Exploring Clinical Predictors of Severe Human Metapneumovirus Respiratory Tract Infections in Children: Insights from a Recent Outbreak
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Design
2.3. Methods
2.4. Analysis
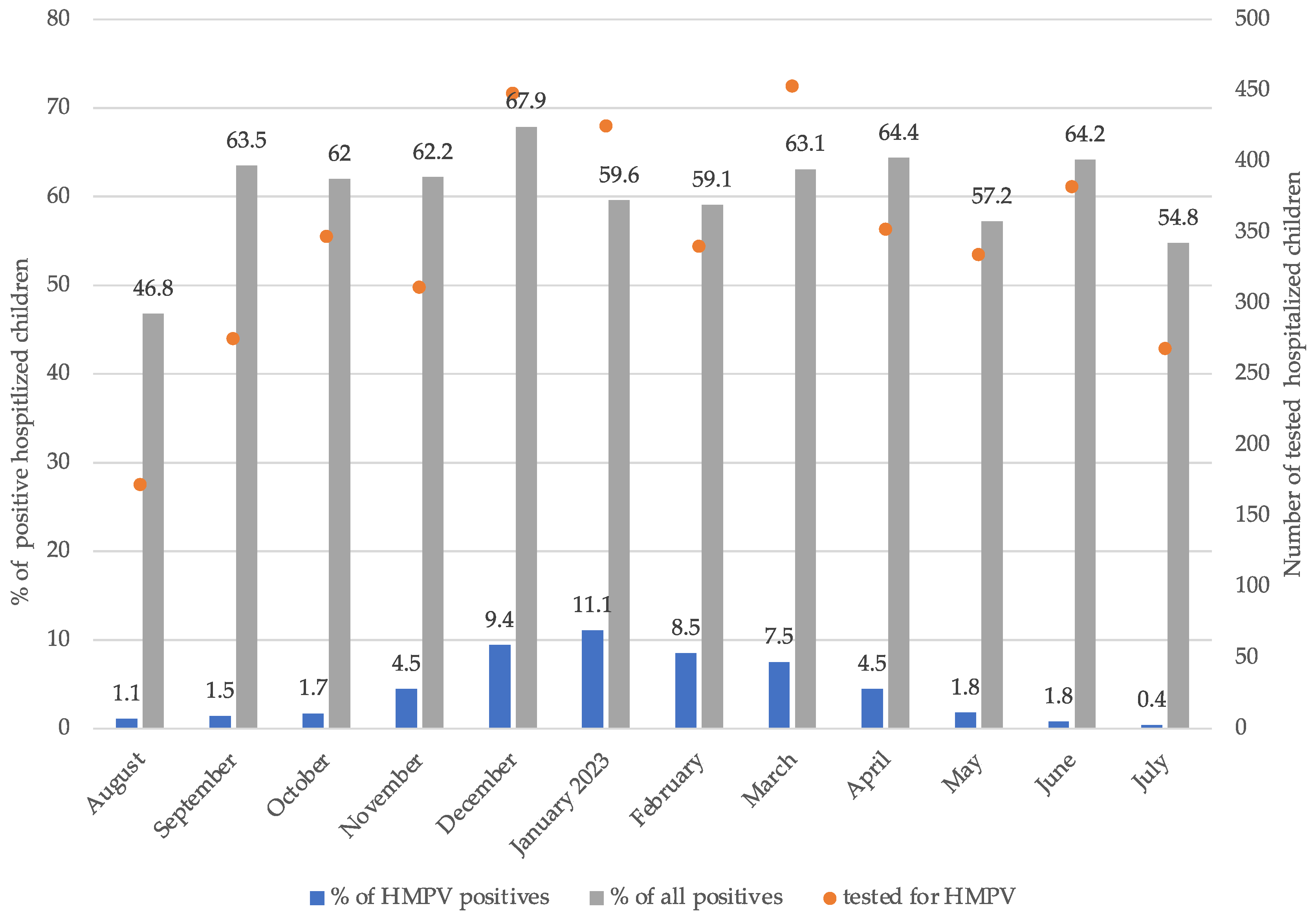
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panda, S.; Mohakud, N.K.; Pena, L.; Kumar, S. Human metapneumovirus: Review of an important respiratory pathogen. Int. J. Infect. Dis. 2014, 25, 45–52. [Google Scholar] [CrossRef]
- Van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, L.; Vankeerberghen, A.; Boel, A.; Van Vaerenbergh, K.; De Beenhouwer, H. Epidemiology of RSV and hMPV in Belgium: A 10-year follow-up. Acta Clin. Belg. 2019, 74, 229–235. [Google Scholar] [CrossRef]
- Van den Hoogen, B.G.; Van Doornum, G.J.J.; Fockens, J.C.; Cornelissen, J.J.; Beyer, W.E.P.; De Groot, R.; Osterhaus, A.D.; Fouchier, R.A. Prevalence and Clinical Symptoms of Human Metapneumovirus Infection in Hospitalized Patients. J. Infect. Dis. 2003, 188, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zou, L.; Peng, J.; Yu, J.; Song, Y.; Liang, L.; Guo, Q.; Kang, M.; Ke, C.; Song, T.; et al. Epidemiology, evolution and transmission of human metapneumovirus in Guangzhou China, 2013–2017. Sci. Rep. 2019, 9, 14022. [Google Scholar] [CrossRef] [PubMed]
- Schildgen, V.; van den Hoogen, B.; Fouchier, R.; Tripp, R.A.; Alvarez, R.; Manoha, C.; Williams, J.; Schildgen, O. Human metapneumovirus: Lessons learned over the first decade. Clin. Microbiol. Rev. 2011, 24, 734–754. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M.; Zhu, Y.; Griffin, M.R.; Weinberg, G.A.; Hall, C.B.; Szilagyi, P.G.; Staat, M.A.; Iwane, M.; Prill, M.M.; Williams, J.V.; et al. Burden of Human Metapneumovirus Infection in Young Children. N. Engl. J. Med. 2013, 368, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Mastrolia, M.V. Metapneumovirus Infections and Respiratory Complications. Semin. Respir. Crit. Care Med. 2016, 37, 512–521. [Google Scholar]
- García-García, M.L.; Calvo, C.; Casas, I.; Bracamonte, T.; Rellán, A.; Gozalo, F.; Tenorio, T.; Pérez-Breña, P. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr. Pulmonol. 2007, 42, 458–464. [Google Scholar] [CrossRef]
- Vicente, D.; Montes, M.; Cilla, G.; Pérez-Trallero, E. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg. Infect. Dis. 2004, 10, 1338–1339. [Google Scholar] [CrossRef]
