Abstract
Despite the introduction of the pneumococcal vaccine, Streptococcus pneumoniae remains a cause of invasive diseases in Brazil. This study provides the distribution of serotypes and antimicrobial susceptibility patterns for pneumococcal isolates before and during the years of the COVID-19 pandemic in two age groups, <5 and ≥50 years. This is a national laboratory-based surveillance study that uses data from the Brazilian national laboratory for invasive S. pneumoniae from the pre-COVID-19 (January 2016 to January 2020) and COVID-19 (February 2020 to May 2022) periods. Antimicrobial resistance was evaluated by disk diffusion and minimum inhibitory concentration. The year 2020 was marked by a 44.6% reduction in isolates received and was followed by an upward trend from 2021 onwards, which became evident in 2022. No differences were observed in serotypes distribution between the studied periods. The COVID-19 period was marked by the high prevalence of serotypes 19A, 3, and 6C in both age groups. Serotypes 19A and 6C were related to non-antimicrobial susceptibility. We observed a reduction in S. pneumoniae, without changes in serotypes distribution and epidemiological capsular switch during the COVID-19 period. We observed elevated resistance rates, mainly to penicillin and ceftriaxone for non-meningitis cases in children under 5 years of age.
1. Introduction
Despite the widespread use of pneumococcal conjugate vaccines (PCVs) in immunization programs, S. pneumoniae continues to cause severe invasive diseases such as meningitis, sepsis, and bacteremic pneumonia, which is associated with high morbidity and mortality worldwide among all age groups, especially among young children and the elderly [1].
S. pneumoniae is a respiratory-borne pathogen that colonizes the upper respiratory tract of humans. Its transmission occurs through close contact between people and through respiratory tract material, such as saliva droplets or aerosols. S. pneumoniae also has several virulence factors that are crucial for its transmission, colonization, and invasion [1]. One of these factors is the polysaccharide capsule, which defines pneumococcal serotypes, induces serotype-specific protective immunity, and is, therefore, the antigen of the pneumococcal vaccines. To date, around 100 pneumococcus serotypes have been described, all of which have the potential to cause disease, although some are more invasive than others [2]. Currently available pneumococcal vaccine formulations contain multiple prevalent antigens from 10, 13, 15, 20, and 23 serotypes [3,4,5,6,7]. In Brazil, the 10-valent PCV was introduced in the national childhood immunization program in 2010 [8] and is still used in routine immunization [9].
The efforts to control the COVID-19 pandemic interfered with the dynamics of transmission of respiratory diseases, modifying the seasonality of S. pneumoniae respiratory diseases associated with an elevated incidence in the winter periods. Non-pharmacological measures to control COVID-19 have deeply reduced S. pneumoniae transmission and contagion, changing the global epidemiology of invasive pneumococcal disease (IPD) [10]. In Brazil, from the pre-COVID period (2016 to 2019) to the pandemic period (considering the years 2020 and 2021), the average annual incidence of confirmed pneumococcal meningitis declined from 0.47 to 0.17 per 100,000 inhabitants, according to data from the Notifiable Diseases Information System. In 2022, the levels returned to the pre-COVID levels, reaching 0.54 per 100,000 inhabitants (Supplementary Figure S1).
Bacterial co-infection in patients with SARS-CoV-2 appears to be uncommon, such as that caused by S. pneumoniae [11,12]. The empirical use of antibiotics as a therapeutic strategy, particularly azithromycin, largely used in hospitalized patients with COVID-19 in Brazil [12,13], could have had an impact on increasing resistance to antibiotics.
Given the progress of the COVID-19 pandemic in Brazil and its impact on IPD, the present study aimed to assess the distribution of serotypes and antimicrobial susceptibility patterns for pneumococcal isolates before and during the years of the COVID-19 pandemic, exploring data from the Brazilian national laboratory surveillance for Streptococcus pneumoniae.
2. Materials and Methods
Supplementary Figure S2 summarizes the methodology used in this study.
2.1. Study Design and Population
This is a national laboratory-based surveillance study conducted from January 2016 to May 2022 using data from the national laboratory for public health surveillance of IPD in Brazil. S. pneumoniae isolates recovered from normally sterile clinical specimens (mainly for cerebrospinal fluid, blood, and pleural fluid) were collected in public health laboratories and hospitals across the country and were routinely sent in a culture medium (blood agar or chocolate agar) to the Centre of Bacteriology at the Institute Adolfo Lutz (IAL), the Brazilian National Reference Laboratory for meningitis and IPD. This network is coordinated by the Brazilian Ministry of Health and encompasses 26 public health laboratories located in each of the Brazilian states, covering the whole country [14]. The isolates were sent to the IAL, along with data on age, gender, clinical diagnosis of the patient, and municipality where the sample was collected.
2.2. Microbiology Methods
All pneumococcal IPD isolates were confirmed at the IAL using standard methods [15]. Briefly, the S. pneumoniae isolates were cultured on sheep blood agar plates for 18–24 h on 5% CO2. The species were identified by the susceptibility on the optochin test and the solubility on the bile solubility test. S. pneumoniae were serotyped by means of the Pneumotest-latex agglutination kit and the Quellung reaction using antisera, both from the Statens Serum Institute (Copenhagen, Denmark). Non-typeable (NT) isolates identified by Quellung were also verified by sequential multiplex PCR; deduction of serotypes/serogroups was achieved using 41 primer pairs for serotype/serogroup discrimination plus a primer pair for S. pneumoniae capsule identification (cps gene) using nine sequential reactions following the gene targets and protocols described by the Center for Diseases Control and Prevention (CDC), Atlanta, GA, USA [16,17]. In summary, the reactions were performed on eight sequential reactions (each with five primer pairs to serotype/serogroup plus the cps gene primer) and one reaction to discriminate serogroup 6 (with two primer pairs to identify serotypes 6A/B and 6C/D plus the cps gene primer).
Susceptibility to oxacillin 1 μg (screening for susceptibility to penicillin and ceftriaxone), erythromycin 15 μg, clindamycin 2 μg, vancomycin 30 μg, levofloxacin 5 μg, sulfamethoxazole-trimethoprim 23.75–1.25 μg, and tetracycline 30 μg was assessed by means of the disk diffusion method as recommended by the Clinical Laboratory Standards Institute guidelines (CLSI) [18,19,20]. Isolates presenting with oxacillin resistance (zone of inhibition ≤ 19 mm) were analyzed for their minimum inhibitory concentration (MIC) by broth microdilution to penicillin (8–0.015 μg/mL) and ceftriaxone (4–0.003 μg/mL). The S. pneumoniae ATCC49619 strain was used as a reference. Interpretative breakpoints to antimicrobial agents (susceptible, intermediate, or resistant) were based on the CLSI guidelines of 2022 [18]. Intermediate and resistant isolates were defined as non-susceptible. Isolates resistant to at least three classes of antibiotics were defined as multidrug-resistant (MDR).
