Molecular Characterization of ‘Candidatus Phytoplasma prunorum’ in the Czech Republic and Susceptibility of Apricot Rootstocks to the Two Most Abundant Haplotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Insect Samples for ‘Ca. P. prunorum’ Molecular Characterization
2.2. Plant Material for Rootstock Susceptibility Testing and Sampling
2.3. DNA Extraction, Phytoplasma Detection and Quantification
2.4. Molecular Characterization
2.5. Statistical Analysis
3. Results
3.1. Diversity of Genotypes
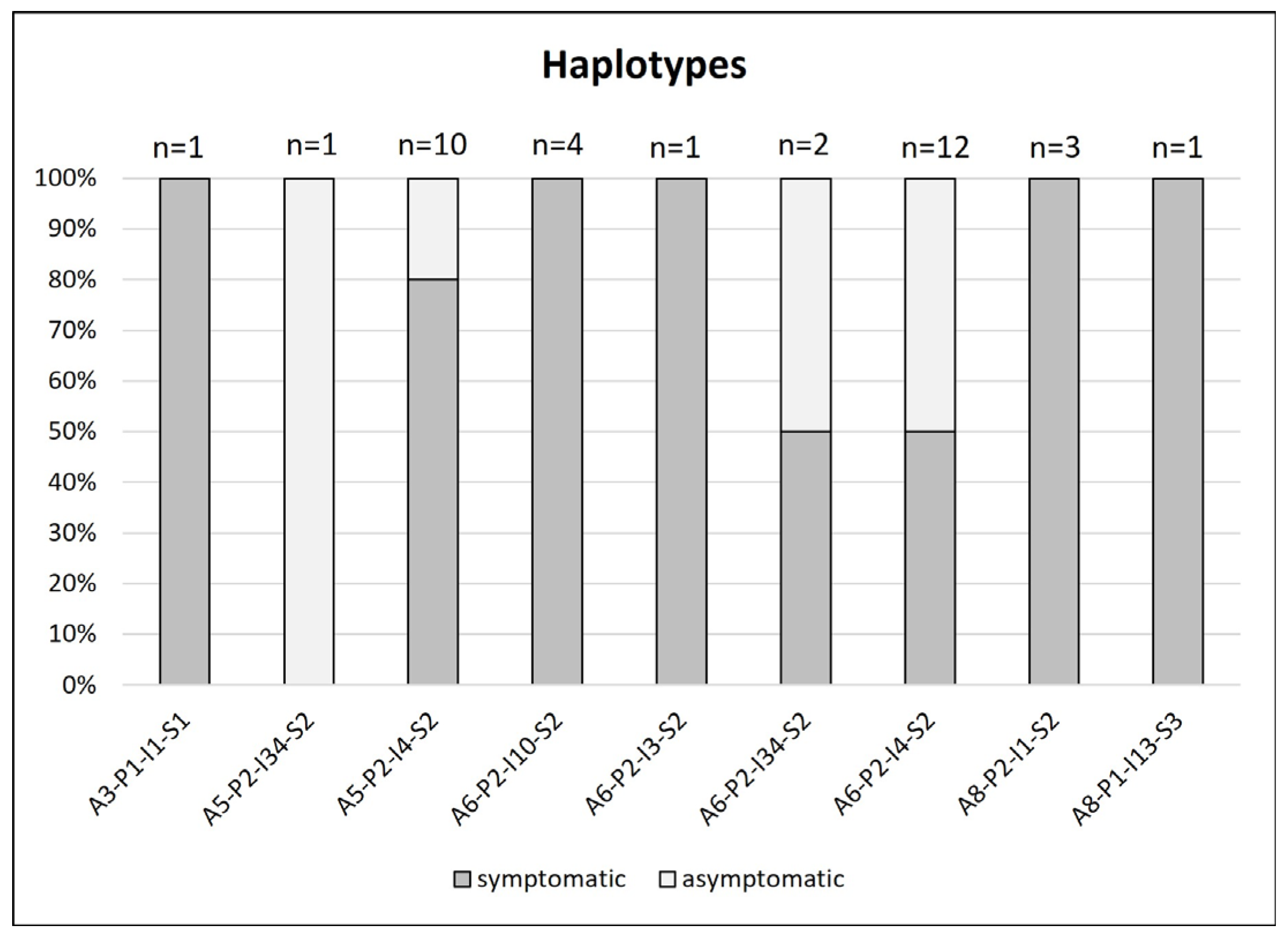
3.2. Haplotype Diversity
3.3. ‘Ca. P. prunorum’ Isolates in Asymptomatic Plants
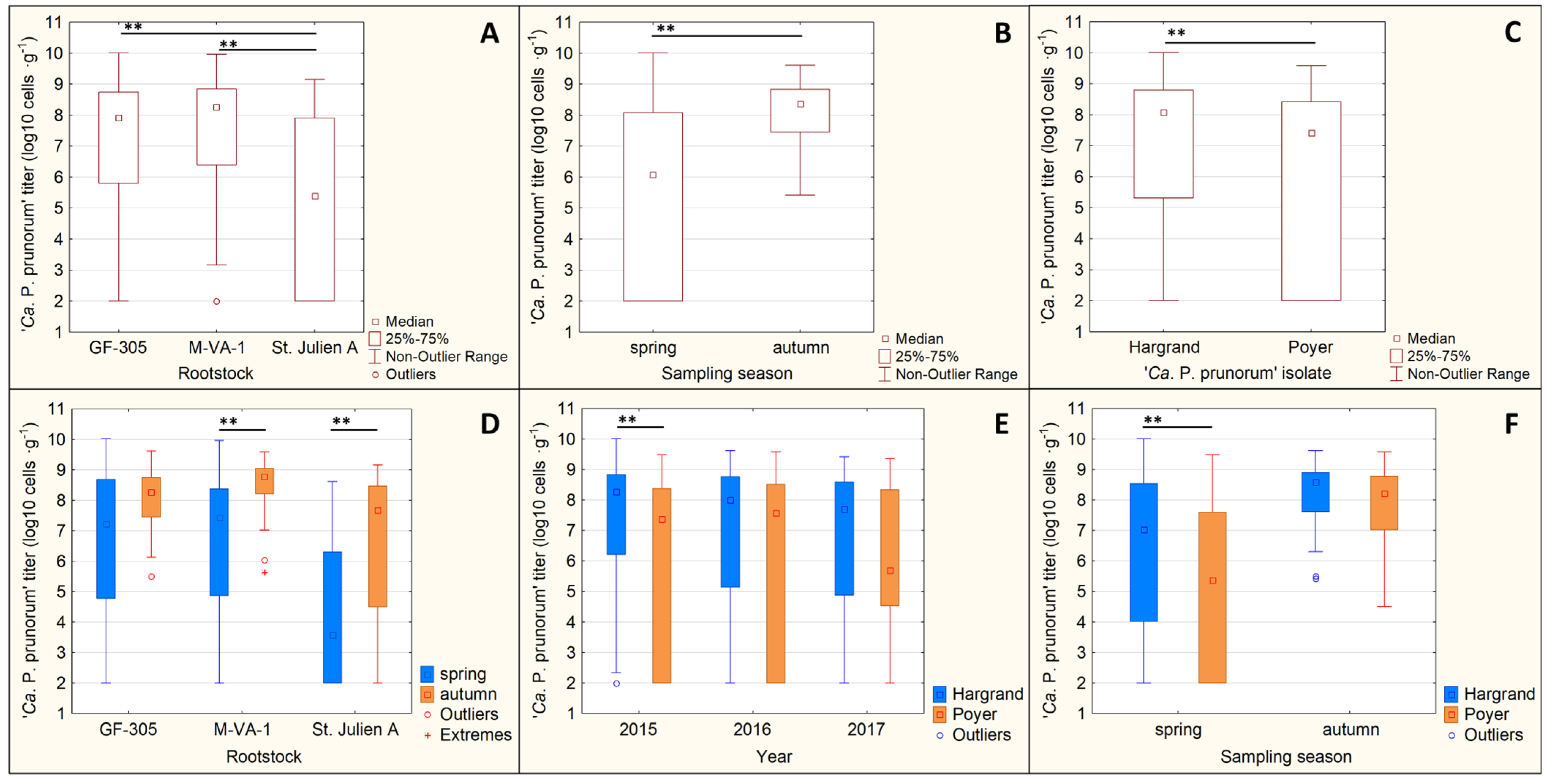
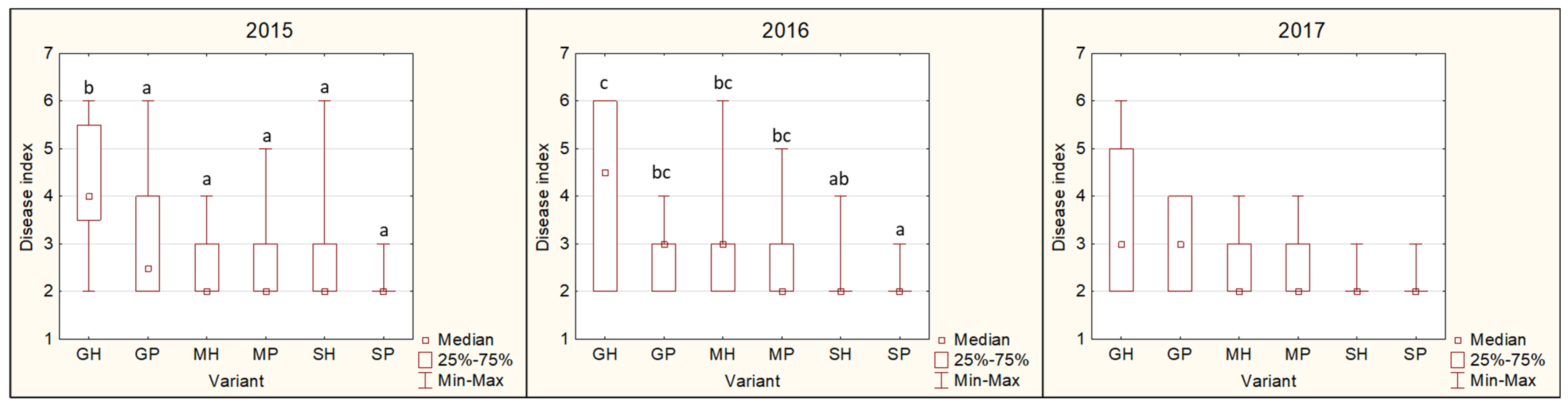
3.4. Rootstock Susceptibility Test
3.4.1. Phytoplasma Titer
3.4.2. Disease Index (DI)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seemuller, E.; Schneider, B. ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef]
- The Irpcm Phytoplasma/Spiroplasma Working Team-Phytoplasma Taxonomy, G. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [CrossRef] [PubMed]
- PM 7/62 (3) ‘Candidatus Phytoplasma mali’, ‘Ca. P. pyri’ and ‘Ca. P. prunorum’. EPPO Bull. 2020, 50, 69–85. [CrossRef]
- Nečas, T.; Kiss, T.; Eichmeier, A.; Nečasová, J.; Ondrášek, I. The effect of phytoplasma disease caused by ‘Candidatus Phytoplasma prunorum’ on the phenological and pomological traits in apricot trees. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 107–114. [Google Scholar] [CrossRef]
- Marcone, C.; Jarausch, B.; Jarausch, W. Phytoplasma prunorum, the causal agent of European Stone Fruit Yellows: An overview. J. Plant Pathol. 2010, 92, 19–34. [Google Scholar]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K.; et al. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Evol. Micr. 2022, 72, 005353. [Google Scholar] [CrossRef]