- Perchetti, G.A.; Wilcox, N.; Chu, H.Y.; Katz, J.; Khatry, S.K.; LeClerq, S.C.; Tielsch, J.M.; Jerome, K.R.; Englund, J.A.; Kuypers, J. Human Metapneumovirus Infection and Genotyping of Infants in Rural Nepal. J. Pediatr. Infect. Dis. Soc. 2021, 10, 408–416. [Google Scholar] [CrossRef]
- Gaillard, E.A.; Kuehni, C.E.; Turner, S.; Goutaki, M.; Holden, K.A.; de Jong, C.C.M.; Lex, C.; Lo, D.K.H.; Lucas, J.S.; Midulla, F.; et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur. Respir. J. 2021, 58, 2004173. [Google Scholar] [CrossRef]
- Jevšnik Virant, M.; Uršič, T.; Kogoj, R.; Korva, M.; Petrovec, M.; Avšič-Županc, T. Evaluation of Two Broadly Used Commercial Methods for Detection of Respiratory Viruses with a Recently Added New Target for Detection of SARS-CoV-2. Viruses 2022, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; De Serres, G.; Côté, S.; Gilca, R.; Abed, Y.; Rochette, L.; Bergeron, M.G.; Déry, P. Human Metapneumovirus Infections in Hospitalized Children. Emerg. Infect. Dis. 2003, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nakamura, M.; Hirano, E.; Kiyota, N.; Omura, T.; Suzuki, Y.; Noda, M.; Kimura, H. Characteristics of Human Metapneumovirus Infection Prevailing in Hospital Wards Housing Patients with Severe Disabilities. Jpn. J. Infect. Dis. 2013, 66, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Harris, P.A.; Tollefson, S.J.; Halburnt-Rush, L.L.; Pingsterhaus, J.M.; Edwards, K.M.; Wright, P.F.; Crowe, J.E., Jr. Human Metapneumovirus and Lower Respiratory Tract Disease in Otherwise Healthy Infants and Children. N. Engl. J. Med. 2004, 350, 443. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, P.; Mikoś, M.; Bręborowicz, A.; Szczepankiewicz, A. Human bocavirus and metapneumovirus in acute wheezing in children—Is there a link with atopy? Clin. Respir. J. 2020, 14, 1201–1207. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. 2008, 178, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Bedolla-Barajas, M.; Montero, H.; Morales-Romero, J.; Landa-Cardeña, A.; Díaz, J.; Delgado-Figueroa, N.; Orozco-Alatorre, L.G. Prevalence of respiratory viruses in wheezing children not older than 24 months of age. Gac. Med. Mex. 2017, 153, 329–334. [Google Scholar]
- Kusel, M.M.H.; de Klerk, N.H.; Kebadze, T.; Vohma, V.; Holt, P.G.; Johnston, S.L.; Sly, P.D. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007, 119, 1105–1110. [Google Scholar] [CrossRef]