2.3. Data Analysis
For this investigation, data on S. pneumoniae isolates, associated serotypes, and antimicrobial resistance were extracted from the IAL database in Brazil. Duplicate isolates of S. pneumoniae from the same patient were excluded from the analysis.
The distribution of serotypes and the susceptibility rates to antimicrobials were evaluated in two study periods, the pre-COVID-19 period (January 2016–January 2020) and the COVID-19 pandemic period (February 2020–May 2022), in two age groups, <5 and ≥50 years old. Non-susceptibility to penicillin and ceftriaxone was analyzed considering meningitis and non-meningitis.
The proportion of serotypes included in each PCV (PCV10, PCV13, PCV15, PCV20, and PCV24) was calculated by the proportion of isolates included in each formulation divided by the total number of invasive isolates, by age group and study period.
Hypothesis testing comparing changes between the COVID-19 period and the pre-COVID-19 reference period in the proportion of serotypes included in the vaccine formulations or the proportion of non-susceptibility to antimicrobials was performed with the chi-square test or Fisher’s exact when appropriate. Given that 30 separate hypothesis tests were carried out in this study, to counteract the problem of multiple comparisons, the Holm–Bonferroni method was used. The K index identified by the method was 0.002, that is, only p values equal to or smaller than this value rejected the null hypothesis. It is important to highlight that, although the Holm–Bonferroni method is considered “uniformly” more powerful than the classical Bonferroni correction, it is still a conservative test; therefore, discretion is recommended in interpreting the p-values presented.
2.4. Ethical Aspects
The S. pneumoniae isolates’ data studied here came from a retrospective collection of bacterial isolates gathered by the Institute Adolfo Lutz as part of national routine surveillance activities. Patient information was obtained through routine clinical care procedures, and, during this study’s analysis, patient identification was completely anonymous. No human or animal tissue or any other biological material was used in this study. This study was approved by the Institutional Review Board, Technical and Scientific Council (CTC40N-2021) of the Institute Adolfo Lutz (São Paulo, Brazil). According to law No. 11794/2008, this study does not require evaluation from the Institute Adolfo Lutz’s Independent Ethics Committee (CEPIAL) or the Independent Ethics Committee on the Use of Experimental Animals of the Institute Adolfo Lutz (CEUA/IAL) since it did not involve any research with human beings or animals and has no ethical implications.
3. Results
3.1. S. pneumoniae Sample
From January 2016 to May 2022, the IAL received a total of 4766 S. pneumoniae isolates of IPD. Of those, 287 (6%) were duplicate isolates from the same patient, plus 1708 (35.8%) isolates from the age group 5 to 49 years old were excluded from the analysis. Therefore, 2771 S. pneumoniae isolates were included in this study, and each was classified as an IPD case; 2004 (72.3%) were classified in the pre-COVID-19 period and 767 (27.7%) in the COVID-19 period. A total of 952 (34.4%) isolates were classified in the <5-year-old and 1819 (65.6%) in the ≥50-year-old group. A total of 884 (31.9%) isolates were from meningitis cases, and 1887 (68.1%) were from non-meningitis cases (Supplementary Table S1).
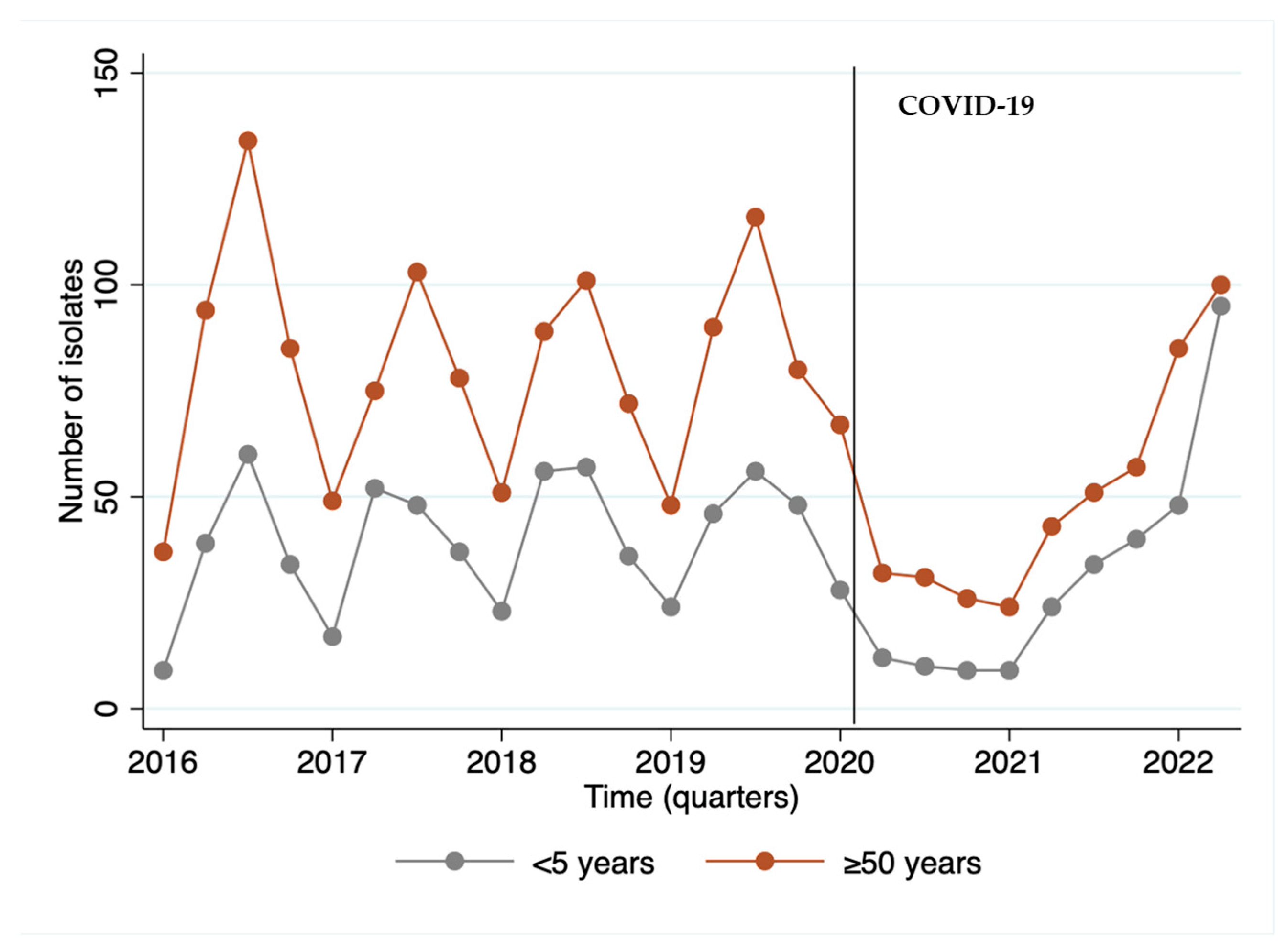
We observed that the year 2020, the beginning of the COVID-19 period, was marked by a reduction of 44.6% in the invasive isolates received by the IAL, with a disruption in seasonality, followed by an upward trend starting in 2021 and becoming more evident in 2022. Remarkably, the largest number of isolates from children occurred in the last quarter of the series, when post-COVID-19 cases resumed (Figure 1).
