J.; Plecko, B.; Köfeler, H.C. A Metabolomics Workflow for Analyzing Complex Biological Samples Using a Combined Method of Untargeted and Target-List Based Approaches. Metabolites 2020, 10, 342. [Google Scholar] [CrossRef]
- Shafy, A.; Molinié, V.; Cortes-Morichetti, M.; Hupertan, V.; Lila, N.; Chachques, J.C. Comparison of the Effects of Adenosine, Inosine, and Their Combination as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction. ISRN Cardiol. 2012, 2012, 326809. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- De Almada, C.N.; De Almada, C.N.; De Souza Sant’Ana, A. Paraprobiotics as Potential Agents for Improving Animal Health. In Probiotics and Prebiotics in Animal Health and Food Safety; Di Gioia, D., Biavati, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 247–268. ISBN 978-3-319-71948-1. [Google Scholar]
- Vallejo-Cordoba, B.; Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A. Postbiotics and Paraprobiotics: A Review of Current Evidence and Emerging Trends. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 1–34. ISBN 978-0-12-820218-0. [Google Scholar]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, G.; Yang, X.-M.; Chen, G.-J.; Chen, H.-B.; Liao, Z.-L.; Zhong, Q.-P.; Wang, L.; Fang, X.; Wang, J. Transcriptomic Analysis Revealed Antimicrobial Mechanisms of Lactobacillus rhamnosus SCB0119 against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 15159. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Viñas, I.; Colás-Medà, P.; Collazo, C.; Serrano, J.C.E.; Abadias, M. Adhesion and Invasion of Listeria monocytogenes and Interaction with Lactobacillus rhamnosus GG after Habituation on Fresh-Cut Pear. J. Funct. Foods 2017, 34, 453–460. [Google Scholar] [CrossRef]
- Muyyarikkandy, M.S.; Amalaradjou, M. Lactobacillus Bulgaricus, Lactobacillus rhamnosus and Lactobacillus paracasei Attenuate Salmonella enteritidis, Salmonella Heidelberg and Salmonella typhimurium Colonization and Virulence Gene Expression In Vitro. Int. J. Mol. Sci. 2017, 18, 2381. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Bhunia, A.K. Salmonella enterica Serovar Typhimurium Adhesion and Cytotoxicity during Epithelial Cell Stress Is Reduced by Lactobacillus rhamnosus GG. Gut Pathog. 2009, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In Vitro Evaluation of Antimicrobial Activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against Pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-G.; Lee, S.