- Williams, J.V.; Crowe, J.E., Jr.; Enriquez, R.; Minton, P.; Peebles, R.S., Jr.; Hamilton, R.G.; Higgins, S.; Griffin, M.; Hartert, T.V. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 2005, 192, 1149–1153. [Google Scholar] [CrossRef]
- Williams, J.V.; Tollefson, S.J.; Heymann, P.W.; Carper, H.T.; Patrie, J.; Crowe, J.E. Human metapneumovirus infection in children hospitalized for wheezing. J. Allergy Clin. Immunol. 2005, 115, 1311–1312. [Google Scholar] [CrossRef]
- Furuta, T.; Hasegawa, S.; Mizutani, M.; Iwai, T.; Ohbuchi, N.; Kawano, S.; Tashiro, N.; Uchida, M.; Hasegawa, M.; Motoyama, M.; et al. Burden of Human Metapneumovirus and Respiratory Syncytial Virus Infections in Asthmatic Children. Pediatr. Infect. Dis. J. 2018, 37, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Kawada, J.I.; Go, K.; Fujishiro, N.; Hosokawa, Y.; Maki, Y.; Sugiyama, Y.; Suzuki, M.; Tsuji, T.; Hoshino, S.; et al. Comparison of Clinical Characteristics of Human Metapneumovirus and Respiratory Syncytial Virus Infections in Hospitalized Young Children. Jpn. J. Infect. Dis. 2019, 72, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.V.; Prince, G.A.; Gomez, A.M.; Kinkead, R.; Boivin, G. Human Metapneumovirus Infection Induces Long-Term Pulmonary Inflammation Associated with Airway Obstruction and Hyperresponsiveness in Mice. J. Infect. Dis. 2006, 193, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; van Den Hoogen, B.G.; Van Riel, D.A.J.; Laman, J.D.; Van Amerongen, G.; Sprong, L.; Fouchier, R.A.; Osterhaus, A.D. Experimental Human Metapneumovirus Infection of Cynomolgus Macaques (Macaca fascicularis) Results in Virus Replication in Ciliated Epithelial Cells and Pneumocytes with Associated Lesions throughout the Respiratory Tract. Am. J. Pathol. 2004, 164, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Tollefson, S.J.; Johnson, J.E.; Crowe, J.E. The Cotton Rat (Sigmodon hispidus) Is a Permissive Small Animal Model of Human Metapneumovirus Infection, Pathogenesis, and Protective Immunity. J. Virol. 2005, 79, 10944–10951. [Google Scholar] [CrossRef] [PubMed]
- Wyde, P.R.; Chetty, S.N.; Jewell, A.M.; Schoonover, S.L.; Piedra, P.A. Development of a cotton rat-human metapneumovirus (hMPV) model for identifying and evaluating potential hMPV antivirals and vaccines. Antivir. Res. 2005, 66, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.O.; Kozakewich, H.P.W.; Perez-Atayde, A.R.; McAdam, A.J. Pathology of human metapneumovirus infection: Insights into the pathogenesis of a newly identified respiratory virus. Pediatr. Dev. Pathol. 2004, 7, 478–486. [Google Scholar] [CrossRef]