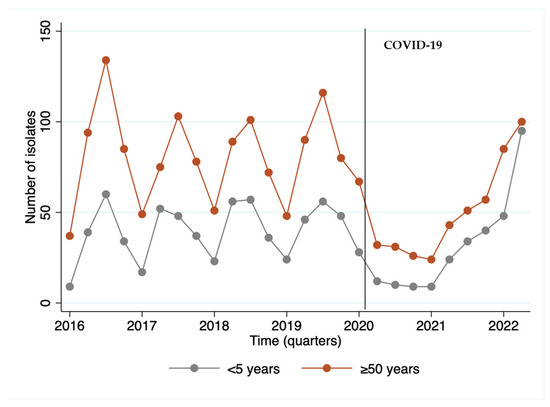
Figure 1.
Number of invasive S. pneumoniae isolates (N = 2.771) per quarter of the period from 2016 to May of 2022 by age group.
3.2. Serotype Distribution
The diversity of the serotypes is displayed by the presence of 62 serotypes associated with IPD, 60 in the pre-COVID-19 period and 50 in the COVID-19 period. The distribution according to study periods and age group is presented for the <5-year-old group (Table 1) and the ≥50-year-old group (Table 2). There was no significant change between the COVID-19 period and the reference pre-COVID-19 period in the proportion of serotypes included or not in the PCV10 vaccine. In both age groups and both periods, serotypes 19A, 3, and 6C were those with the greatest contributions. When analyzed separately, there was a statistically significant increase in the contribution of serotype 19A between the periods for children aged 5 and under (from 37.2% to 48.1%; p-value: 0.001) and a non-significant increase in adults aged 50 years and over (from 12.5% to 17.8%, p-value: 0.004) when using the Holm–Bonferroni significance-level correction.

Table 1.
Distribution of and percentage change in invasive S. pneumoniae serotypes in <5-year-old group by period studied.

Table 2.
Distribution of and percentage change in invasive S. pneumoniae serotypes in ≥50-year-old group by period studied.
The analysis of the cumulative percentage obtained by summing the serotypes corresponding to the different vaccine formulations in each age group showed a trend towards increased protection against IPD, caused by the additional serotypes included in these higher-valence vaccines, independently of the age group or period. This occurred mainly due to the addition of serotypes 19A (n = 637/2763, 23.0%) and 3 (n = 333/2763, 12.0%), the most frequently identified in this study. Although there is a percentage increase in the contribution of serotypes contained in vaccines with a higher valence than PCV10 between the pre-COVID and COVID periods, both for children and adults over 50 years of age, considering the level of significance corrected for the multiplicity of hypothesis tests (≤0.002), none of these increases would be considered statistically significant (Table 3).

Table 3.
Distribution of the invasive S. pneumoniae serotypes by vaccine formulations (PCV10, PCV13, PCV15, PCV20, and PCV24) by age group. Hypothesis testing of the difference in distribution between periods by formulation and age group.
3.3. Antimicrobial Resistance
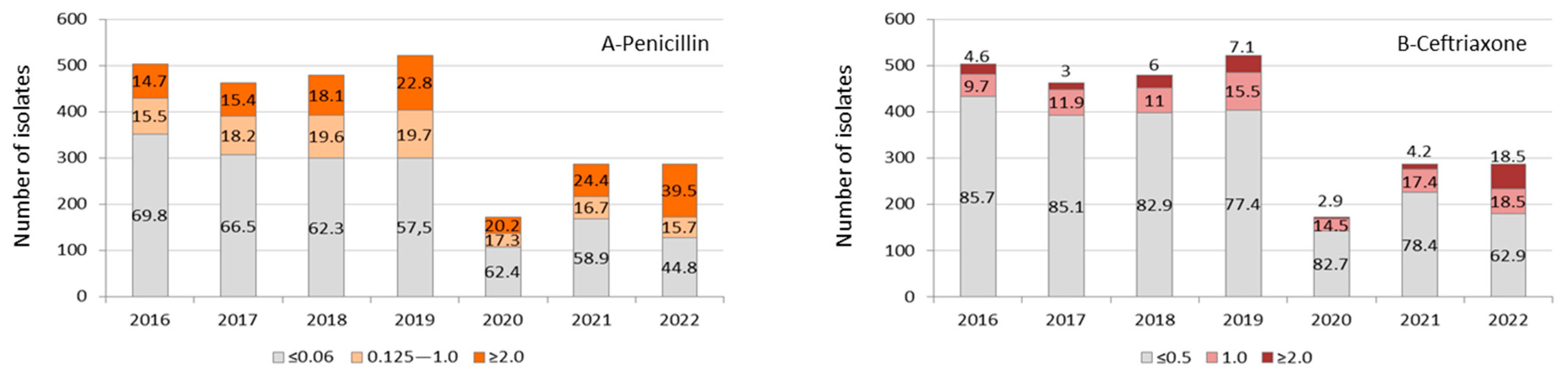
Except for the year 2020 due to the low number of isolates, we observed a gradual increase in the proportion of S. pneumoniae isolates with MIC values for penicillin ≥0.125 μg/mL and ceftriaxone ≥1.0 μg/mL during the period studied, reaching a proportion of 55.2% and 37% of isolates, respectively, by the first months of 2022. We also observed an increasing trend towards higher levels of MIC values (≥2.0 μg/mL) for both antimicrobials, especially when analyzing the COVID-19 year of 2021 (24.4% for penicillin) and 2022 (39.5% for penicillin and 18.5% for ceftriaxone) (Figure 2A,B).