- Foissac, X.; Danet, J.L.; Malembic-Maher, S.; Salar, P.; Safarova, D.; Valova, P.; Navratil, M. Tuf and secY PCR amplification and genotyping of phytoplasmas. Methods Mol. Biol. 2013, 938, 189–204. [Google Scholar] [CrossRef]
- Danet, J.L.; Bahriz, H.; Cimerman, A.; Foissac, X. New molecular typing tools to monitor fruit tree phytoplasma variability in the 16SrX taxonomic group. Acta Hortic. 2008, 781, 343–349. [Google Scholar] [CrossRef]
- Danet, J.L.; Balakishiyeva, G.; Cimerman, A.; Sauvion, N.; Marie-Jeanne, V.; Labonne, G.; Lavina, A.; Batlle, A.; Krizanac, I.; Skoric, D.; et al. Multilocus sequence analysis reveals the genetic diversity of European fruit tree phytoplasmas and supports the existence of inter-species recombination. Microbiology 2011, 157, 438–450. [Google Scholar] [CrossRef]
- Marcone, C.; Schneider, B.; Seemüller, E. Comparison of European stone fruit yellows phytoplasma strains differing in virulence by multi-gene sequence analyses. In Proceedings of the 21st International Conference on Virus and Other Graft Transmissible Diseases of Fruit Crops, Neustadt, Germany, 5–10 July 2010. [Google Scholar]
- Valasevich, N.; Schneider, B. Detection, Identification and molecular diversity of ‘Candidatus Phytoplasma prunorum’ in Belarus. J. Plant Pathol. 2016, 98, 625–629. [Google Scholar] [CrossRef]
- Riedle-Bauer, M.; Paleskic, C.; Schwanzer, J.; Kölber, M.; Bachinger, K.; Antonielli, L.; Schönhuber, C.; Elek, R.; Stradinger, J.; Emberger, M.; et al. Epidemiological and molecular study on ‘Candidatus Phytoplasma prunorum’ in Austria and Hungary. Ann. Appl. Biol. 2019, 175, 400–414. [Google Scholar] [CrossRef]