- Kinder, J.T.; Moncman, C.L.; Barrett, C.; Jin, H.; Kallewaard, N.; Dutch, R.E. Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies. J. Virol. 2020, 94, e01068-20. [Google Scholar] [CrossRef]
- Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Soto, J.A.; Stranger, V.; Rivera, T.; Vásquez, A.E.; Kalergis, A.M. Host components that modulate the disease caused by hMPV. Viruses 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Thomas, B.J.; Zaid, A.; MacDonald, M.; Kan-O, K.; Rolph, M.S.; Soorneedi, A.R.; Bardin, P.G.; Mahalingam, S. Role of human metapneumovirus and respiratory syncytial virus in asthma exacerbations: Where are we now? Clin. Sci. 2017, 131, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | hMPV URTI (n = 28) | hMPV LRTI (n = 50) | Test Statistic, p-Value |
|---|---|---|---|
| Age (y), mean (SD) | 4.2 (SD 3.7) | 2.5 (SD 2.4) | t = 2.172, 0.036 |
| Male, no. (%) | 17 (61%) | 30 (60%) | χ2 = 0.004, 0.951 |
| Chronic disease, no. (%) | 8 (29%) | 1 (2%) | χ2 = 12.415, <0.001 |
| Viral co-infection, no. (%) | 14 (50%) | 19 (38%) | χ2 = 1.059, 0.303 |
| Atopy, no. (%) | 1 (4%) | 4 (8%) | χ2 = 0.587, 0.444 |
| Prematurity, no. (%) | 0 (0%) | 3 (6%) | χ2 = 1.747, 0.186 |
| Disease presentation | |||
| Fever, no. (%) | 19 (68%) | 41 (82%) | χ2 = 2.022, 0.155 |
| Fever duration (days), mean (SD) | 4 (SD 2) | 5 (SD 3) | t = -0.924, 0.359 |
| Rhinorrea, no. (%) | 18 (64%) | 43 (86%) | χ2 = 4.965, 0.026 |
| Cough, no. (%) | 23 (82%) | 49/50 (98%) | χ2 = 6.356, 0.012 |
| Dyspnea, no. (%) | 1 (4%) | 25 (50%) | χ2 = 17.411, <0.001 |
| Tachypnea, no. (%) | 1 (4%) | 29 (58%) | χ2 = 22.465, <0.001 |
| Laboratory results | |||
| CRP (mg/L), median (IQR) | 10.0 (IQR 5.0–41.0) | 42.0 (IQR 10.0–89.0) | U = 857.000, 0.034 |
| WBC (×109/L), mean (SD) | 11.1 (SD 5.6) | 12.9 (SD 5.3) | t = −1.457, 0.149 |
| Bacterial pneumonia, no. (%) | 3 (11%) | 20 (40%) | χ2 = 7.404, 0.007 |
| Systemic steroids, no. (%) | 0 (0%) | 7 (14%) | χ2 = 4.306, 0.038 |
| Hospitalization, no. (%) | 15 (54%) | 35 (70%) | χ2 = 2.105, 0.147 |
| Hospital stay (days), median (IQR) | 2 (IQR 1–4) | 4 (IQR 2–6) | U = 355.500, 0.046 |
| Causes of hospitalization | |||
| Dehydration, no. (%) | 3 (20%) | 17 (49%) | χ2 = 3.571, 0.059 |
| UTI, no. (%) | 4 (27%) | 1 (3%) | χ2 = 6.614, 0.010 |