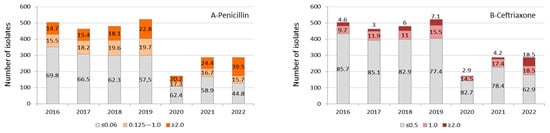
Figure 2.
Invasive S. pneumoniae (n = 2.714) isolates’ distribution and proportion of values for minimum inhibitory concentration (μg/mL) to penicillin (A) and ceftriaxone (B) by year. Data from the year 2022 corresponded to a period from January to May. The antimicrobial susceptibility profile was not determined in 57 S. pneumoniae isolates identified by molecular methodology.
The non-susceptibility rates to penicillin and ceftriaxone were higher for patients with meningitis than for patients with other clinical forms in both age groups and periods. They were also higher in the COVID period than in the pre-COVID period. The statistical comparison showed that the levels were significantly higher in the COVID period, particularly for cases of clinical forms other than meningitis in both age groups, except for ceftriaxone in adults (Table 4).

Table 4.
Non-susceptibility rates to penicillin and ceftriaxone of invasive S. pneumoniae isolates from meningitis and non-meningitis clinical diagnoses by age group and study period.
Except for levofloxacin, the overall trend of increased non-susceptibility rates in the COVID-19 period compared to the pre-COVID-19 period was also documented for the non-beta-lactam antimicrobials erythromycin, clindamycin, tetracycline, and sulfamethoxazole-trimethoprim, increasing multidrug resistance (Table 5). All S. pneumoniae isolates were susceptible to vancomycin.

Table 5.
Non-susceptibility * rates to non-beta-lactam antimicrobials of invasive S. pneumoniae isolates per study period $.
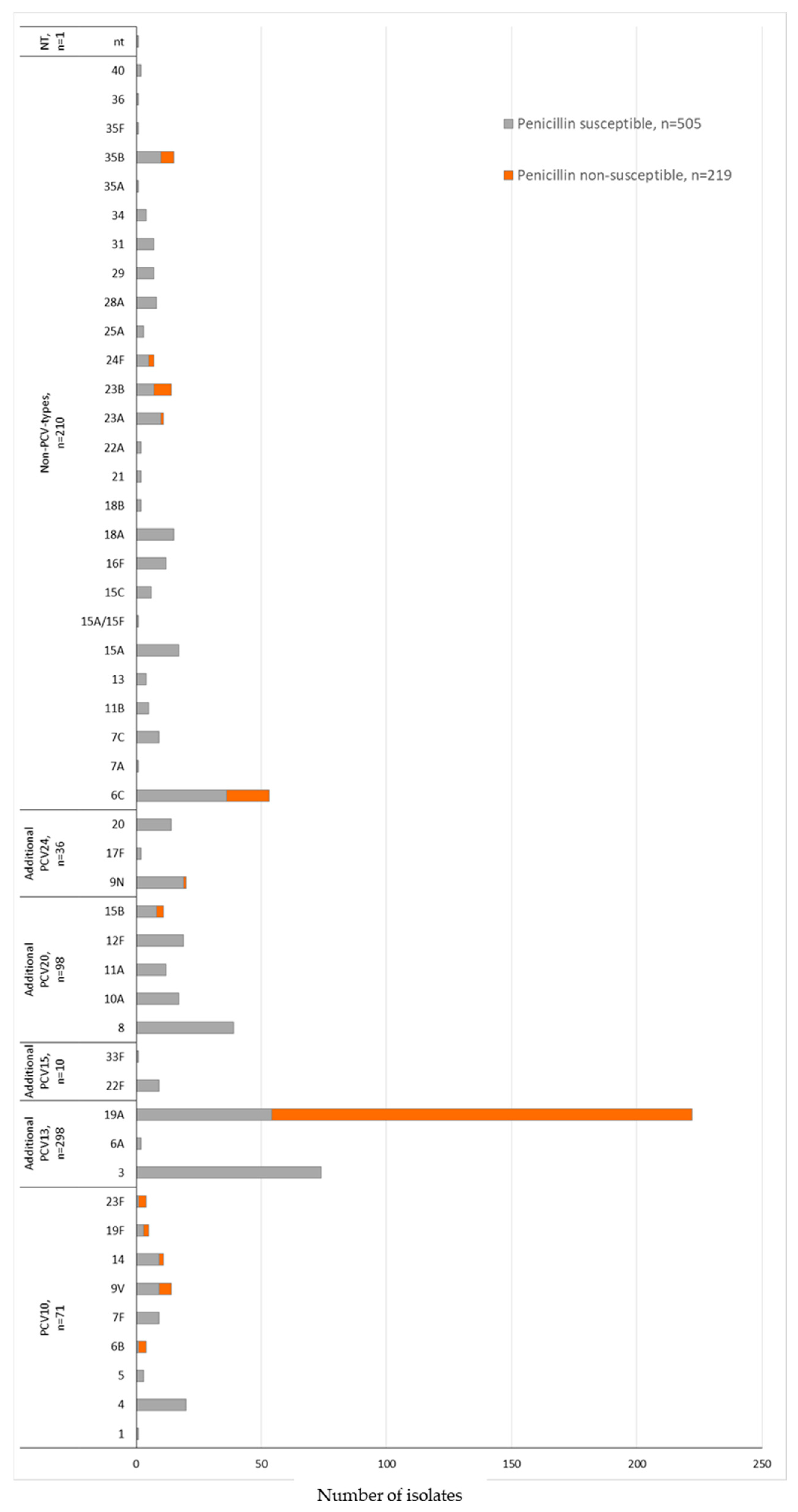
The serotype distribution according to penicillin non-susceptibility (MIC ≥ 0.125 μg/mL) in the COVID-19 period identified 13 serotypes. We also observed that 89.9% (n = 197/219) of the S. pneumoniae isolates belonged to the non-PCV10 types 19A (n = 168), 6C (n = 17), 23B (n = 7), and 35B (n = 5) (Figure 3 and Table 6). The MDR was associated mainly with non-PCV10 types 19A (n = 194, 87.4%) and 6C (n = 43, 81.1%) (Table 6). Serotype 3, the second most prevalent serotype, presented 100% susceptibility to penicillin and ceftriaxone and displayed low non-susceptibility rates to the other antimicrobials or multidrug resistance (Table 6).