- Marie-Jeanne, V.; Bonnot, F.; Thébaud, G.; Peccoud, J.; Labonne, G.; Sauvion, N. Multi-scale spatial genetic structure of the vector-borne pathogen ‘Candidatus Phytoplasma prunorum’ in orchards and in wild habitats. Sci. Rep. 2020, 10, 5002. [Google Scholar] [CrossRef]
- Krizanac, I.; Plavec, J.; Budinscak, Z.; Ivic, D.; Skoric, D.; Music, M.S. Apple proliferation disease in Croatian orchards: A molecular characterization of ‘Candidatus Phytoplasma mali’. J. Plant Pathol. 2017, 99, 95–101. [Google Scholar] [CrossRef]
- Morton, A.; Davies, D.L.; Blomquist, C.L.; Barbara, D.J. Characterization of homologues of the apple proliferation immunodominant membrane protein gene from three related phytoplasmas. Mol. Plant Pathol. 2003, 4, 109–114. [Google Scholar] [CrossRef]
- Lee, I.M.; Bottner-Parker, K.D.; Zhao, Y.; Davis, R.E.; Harrison, N.A. Phylogenetic analysis and delineation of phytoplasmas based on secY gene sequences. Int. J. Syst. Evol. Micr. 2010, 60, 2887–2897. [Google Scholar] [CrossRef]
- Danet, J.L.; Bonnet, P.; Jarausch, W.; Carraro, L.; Skoric, D.; Labonne, G.; Foissac, X. Imp and secY, two new markers for MLST (multilocus sequence typing) in the 16SrX phytoplasma taxonomic group. Bull. Insectol. 2007, 60, 339–340. [Google Scholar]
- Dermastia, M.; Dolanc, D.; Mlinar, P.; Mehle, N. Molecular diversity of ‘Candidatus Phytoplasma mali’ and ‘Ca. P. prunorum’ in orchards in Slovenia. Eur. J. Plant Pathol. 2018, 152, 791–800. [Google Scholar] [CrossRef]
- Seemüller, E.; Moll, E.; Schneider, B. Pear decline resistance in progenies of Pyrus taxa used as rootstocks. Eur. J. Plant Pathol. 2009, 123, 217–223. [Google Scholar] [CrossRef]