| Febrile convulsions, no. (%) | 2 (13%) | 1 (3%) | χ2 = 2.043, 0.153 |
| Hypoxemia, no. (%) | 0 (0%) | 21 (42%) | χ2 = 16.093, <0.001 |
| ICU admission, no. (%) | 0 (0%) | 2 (4%) | χ2 = 1.149, 0.284 |
| Characteristic | hMPV LRTI Hypoxemia (n = 21) | hMPV LRTI No Hypoxemia (n = 29) | Test Statistic, p-Value |
|---|---|---|---|
| Age (y), mean (SD) | 2.6 (SD 2.6) | 2.5 (SD 2.3) | t = −0.078, 0.938 |
| Male, no. (%) | 14 (67%) | 16 (55%) | χ2 = 0.670, 0.413 |
| Chronic condition, no. (%) | 2 (10%) | 1 (3%) | χ2 = 0.797, 0.372 |
| Viral co-infection, no. (%) | 7 (33%) | 12 (41%) | χ2 = 0.335, 0.563 |
| Atopy, no. (%) | 4 (19%) | 0 (0%) | χ2 = 6.004, 0.014 |
| Prematurity, no. (%) | 2 (10%) | 1 (3%) | χ2 = 0.797, 0.372 |
| Fever, no. (%) | 17 (81%) | 24 (83%) | χ2 = 0.027, 0.870 |
| Fever duration (days), mean (SD) | 5 (SD 3) | 5 (SD 3) | t = 0.033, 0.974 |
| Dyspnea, no. (%) | 18 (86%) | 7 (24%) | χ2 = 18.473, <0.001 |
| Tachypnea, no. (%) | 19 (90%) | 10 (34%) | χ2 = 15.676, <0.001 |
| Asthma attack, no. (%) | 5 (24%) | 0 (0%) | χ2 = 7.672, 0.006 |
| Lung auscultation | |||
| Wheezing, (%) | 57% | 31% | χ2 = 3.408, 0.065 |
| Unilateral/bilateral crackles, (%) | 5%/86% | 36%/54% | χ2 = 6.797, 0.031 |
| CRP (mg/L), median (IQR) | 29.0 (IQR 10.0–59.5) | 56.0 (IQR 8.3–105.5) | U = 240.500, 0.279 |
| WBC (×109/L), mean (SD) | 11.1 (SD 4.5) | 14.2 (SD 5.6) | t = 2.133, 0.038 |
| X-ray findings | |||
| Infiltrates, no. (%) | 15/16 (94%) | 15/16 (94%) | χ2 = 0.000, 1.000 |
| Effusion, no. (%) | 2/16 (13%) | 2/16 (13%) | χ2 = 0.000, 1.000 |
| Bacterial pneumonia, no. (%) | 9 (43%) | 11 (38%) | χ2 = 0.123, 0.726 |
| Systemic steroids, no. (%) | 6 (29%) | 1 (3%) | χ2 = 6.385, 0.012 |
| Hospitalization, no. (%) | 21 (100%) | 14 (48%) | χ2 = 15.517, <0.001 |
| Hospital stay (days), median (IQR) | 4 (IQR 3–7) | 2 (IQR 2–5) | U = 200.000, 0.077 |
| ICU admission, no. (%) | 1 (5%) | 1 (3%) | χ2 = 0.055, 0.815 |
| Characteristic | hMPV LRTI Hypoxemia No Viral Codetection (n = 14) | hMPV LRTI No Hypoxemia No Viral Codetection (n = 17) | Test Statistic, p-Value |
|---|---|---|---|
| Age (y), mean (SD) | 2.9 (SD 2.9) | 3.1 (SD 2.6) | t = 0.189, 0.851 |
| Male, no. (%) | 8 (57%) | 12 (71%) | χ2 = 0.606, 0.436 |
| Chronic condition, no. (%) | 1 (7%) | 1 (6%) | χ2 = 0.020, 0.887 |
| Atopy, no. (%) | 3 (21%) | 0 (0%) | χ2 = 4.033, 0.045 |
| Prematurity, no. (%) | 1 (7%) | 1 (6%) | χ2 = 0.020, 0.887 |
| Fever, no. (%) | 11 (79%) | 14 (82%) | χ2 = 0.070, 0.791 |