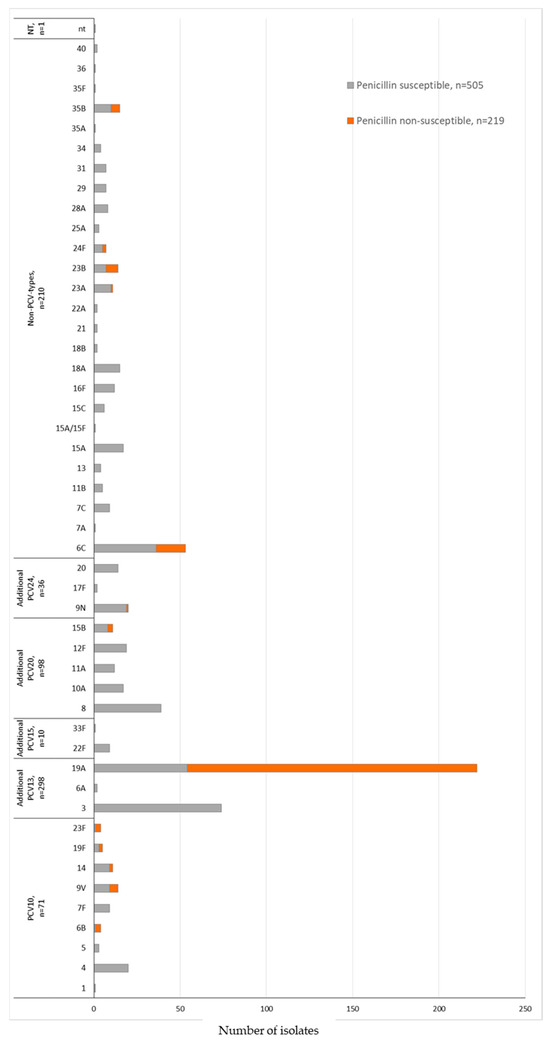
Figure 3.
Penicillin susceptibility profile and serotyping distribution of invasive S. pneumoniae (N = 724) isolates in the COVID-19 period (2020 February–2022 May) by PCV formulations (PCV10; additional serotypes PCV13, PCV15, PCV20, or PCV24; non-PCVs types; and non-typable). Penicillin non-susceptibility corresponding to S. pneumoniae isolates with MIC ≥ 0.125 μg/mL according to the CLSI, 2022. The susceptibility profile was not determined in 43 S. pneumoniae isolates identified by molecular methodology.

Table 6.
Rates of antimicrobial non-susceptibility * of invasive S. pneumoniae (n = 720) isolates by pneumococcal vaccine formulation in the COVID-19 period.
4. Discussion
Limited information on Streptococcus pneumoniae laboratory data is available from emerging countries recovered from the COVID-19 pandemic era. This study presents a detailed analysis of a large collection of IPD isolates from Brazil, comparing pre- and COVID-19 periods, focusing on serotype antigens and antimicrobial resistance profiles. Our data indicate no emergence or epidemiological capsular switch of the pneumococcal serotype occurred, but changes in antimicrobial patterns were observed.
Studies comprising data from 30 countries, including Brazil, showed the sustained reduction in the isolation of S. pneumoniae [21,22,23,24] and other respiratory transmission pathogens (H. influenzae and N. meningitidis) [21,22] from severe invasive diseases during the COVID-19 pandemic, associated mainly to the introduction of containment policies, such as non-pharmacological measures and restriction of people circulation, leading to a significant reduction in life-threatening invasive diseases in many countries worldwide [21,22,23,24], a phenomenon which was also documented in our study. We observed an overall reduction of 44.6% in the invasive S. pneumoniae isolates received by the IAL in the COVID-19 period when compared with the pre-COVID-19 period, despite the gradual increase in invasive S. pneumoniae isolates during the last few months of the COVID-19 period in 2022, which coincided with the gradual loosening of the non-pharmacological measures to control virus transmission. Our study also revealed no changes in the diversity and frequency of S. pneumoniae serotypes between the periods and age groups analyzed, as reported in some European and Latin American countries [21,22].
Brazilian studies conducted on PCV10’s impact showed a great effect of this vaccine in the reduction in IPD and nasopharyngeal colonization by PCV10 types, in addition to the increase in non-PCV10 types [14,25,26,27,28]. A high diversity of non-PCV10 types was found in IPD, mainly by serotypes 3, 19A, 6C, 8, and 23A, the most prevalent in Brazil in the 2017–2019 period [14]. Our data support the consistency of a long-term PCV10 impact on IPD due to the lower prevalence of PCV10 types in the COVID-19 period in both of the age groups studied and highlights the sustained transmission of non-PCV10 types 19A, 3, and 6C during the periods studied. This was observed in both age groups but mainly in the <5-year-old group represented by 63.4% of the isolates described by our study in the COVID-19 period.
Despite a decrease in the overall numbers, we observed a significant increase in serotype 19A during the COVID-19 pandemic period in the group of people <5 years old. Our data highlight the persistence of serotype 19A in the population, as observed in the first year of the COVID-19 pandemic in the Netherlands [29], demonstrating the ability of serotype 19A to adapt and persist within a community. The expansion of serotype 19A’s lineage clonal complex 320 associated with antimicrobial resistance has been well documented in Brazil since PCV10’s introduction in 2010 [30,31,32], suggesting that vaccination pressure added to the pressure resulting from the use of antimicrobials, such as beta-lactams, mainly in <5-year-old children, resulting in the selection of this multidrug-resistant serotype 19A lineage of pneumococcus which presents a competitive advantage for transmission and pathogenicity.
The data presented suggest that the use of conjugate vaccines with a higher valence than PCV10, licensed (PCV13, PCV15, or PCV20) or under development (PCV24), would increase protection against IPD in both of the studied populations, given that their formulation includes serotypes 19A and 3, the most prevalent serotypes in Brazil. Studies have demonstrated the presence of cross-protection between serotype 6A, an antigen present in vaccine formulations other than PCV10, and serotype 6C [33,34,35,36]. Serotype 6C was the third most prevalent serotype in Brazil described in this study, so a potential expansion in IPD protection could be obtained with the introduction of vaccines which include serotype 6A.