- Jarausch, W.; Bisognin, C.; Schneider, B.; Grando, S.; Velasco, R.; Seemüller, E. Breeding apple proliferation-resistant rootstocks: Durability of resistance and pomological evaluation. Bull. Insectol. 2011, 64, S275–S276. [Google Scholar]
- Dosba, F.; Lansac, M.; Mazy, K.; Garnier, M.; Eyquard, J.P. Incidence of different diseases associated with Mycoplasma Like Organisms in different species of Prunus. Acta Hortic. 1990, 283, 311–320. [Google Scholar] [CrossRef]
- Kison, H.; Seemüller, E. Differences in strain virulence of the European stone fruit yellows phytoplasma and susceptibility of stone fruit trees on various rootstocks to this pathogen. J. Phytopathol. 2001, 149, 533–541. [Google Scholar] [CrossRef]
- Martini, M.; Ermacora, P.; Magris, G.; Ferrini, F.; Loi, N. Symptom expression and ‘Candidatus Phytoplasma prunorum’ concentration in different species. Bull. Insectol. 2011, 64, S171–S172. [Google Scholar]
- Jarausch, W.; Eyquard, J.P.; Mazy, K.; Lansac, M.; Dosba, F. High level of resistance of sweet cherry (Prunus avium L.) towards European stone fruit yellows phytoplasmas. Adv. Hortic. Sci. 1999, 13, 108–112. [Google Scholar]
- Maixner, M.; Ahrens, U.; Seemuller, E. Detection of the German grapevine yellows (Vergilbungskrankheit) MLO in grapevine, alternative hosts and a vector by a specific PCR procedure. Eur. J. Plant Pathol. 1995, 101, 241–250. [Google Scholar] [CrossRef]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Schneider, B.; Seemueller, E.; Smart, C.D.; Kirkpatrick, B.C. Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. Mol. Diagn. Proced. Mycoplasmol. 1995, 369–380. [Google Scholar] [CrossRef]
- Lorenz, K.H.; Schneider, B.; Ahrens, U.; Seemuller, E. Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA. Phytopathology 1995, 85, 771–776. [Google Scholar] [CrossRef]