| Fever duration (days), mean (SD) | 5 (SD 3) | 4 (SD 3) | t = −0.507, 0.617 |
| Dyspnea, no. (%) | 12 (86%) | 3 (18%) | χ2 = 14.243, <0.001 |
| Tachypnea, no. (%) | 13 (93%) | 5 (29%) | χ2 = 12.692, <0.001 |
| Asthma attack, no. (%) | 3 (21%) | 0 (0%) | χ2 = 4.033, 0.045 |
| Lung auscultation | |||
| Wheezing, (%) | 64% | 12% | χ2 = 9.251, 0.002 |
| Unilateral/bilateral crackles, (%) | 7%/86% | 56%/38% | χ2 = 8.304, 0.016 |
| CRP (mg/L), median (IQR) | 34.5 (IQR 5.8–82.3) | 33.0 (IQR 9.0–112.0) | U = 100.000, 0.468 |
| WBC (×109/L), mean (SD) | 10.6 (SD 4.4) | 14.7 (SD 6.5) | t = 2.023, 0.052 |
| Bacterial pneumonia, no. (%) | 6 (43%) | 9 (53%) | χ2 = 0.313, 0.576 |
| Systemic steroids, no. (%) | 4 (29%) | 0 (0%) | χ2 = 5.577, 0.018 |
| Hospitalization, no. (%) | 14 (100%) | 11 (65%) | χ2 = 6.127, 0.013 |
| Hospital stay (days), median (IQR) | 5 (IQR 4–8) | 2 (IQR 2–5) | U = 109.500, 0.075 |
| ICU admission, no. (%) | 1 (7%) | 1 (6%) | χ2 = 0.020, 0.887 |
| p-Value | Odds Ratio (OR) | 95% CI for OR | |
|---|---|---|---|
| Age | 0.329 | 1.31 | 0.77–2.22 |
| Gender | 0.347 | 2.80 | 0.33–23.89 |
| Wheezing | 0.026 | 10.32 | 1.33–80.40 |
| Bilateral crackles | 0.111 | 7.11 | 0.64–79.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronese, A.; Uršič, T.; Bizjak Vojinovič, S.; Rodman Berlot, J. Exploring Clinical Predictors of Severe Human Metapneumovirus Respiratory Tract Infections in Children: Insights from a Recent Outbreak. Microorganisms 2024, 12, 641. https://doi.org/10.3390/microorganisms12040641
Veronese A, Uršič T, Bizjak Vojinovič S, Rodman Berlot J. Exploring Clinical Predictors of Severe Human Metapneumovirus Respiratory Tract Infections in Children: Insights from a Recent Outbreak. Microorganisms. 2024; 12(4):641. https://doi.org/10.3390/microorganisms12040641
Chicago/Turabian StyleVeronese, Airin, Tina Uršič, Simona Bizjak Vojinovič, and Jasna Rodman Berlot. 2024. "Exploring Clinical Predictors of Severe Human Metapneumovirus Respiratory Tract Infections in Children: Insights from a Recent Outbreak" Microorganisms 12, no. 4: 641. https://doi.org/10.3390/microorganisms12040641
APA StyleVeronese, A., Uršič, T., Bizjak Vojinovič, S., & Rodman Berlot, J. (2024). Exploring Clinical Predictors of Severe Human Metapneumovirus Respiratory Tract Infections in Children: Insights from a Recent Outbreak. Microorganisms, 12(4), 641. https://doi.org/10.3390/microorganisms12040641