-H. Inhibitory Effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida Biofilm of Denture Surface. Arch. Oral Biol. 2017, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 Exhibit Strong Antifungal Effects against Vulvovaginal Candidiasis-causing Candida glabrata Isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Alseth, I.; Dalhus, B.; Bjørås, M. Inosine in DNA and RNA. Curr. Opin. Genet. Dev. 2014, 26, 116–123. [Google Scholar] [CrossRef]
- Licht, K.; Hartl, M.; Amman, F.; Anrather, D.; Janisiw, M.P.; Jantsch, M.F. Inosine Induces Context-Dependent Recoding and Translational Stalling. Nucleic Acids Res. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Jing, Y.; Tabibiazar, R.; Jo, S.A.; Petrausch, B.; Stuermer, C.A.; Rosenberg, P.A.; Irwin, N. Axon Outgrowth Is Regulated by an Intracellular Purine-Sensitive Mechanism in Retinal Ganglion Cells. J. Biol. Chem. 1998, 273, 29626–29634. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.; Li, Y.-M.; O’Toole, J.E.; Benowitz, L.I. Mst3b, a Purine-Sensitive Ste20-like Protein Kinase, Regulates Axon Outgrowth. Proc. Natl. Acad. Sci. USA 2006, 103, 18320–18325. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Goldberg, D.E.; Madsen, J.R.; Soni, D.; Irwin, N. Inosine Stimulates Extensive Axon Collateral Growth in the Rat Corticospinal Tract after Injury. Proc. Natl. Acad. Sci. USA 1999, 96, 13486–13490. [Google Scholar] [CrossRef] [PubMed]
- Muto, J.; Lee, H.; Lee, H.; Uwaya, A.; Park, J.; Nakajima, S.; Nagata, K.; Ohno, M.; Ohsawa, I.; Mikami, T. Oral Administration of Inosine Produces Antidepressant-like Effects in Mice. Sci. Rep. 2014, 4, 4199. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Sitkovsky, M.V.; Szabó, C. Immunomodulatory and Neuroprotective Effects of Inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Kuhel, D.G.; Németh, Z.H.; Mabley, J.G.; Stachlewitz, R.F.; Virág, L.; Lohinai, Z.; Southan, G.J.; Salzman, A.L.; Szabó, C. Inosine Inhibits Inflammatory Cytokine Production by a Posttranscriptional Mechanism and Protects Against Endotoxin-Induced Shock. J. Immunol. 2000, 164, 1013–1019. [Google Scholar] [CrossRef]
- Liaudet, L.; Mabley, J.G.; Soriano, F.G.; Pacher, P.; Marton, A.; Haskó, G.; Szabó, C. Inosine Reduces Systemic Inflammation and Improves Survival in Septic Shock Induced by Cecal Ligation and Puncture. Am. J. Respir. Crit. Care Med. 2001, 164, 1213–1220. [Google Scholar] [CrossRef]


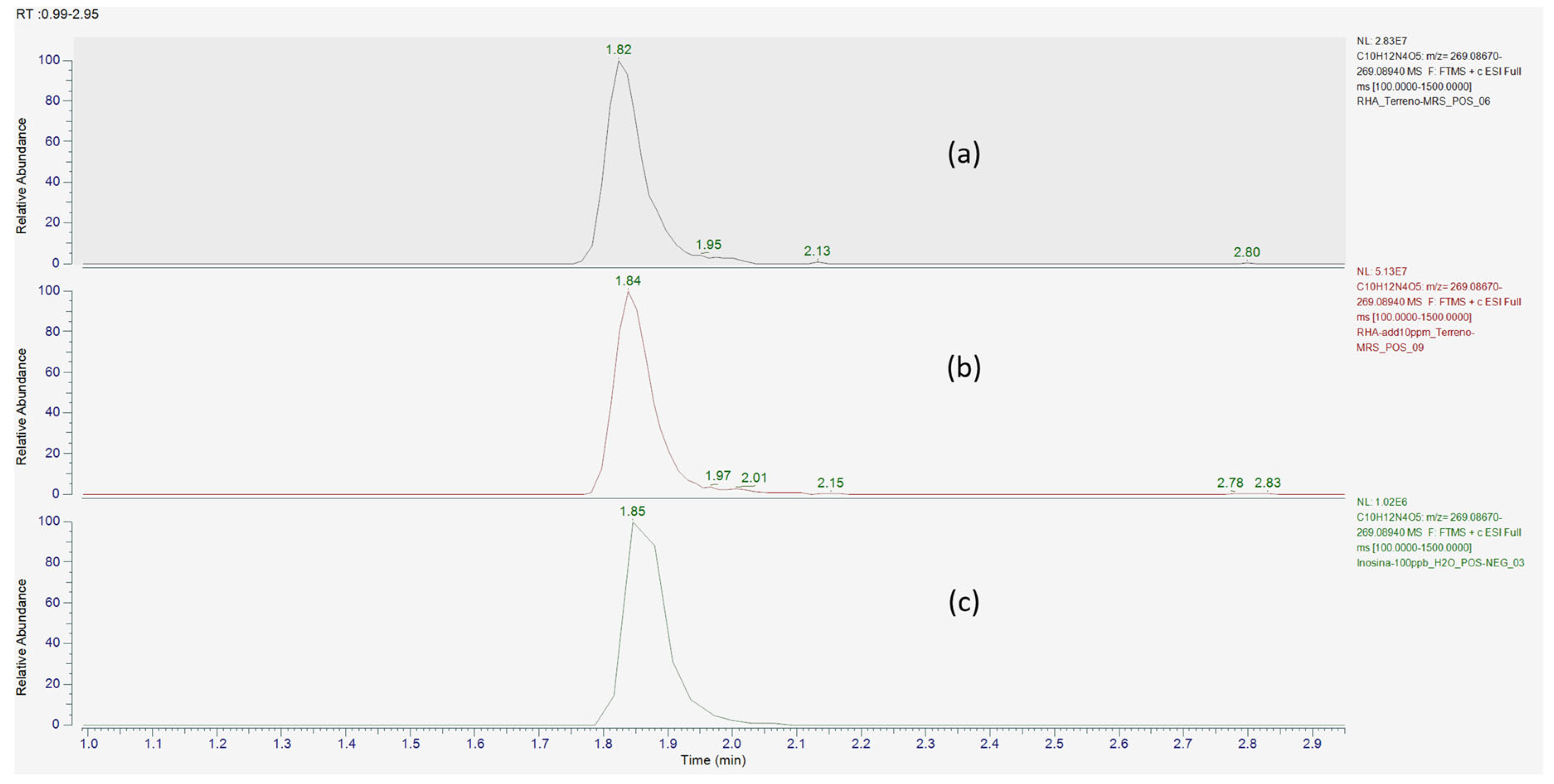