- Nikolic, P.; Mehle, N.; Gruden, K.; Ravnikar, M.; Dermastia, M. A panel of real-time PCR assays for specific detection of three phytoplasmas from the apple proliferation group. Mol. Cell. Probes 2010, 24, 303–309. [Google Scholar] [CrossRef]
- Christensen, N.M.; Nicolaisen, M.; Hansen, M.; Schulz, A. Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Mol. Plant-Microbe Interact. 2004, 17, 1175–1184. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A.; Cociu, V.; Hough, F.L. APRICOTS (PRUNUS). Acta Hortic. 1991, 290, 65–110. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef]
- Audergon, J.; Castelain, C.; Morvan, G.; Chastellière, M. Behaviour of 150 apricot varieties after an apricot chlorotic leaf roll inoculation. Acta Hortic. 1991, 293, 593–598. [Google Scholar] [CrossRef]
- Osler, R.; Borselli, S.; Ermacora, P.; Ferrini, F.; Loschi, A.; Martini, M.; Moruzzi, S.; Musetti, R.; Giannini, M.; Serra, S.; et al. Transmissible tolerance to European stone fruit yellows (ESFY) in apricot: Cross-protection or a plant mediated process? Phytoparasitica 2016, 44, 203–211. [Google Scholar] [CrossRef]
- Martini, M.; Ferrini, F.; Danet, J.-L.; Ermacora, P.; Sertkaya, G.; Delić, D.; Loi, N.; Foissac, X.; Carraro, L. PCR/RFLP-based method for molecular characterization of ‘Candidatus Phytoplasma prunorum’strains using the aceF gene. Jul. Kühn-Arch. 2010, 386. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kiss, T.; Necas, T.; Penazova, E.; Tekielska, D.; Bohunicka, M.; Valentova, L.; Cmejla, R.; Morais, D.; Baldrian, P. High-throughput sequencing analysis of the bacterial community in stone fruit phloem tissues infected by “Candidatus Phytoplasma prunorum”. Microb. Ecol. 2019, 77, 664–675. [Google Scholar] [CrossRef]