An important concern has arisen due to the containment measures created to block the dissemination of COVID-19. It has been argued that, by circulating pneumococcal serotypes, especially non-vaccine serotypes, there may be a possible debt in natural or innate immunity due to low colonization [10]. Also, the possibility of compromising the indirect effect of vaccination in the unvaccinated population has been argued. These factors could increase the risk for IPD by non-PCV types and PCV types after the COVID-19 pandemic era [10] and reinforce the importance of continuous surveillance of pneumococcal infections in understanding the possible future role of new vaccine formulations.
Cohen and collaborators (2021) highlighted an emergency scenario when they mentioned that the COVID-19 pandemic has led to the risk of a resurgence of vaccine-preventable diseases. This is due to the fact that low vaccination coverage, the absence of catch-up campaigns, and less exposure of individuals to viral or bacterial infections have been observed globally [10]. Data from the Brazilian National Immunization Program indicated a reduction in PCV10 coverage in Brazil since the pre-COVID period, with an aggravation in the COVID-19 period. The coverage of 81.5% between the years 2016 and 2019 (pre-COVID-19) fell to 69.9% between the years 2020 and 2022 (COVID-19) (Supplementary Figure S3). This alerts us to a risk of resurgence of PCV10 serotypes in this vaccine’s target population, with potential impairment of the indirect effect of the vaccine in the unvaccinated population.
An increase in antibiotic consumption in emerging countries, including Brazil, presenting high rates, from 2000 to 2010 has previously been reported [37]. Faced with this situation, since 2010, Brazil has introduced restrictions for over-the-counter sales of antimicrobials, determining that sales should be associated with the presentation and retention of a medical prescription, promoting control over the use of these products in the country [38]. This measure, associated with the introduction of PCVs, which contained the prevalent serotypes associated with the antimicrobial resistance causing the IPD in question, resulted in an initial reduction in the non-susceptibility rates to antimicrobials, mainly to beta-lactams, used for the treatment of pneumococcal infections [38,39,40,41].
In Brazil, studies evaluating the long-term impact of the use of PCV10 on antimicrobial resistance have demonstrated that the benefit of controlling antimicrobial resistance has impacted by pneumococcal infections caused by non-PCV10 serotypes associated with antimicrobial resistance, mainly serotypes 19A and 6C [25,30,31,39,40,42].
Our study showed a concerning situation with a higher rate of non-susceptibility related to beta-lactams antimicrobials, mainly in the <5-year-old group of non-meningitis infections, and other antimicrobials classes. This resulted in an elevation of multidrug-resistant S. pneumoniae isolates that may be related not only to the higher prevalence of non-PCV10 types 19A and 6C but also to the gross misuse of antimicrobials to avoid possible co-infections in severe cases of SARS-CoV-2 infections during the COVID-19 pandemic [13,43]. In our study. it was documented that beta-lactam non-susceptibility was associated with some risk factors such as recent antibiotic use, age (mainly in children <5 years of age), hospitalization, living in urban areas, attending daycare, and individuals with immunosuppression [44]. Antimicrobial misuse, mainly for viral infections, and the excessive use of broad-spectrum antibiotics, such ceftriaxone, were also related to the emergence of multidrug-resistant microorganisms [45].
The diversity of the emerging non-PCV10 serotypes with antimicrobial resistance may be related to many factors. The presence of pneumococcus in the nasopharynx favors its natural ability to acquire or exchange genetic material, via transformation or recombination, with other pneumococci or microorganisms, facilitating the development of antimicrobial resistance [1]. In an attempt to understand the nasopharyngeal pneumococcal carriage pattern, Weinberger and collaborators (2009) proposed a model based on the importance of the role of the biochemical structure of the capsular polysaccharide. The model proposed suggested that some serotypes, such as 19A and 6A, that presented polysaccharides less metabolically costly (with fewer carbons per repeat unit, for example) were more encapsulated, able to avoid the host immune system, and capable of persisting in the nasopharynx [46]. So, this persistence carriage pattern may explain the selection of prevalent MDR lineages of serotypes 19A and 6C in our study. Point mutations, capsular switch, and vaccine pressure are also factors related to the emergence of non-vaccine serotypes lineages associated with antimicrobial resistance [44], as well-described for the lineage 19A MDR clonal complex 320 in the post-PCV10 period in Brazil [30,31,32]. The plasticity of the pneumococcus results in a genetic structure that can determine variations in the capacity to adapt, transmit, and be virulent, which could explain the success of some non-vaccine serotypes, and future genomic studies will provide enhanced understanding of the behavior of the pneumococcal population.
This study has important limitations that should be considered. The quality of epidemiological surveillance differs between the various geographic regions of the country. Our data are obtained from laboratory-based surveillance, and they are subject to variations in the healthcare system, which may have involved restrictions on healthcare, medical prescription of antimicrobials, and also failures in sample collection, processing, reporting, and recording; therefore, our data certainly underestimate the real burden of IPD cases in Brazil.
5. Conclusions
This study contributes to understanding the pneumococcal antigen vaccines in circulation as well as the antibiotic resistance profile during the global spread of COVID-19 in our region. We observed no differences in serotype distribution between the studied periods; the COVID-19 period was marked by the high prevalence of serotypes 19A, 3, and 6C in both age groups and a reduction in the S. pneumoniae isolates received by the IAL. We also showed elevated resistance rates, mainly to penicillin and ceftriaxone for non-meningitis cases in children under 5 years of age, and these non-antimicrobial susceptibilities were related to serotypes 19A and 6C. The present data strengthen the notion that continued laboratory surveillance supports local epidemiological data in making prompt decisions for the treatment, prevention, and control of IPDs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12020401/s1: Figure S1. Confirmed cases and incidence of pneumococcal meningitis in Brazil from 2010 to 2022 (N = 12,406). Source: Information System for Notifiable Diseases—Sinan, Ministry of Health, Brazil (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def, accessed on 3 August 2023); Figure S2. Flow chart describing the methodology used in this study; Figure S3. Vaccination coverage for PCV10 in Brazil from 2013 to 2022. Source: National Immunization Program Information System (http://tabnet.datasus.gov.br/cgi/webtabx.exe?bd_pni/cpnibr.def, accessed on 7 August 2023). In 2010 Brazil introduced the PCV10 into the national childhood immunization program; and Table S1. Epidemiologic data from invasive S. pneumoniae isolates by age group and period studied.