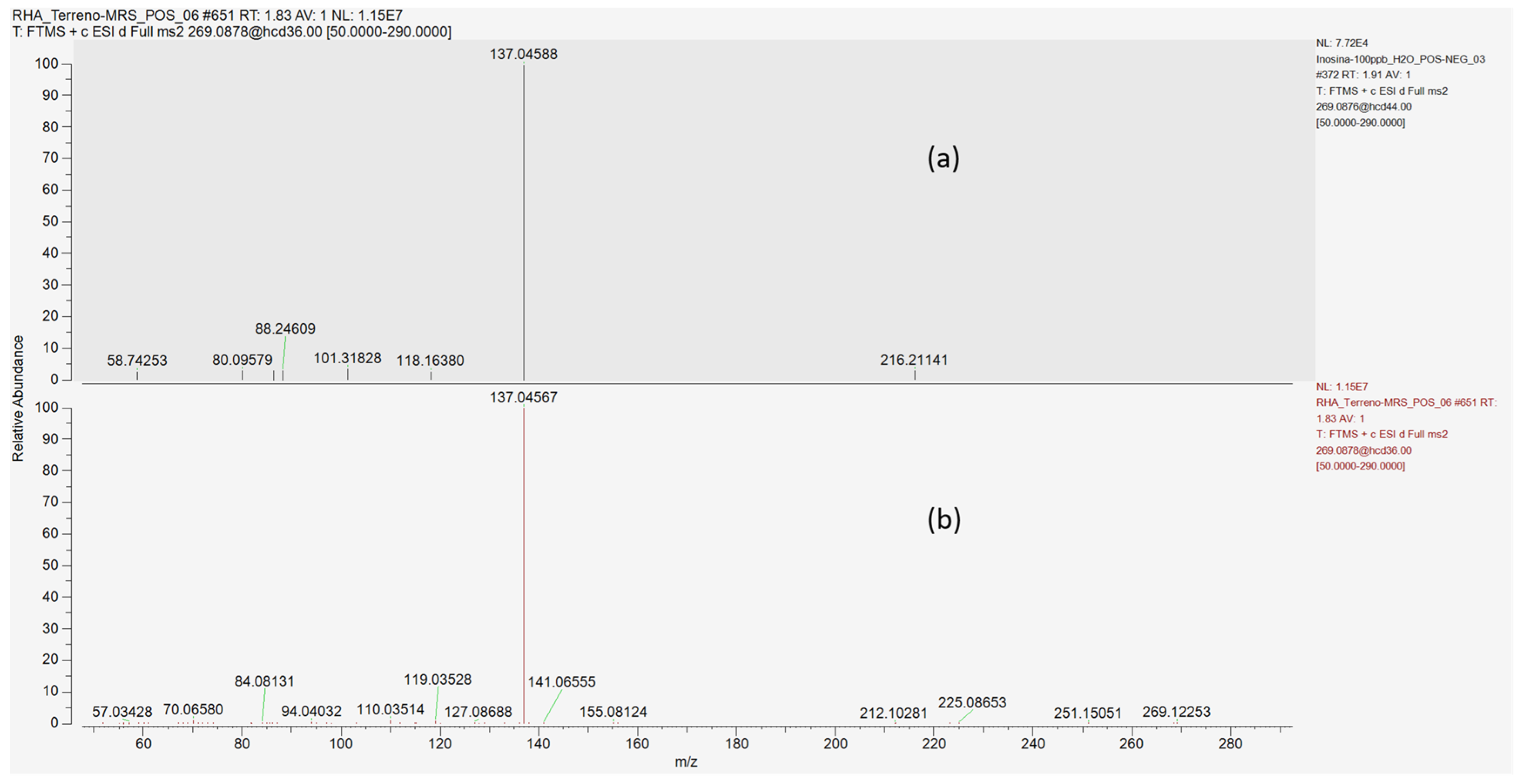
| Compound Name | Predicted Composition | mzCloud Search | mzValue Search | Metabolika Search | ChemSpider Search | MassList Search |
|---|---|---|---|---|---|---|
| Mevalonolactone | Full Match | Full Match | - | Partial Match | Full Match | Partial Match |
| N-α-L-Acetyl-arginine | Full Match | Full Match | - | - | Full Match | Full Match |
| (Ac)2-L-Lys-D-Ala | Full Match | - | - | - | Full Match | - |
| Inosine | Full Match | Full Match | Full Match | Full Match | Partial Match | Partial Match |
| (2S,4S)-4-Amino-2-hydroxy-2-methylpentanedioic acid | Full Match | - | - | - | Full Match | - |
| Confluenine A | Full Match | - | - | - | Partial Match | Full Match |
| Ala-Leu | Full Match | - | - | - | Full Match | - |
| O-Succinyl-L-homoserine | Full Match | - | - | Partial Match | Partial Match | Full Match |
| N-Acetyl-L-leucine | Full Match | Full Match | - | - | Full Match | Full Match |
| (+)-Flavipucine | Full Match | - | - | - | Partial Match | Full Match |
| 2-(2-amino-3-methylbutanamido)-3-phenylpropanoic acid | Full Match | Full Match | - | - | Partial Match | Partial Match |
| Acetyl-L-carnitine | Full Match | - | - | - | Partial Match | Full Match |
| methyl 4-(3,4-dihydroxybenzamido)butanoate | Full Match | - | - | - | - | Full Match |
| 3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one | Full Match | - | - | - | Partial Match | Full Match |
| (2S)-2-{[1-(R)-Carboxyethyl]amino}pentanoate | Full Match | - | - | - | Partial Match | Full Match |
| 2-Amino-5-methyl-5-hexencarbonsaeure | Full Match | - | - | - | Partial Match | Full Match |
| (1S,2R,8aS)-1,2-Dihydroxyindolizidine | Full Match | - | - | - | Full Match | Full Match |
| Compound Name | Predicted Composition | mzCloud Search | mzValue Search | Metabolika Search | ChemSpider Search | MassList Search |
|---|---|---|---|---|---|---|
| Acetylcholine | Full Match | Full Match | - | - | Partial Match | Full Match |
| N-Acetyl-S-2-hydroxyethyl-L-cysteine | Full Match | - | - | - | Full Match | - |
| Palmyrrolinone | Full Match | - | - | - | Partial Match | Full Match |
| Indole-3-lactic acid | - | Full Match | - | Partial Match | Full Match | Full Match |
| Gaburedin A | Full Match | - | - | - | Partial Match | Full Match |
| 3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one | Full Match | - | - | - | Partial Match | Full Match |
| Louisianin B | Full Match | - | - | Partial Match | Partial Match | Full Match |
| γ-L-glutamyl-L-leucine | - | - | - | - | - | Full Match |
| Sistodiolynne | Full Match | - | - | Partial Match | Partial Match | Full Match |
| Quinoline-2-methanol | Full Match | - | - | - | Partial Match | Full Match |
| Compound Name | Predicted Composition | mzCloud Search | mzValue Search | Metabolika Search | ChemSpider Search | MassList Search |
|---|---|---|---|---|---|---|
| Mevalonic acid | - | - | - | - | - | - |
| O-Succinyl-L-homoserine | Full Match | - | - | Partial Match | Partial Match | Full Match |
| N-Acetyl-D-alloisoleucine | - | - | - | - | - | - |
| O-Succinyl-L-homoserine | Full Match | - | - | Partial Match | Partial Match | Full Match |
| N-Acetylvaline | - | - | - | - | - | - |
| Birnbaumin A | Full Match | - | - | - | - | Full Match |
| methyl 4-(3,4-dihydroxybenzamido)butanoate | Full Match | - | - | - | - | Full Match |
| 3,11-dihydroxy-6,8-dimethyldodecanoic acid | Full Match | - | - | - | - | Full Match |
| Ala-Leu | - | - | - | - | Full Match | - |
| Taurochenodeoxycholic acid | - | - | - | - | - | - |
| Phenamide | Full Match | - | - | - | Partial Match | Full Match |
| Lorbamate | Full Match | - | - | - | Full Match | - |
| Pochonicine | Full Match | - | - | - | - | Full Match |
| Compound Name | Predicted Composition | mzCloud Search | mzValue Search | Metabolika Search | ChemSpider Search | MassList Search |
|---|---|---|---|---|---|---|
| 3-Phenyllactic acid | - | - | - | - | - | - |
| DL-4-Hydroxyphenyllactic acid | - | Full Match | Full Match | Full Match | - | - |
| 2-Hydroxycaproic acid | - | - | - | - | - | - |
| 2-Hydroxyvaleric acid | - | - | - | - | - | - |
| trans-Cinnamic acid | - | - | - | - | - | - |
| (+)-Flavipucine | Full Match | - | - | - | Partial Match | Full Match |
| Indole-3-lactic acid | - | Full Match | - | Partial Match | - | - |
| zidometacin | Full Match | - | - | - | Full Match | - |
| (+)-(17R)-apralactone A | Full Match | - | - | - | - | Full Match |
| 2-Oxoglutaric acid | - | - | - | - | - | - |
| N-Acetyl-L-glutamine | - | Full Match | Full Match | - | - | - |
| 2-Hydroxycinnamic acid | - | - | - | - | - | - |
| Pochonicine | Full Match | - | - | - | - | Full Match |
| Peniamidone B | Full Match | - | - | - | - | Full Match |
| 2-Methoxyestradiol | - | - | - | - | - | - |
| Hopantenic acid | Full Match | - | - | - | Full Match | - |
| Pantetheine | Full Match | - | - | - | Partial Match | - |