Author Contributions
Conceptualization, S.C.G.A. and M.C.d.C.B.; formal analysis, S.C.G.A., A.P.S.d.L., A.L.B. and M.C.d.C.B.; funding acquisition, S.C.G.A., J.C.d.M. and M.C.d.C.B.; methodology, S.C.G.A. and M.C.d.C.B.; writing—original draft, S.C.G.A. and A.P.S.d.L.; writing—review and editing, S.C.G.A., A.P.S.d.L., A.L.B., J.C.d.M. and M.C.d.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institute Adolfo Lutz, Secretary of Health of the State of São Paulo, and by an investigator-initiated researcher proposal for the Pfizer Global Medical Grant (Pfizer, Inc., USA) with grant number 69687921. The authors are thankful to FAPESP for indirectly supporting this study via grant 2021/14465-1 from the São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Data Availability Statement
The data presented in the study are available upon request to the corresponding author.
Acknowledgments
We thank Lincoln S. Prado, Maria Helena C. Cavalcante, Maria Luiza L. S. Guerra, Marta Galhardo, Rosemeire C. Almendros, and Ueslei J. Dias for their support in the laboratory. We express our gratitude to Carlos Henrique Camargo for his valuable feedback in reviewing the manuscript. We gratefully acknowledge the surveillance units, hospital staff, and Public Health Laboratory (LACENs) staff at the local, state, and federal levels for sending the pneumococcal isolates to the Institute Adolfo Lutz, and the Secretary of Health Surveillance (COVER, CGLAB, SVS), Brazilian Ministry of Health, for the coordination of the laboratory network. The authors thank the Meningitis Division, Ministry of Health, Brasília, Brazil, for providing epidemiological invasive pneumococcal disease data.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the following aspects of the study: design; collection, analyses, or interpretation of data; preparation of the manuscript; or decision to publish the results.
References
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Prymula, R.; Schuerman, L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev. Vaccines 2009, 8, 1479–1500. [Google Scholar] [CrossRef]
- Jefferies, J.M.C.; Macdonald, E.; Faust, S.N.; Clarke, S.C. 13-valent pneumococcal conjugate vaccine (PCV13). Hum. Vaccines 2011, 7, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Hoover, P.A.; Vesikari, T.; Peltier, C.; Hurley, D.C.; McFetridge, R.D.; Dallas, M.; Hartzel, J.; Marchese, R.D.; Coller, B.-A.G.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine 2018, 36, 6883–6891. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.; Griffin, C.; Young, M.; Scott, D.A.; Pride, M.W.; Scully, I.L.; Ginis, J.; Severs, J.; Jansen, K.U.; Gruber, W.C.; et al. Safety, Tolerability, and Immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (PCV20) in Adults 60 to 64 Years of Age. Clin. Infect. Dis. 2021, 73, e1489–e1497. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bacterial Vaccines in Clinical and Preclinical Development: An Overview and Analysis, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; pp. 18–23. [Google Scholar]
- Domingues, C.M.A.S.; Verani, J.R.; Montenegro Renoiner, E.I.; Brandileone, M.C.D.C.; Flannery, B.; de Oliveira, L.H.; Santos, J.B.; de Moraes, J.C. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: A matched case-control study. Lancet Respir. Med. 2014, 2, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde, Brasil. Calendário Nacional de Vacinação—Criança. Available online: https://www.gov.br/saude/pt-br/vacinacao/calendario (accessed on 15 January 2024).
- Cohen, R.; Ashman, M.; Taha, M.-K.; Varon, E.; Angoulvant, F.; Levy, C.; Ryback, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A.; Unit of Antibiotic Resistance and Special Pathogens. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Ist. Super. Sanità 2020, 56, 359–364. [Google Scholar] [CrossRef]
- Del Fiol, F.S.; Bergamaschi, C.C.; Andrade, I.P.; Lopes, L.C.; Silva, M.T.; Barberato-Filho, S. Consumption Trends of Antibiotics in Brazil During the COVID-19 Pandemic. Front. Pharmacol. 2022, 13, 844818. [Google Scholar] [CrossRef] [PubMed]
- Brandileone, M.C.C.; Almeida, S.C.G.; Minamisava, R.; Andrade, A.L. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine 2018, 36, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 73–86. Available online: https://apps.who.int/iris/handle/10665/70765 (accessed on 9 January 2024).
- Centers for Diseases Control and Prevention, Streptococcus Laboratory. Available online: https://www.cdc.gov/streplab/pneumococcus/resources.html (accessed on 29 December 2023).