| methyl 4-(3,4-dihydroxybenzamido)butanoate | Full Match | - | - | - | - | Full Match |
| Compound Name | Formula | n° Identified Pathways | Pathways | Mapped Compounds | Matched Compounds | Compoundsin Pathways |
|---|---|---|---|---|---|---|
| Inosine | C10H12N4O5 | 1 | Superpathway of purine nucleotide salvage | 14 | 10 | 54 |
| 2 | Purine nucleotides degradation II (aerobic) | 12 | 8 | 27 | ||
| 3 | Purine nucleotides degradation I (plants) | 10 | 7 | 23 | ||
| 4 | Superpathway of purine degradation in plants | 10 | 7 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaggiari, L.; Pedretti, N.; Ricchi, F.; Pinetti, D.; Campisciano, G.; De Seta, F.; Comar, M.; Kenno, S.; Ardizzoni, A.; Pericolini, E. An Untargeted Metabolomic Analysis of Lacticaseibacillus (L.) rhamnosus, Lactobacillus (L.) acidophilus, Lactiplantibacillus (L.) plantarum and Limosilactobacillus (L.) reuteri Reveals an Upregulated Production of Inosine from L. rhamnosus. Microorganisms 2024, 12, 662. https://doi.org/10.3390/microorganisms12040662
Spaggiari L, Pedretti N, Ricchi F, Pinetti D, Campisciano G, De Seta F, Comar M, Kenno S, Ardizzoni A, Pericolini E. An Untargeted Metabolomic Analysis of Lacticaseibacillus (L.) rhamnosus, Lactobacillus (L.) acidophilus, Lactiplantibacillus (L.) plantarum and Limosilactobacillus (L.) reuteri Reveals an Upregulated Production of Inosine from L. rhamnosus. Microorganisms. 2024; 12(4):662. https://doi.org/10.3390/microorganisms12040662
Chicago/Turabian StyleSpaggiari, Luca, Natalia Pedretti, Francesco Ricchi, Diego Pinetti, Giuseppina Campisciano, Francesco De Seta, Manola Comar, Samyr Kenno, Andrea Ardizzoni, and Eva Pericolini. 2024. "An Untargeted Metabolomic Analysis of Lacticaseibacillus (L.) rhamnosus, Lactobacillus (L.) acidophilus, Lactiplantibacillus (L.) plantarum and Limosilactobacillus (L.) reuteri Reveals an Upregulated Production of Inosine from L. rhamnosus" Microorganisms 12, no. 4: 662. https://doi.org/10.3390/microorganisms12040662
APA StyleSpaggiari, L., Pedretti, N., Ricchi, F., Pinetti, D., Campisciano, G., De Seta, F., Comar, M., Kenno, S., Ardizzoni, A., & Pericolini, E. (2024). An Untargeted Metabolomic Analysis of Lacticaseibacillus (L.) rhamnosus, Lactobacillus (L.) acidophilus, Lactiplantibacillus (L.) plantarum and Limosilactobacillus (L.) reuteri Reveals an Upregulated Production of Inosine from L. rhamnosus. Microorganisms, 12(4), 662. https://doi.org/10.3390/microorganisms12040662