- Carvalho, M.d.G.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). M100 Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2022; pp. 94–99. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institue (CLSI): Malvern, PA, USA, 2018. [Google Scholar]
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Abad, R.; Amin-Chowdhury, Z.; Bautista, A.; Bennett, D.; Broughton, K.; Cao, B.; Casanova, C.; Choi, E.H.; Chu, Y.-W.; et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: Analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Glob. Health 2023, 5, e582–e593. [Google Scholar] [CrossRef] [PubMed]
- Bertran, M.; Amin-Chowdhury, Z.; Sheppard, C.L.; Eletu, S.; Zamarreño, D.V.; Ramsay, M.E.; Litt, D.; Fry, N.K.; Ladhani, S.N. Increased incidence of invasive Pneumococcal Disease among children after COVID-19 Pandemic, England. Emerg. Infect. Dis. 2022, 28, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Perniciaro, S.; van der Linden, M.; Weinberger, D.M. Reemergence of invasive pneumococcal disease in Germany during the spring and summer of 2021. Clin. Infect. Dis. 2022, 75, 1149–1153. [Google Scholar] [CrossRef]
- Jarovsky, D.; Berezin, E.N. Impact of PCV10 on pediatric pneumococcal disease burden in Brazil: Time for new recommendations? J. Pediatr. 2023, 99, S26–S56. [Google Scholar] [CrossRef]
- Duarte, F.G.; Barberino, M.G.; Moreira, S.d.S.; Reis, J.N.; Spinardi, J.R.; de Almeida, R.S.; Allen, K.E.; Alexander-Parrish, R.; Brim, R.; Neto, C.A.d.A.; et al. Incidence, aetiology and serotype coverage for pneumococcal vaccines of community-acquired pneumonia in adults: A population-based prospective active surveillance study in Brazil. BMJ Open 2022, 12, e059824. [Google Scholar] [CrossRef]
- Brandileone, M.-C.d.C.; Zanella, R.C.; Almeida, S.C.; Cassiolato, A.P.; de Lemos, A.P.S.; Salgado, M.M.; Higa, F.T.; Minamisava, R.; Andrade, A.L. Long-term effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae in children in Brazil. Vaccine 2019, 37, 5357–5363. [Google Scholar] [CrossRef]
- Neves, F.P.; Cardoso, N.T.; Snyder, R.E.; Marlow, M.A.; Cardoso, C.A.; Teixeira, L.M.; Riley, L.W. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: The emergence of multidrug resistant serotype 6C. Vaccine 2017, 35, 2794–2800. [Google Scholar] [CrossRef]
- Steens, A.; Knol, M.J.; Freudenburg-de Graaf, W.; de Melker, H.E.; van der Ende, A.; van Sorge, N.M. Pathogen- and Type-Specific Changes in Invasive Bacterial Disease Epidemiology during the First Year of the COVID-19 Pandemic in The Netherlands. Microorganisms 2022, 10, 972. [Google Scholar] [CrossRef]
- Cassiolato, A.P.; Almeida, S.C.G.; Andrade, A.L.; Minamisava, R.; Brandileone, M.C.C. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS ONE 2018, 13, e0208211. [Google Scholar] [CrossRef]
- Mott, M.; Caierão, J.; Cunha, G.; Del Maschi, M.; Pizzutti, K.; D’Azevedo, P.; Dias, C. Emergence of serotype 19A Streptococcus pneumoniae after PCV10 associated with a ST320 in adult population, in Porto Alegre. Epidemiol. Infect. 2019, 147, e93. [Google Scholar] [CrossRef]
- Moreno, J.; Duarte, C.; Cassiolato, A.P.; Chacón, G.C.; Alarcon, P.; Sánchez, J.; Martín, Y.N.S.; Valenzuela, C.; Castillo, W.; Gabarrot, G.G.; et al. Molecular characterization of Latin American invasive Streptococcus pneumoniae serotype 19A isolates. Vaccine 2020, 38, 3524–3530. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.R.; O’brien, S.E.; Burbidge, P.; Haston, M.; Zancolli, M.; Cowell, L.; Johnson, M.; Weatherholtz, R.C.; Reid, R.; Santosham, M.; et al. Comparative immunogenicity of 7 and 13-Valent Pneumococcal Conjugate Vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS ONE 2013, 8, e74906. [Google Scholar] [CrossRef]
- Naucler, P.; Galanis, I.; Morfeldt, E.; Darenberg, J.; Örtqvist, Å.; Henriques-Normark, B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin. Infect. Dis. 2017, 65, 1780–1789. [Google Scholar] [CrossRef]
- Diamantino-Miranda, J.; Aguiar, S.I.; Carriço, J.A.; Melo-Cristino, J.; Ramirez, M. Clonal and serotype dynamics of serogroup 6 isolates causing invasive pneumococcal disease in Portugal: 1999–2012. PLoS ONE 2017, 12, e0170354. [Google Scholar] [CrossRef]
- Shi, Y.; Nolan, K.M.; Burton, R.L.; Shekar, T.; Murphy, R.D.; Banniettis, N.; Musey, L.; Buchwald, U.K. The 15-valent pneumococcal conjugate vaccine V114 induces cross-reactive antibodies against pneumococcal serotype 6C. Hum. Vaccines Immunother. 2023, 19, 2235238. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Moura, M.L.; Boszczowski, I.; Blaque, M.; Mussarelli, R.M.; Fossaluza, V.; Pierrotti, L.C.; Campana, G.; Brandileone, M.C.; Zanella, R.; Almeida, S.C.; et al. Effect on Antimicrobial Resistance of a Policy Restricting Over-the-Counter Antimicrobial Sales in a Large Metropolitan Area, São Paulo, Brazil. Emerg. Infect. Dis. 2022, 28, 180–187. [Google Scholar] [CrossRef]
- Knupp-Pereira, P.A.; Cabral, A.S.; Dolores, Í.M.; Beiral, A.; Póvoa, H.C.C.; Neves, F.P.G. Antimicrobial resistance in Streptococcus pneumoniae before and after the introduction of Pneumococcal Conjugate Vaccines in Brazil: A systematic review. Antibiotics 2024, 13, 66. [Google Scholar] [CrossRef]
- Brandileone, M.-C.C.; Almeida, S.C.; Bokermann, S.; Minamisava, R.; Berezin, E.N.; Harrison, L.H.; Andrade, A.-L. Dynamics of antimicrobial resistance of Streptococcus pneumoniae following PCV10 introduction in Brazil: Nationwide surveillance from 2007 to 2019. Vaccine 2021, 39, 3207–3215. [Google Scholar] [CrossRef]
- Jansen, K.U.; Anderson, A.S. The role of vaccines in fighting antimicrobial resistance (AMR). Hum. Vaccines Immunother. 2018, 14, 2142–2149. [Google Scholar] [CrossRef]
- Pinto, T.C.A.; Neves, F.P.G.; Souza, A.R.V.; Oliveira, L.M.A.; Costa, N.S.; Castro, L.F.S.; Mendonça-Souza, C.R.d.V.; Peralta, J.M.; Teixeira, L.M. Evolution of penicillin non-susceptibility among Streptococcus pneumoniae isolates recovered from asymptomatic carriage and invasive disease over 25 years in Brazil, 1990–2014. Front. Microbiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef]
- Kim, L.; McGee, L.; Tomczyk, S.; Beall, B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: A United States perspective. Clin. Microbiol. Rev. 2016, 29, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.L.; Boszczowski, I.; Mortari, N.; Barrozo, L.V.; Neto, F.C.; Lobo, R.D.; Pedroso de Lima, A.C.; Levin, A.S. The Impact of Restricting Over-the-Counter Sales of Antimicrobial Drugs: Preliminary Analysis of National Data. Medicine 2015, 94, e1605. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Trzciński, K.; Lu, Y.-J.; Bogaert, D.; Brandes, A.; Galagan, J.; Anderson, P.W.; Malley, R.; Lipsitch, M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009, 5, e1000476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).