- Morvan, G. Apricot chlorotic leaf roll. EPPO Bull. 1977, 7, 37–55. [Google Scholar] [CrossRef]
- Jarausch, W.; Jarausch, B.; Fritz, M.; Runne, M.; Etropolska, A.; Pfeilstetter, E. Epidemiology of European stone fruit yellows in Germany: The role of wild Prunus spinosa. Eur. J. Plant Pathol. 2019, 154, 463–476. [Google Scholar] [CrossRef]
- Carraro, L.; Ferrini, F.; Ermacora, P.; Loi, N. Role of wild Prunus species in the epidemiology of European stone fruit yellows. Plant Pathol. 2002, 51, 513–517. [Google Scholar] [CrossRef]
- Nečas, T.; Ondrášek, I.; Krška, B. ‘Candidatus Phytoplasma prunorum’–a pathogen spreading uncontrollably in apricot orchards in the Czech Republic. Acta Hortic. 2015, 1105, 131–136. [Google Scholar] [CrossRef]
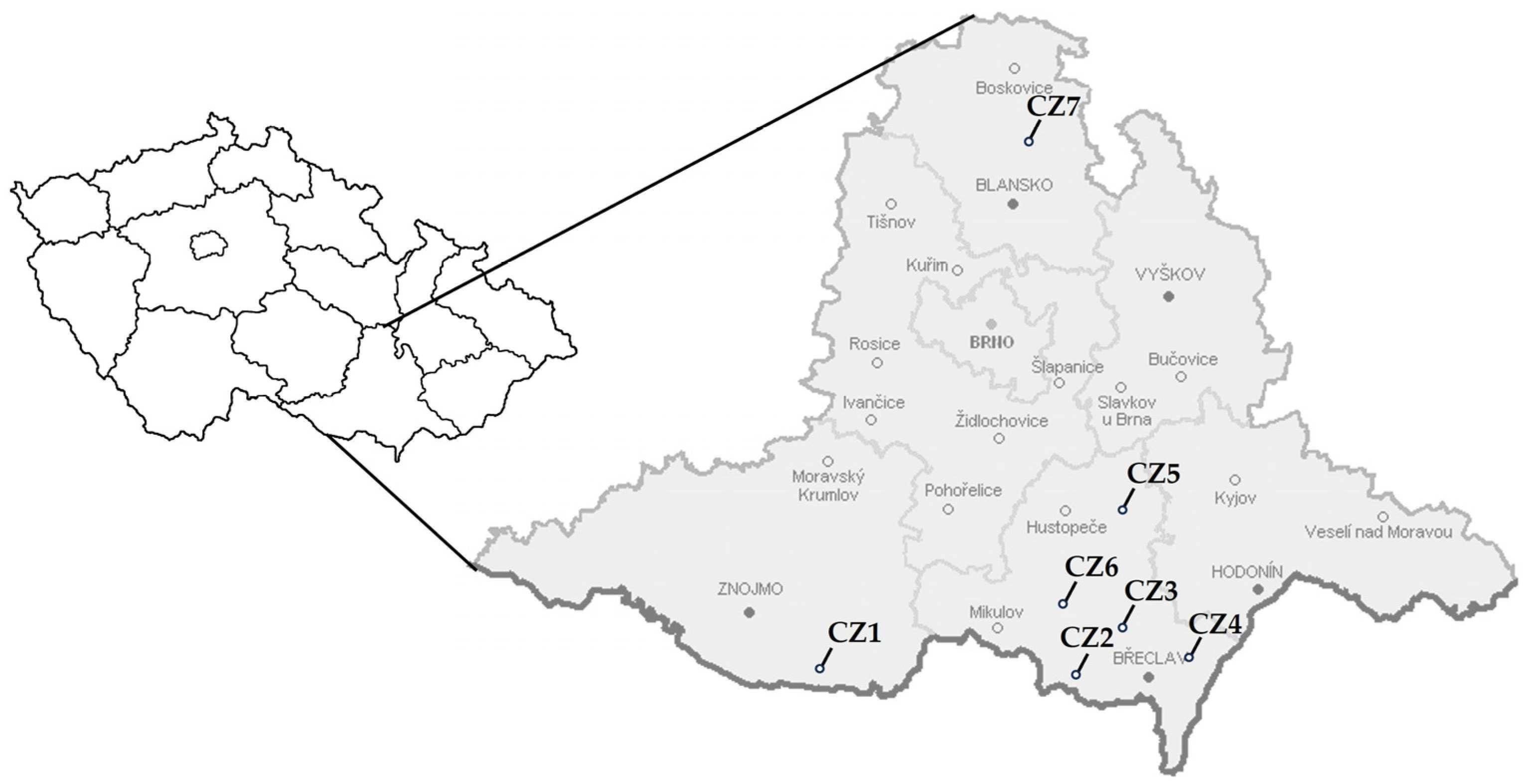


| Sampling Site | Area | Host Species | Plant Samples | C. pruni | |
|---|---|---|---|---|---|
| Emigrants | Remigrants | ||||
| CZ1 | Slup | P. armeniaca, P. persica | 3 | - | - |
| CZ 2 | Valtice | P. armeniaca, P. persica | 11 | - | - |
| CZ 3 | Lednice | P. armeniaca, P. persica, P. salicina, P. cerasifera | 38 | 8 | 7 |
| CZ 4 | Hrušky | P. armeniaca | 1 | - | - |
| CZ 5 | Kobylí | P. armeniaca | 3 | - | - |
| CZ 6 | Bulhary | P. spinosa | - | - | 3 |
| CZ 7 | Kalečník | Picea sp. | - | 5 | 10 |
| Total | 56 | 13 | 20 | ||
| Gene | Genotype 1 | GenBank Acc. No. of Type Sequences Obtained in This Study | Plant Source (No. of Samples/%) | Insect Source (No. of Samples/%) | Sampling Site (CZ) |
|---|---|---|---|---|---|
| aceF | A3 | PP176477, PP176481 | 1/2.1 | 4/14.3 | 5, 7 |
| A5 | PP176478, PP176482 | 13/27.7 | 9/32.1 | 1, 2, 3, 5, 6, 7 | |
| A6 | PP176479, PP176483, PP176486, PP176488 | 24/51.1 | 9/32.1 | 2, 3, 5, 6, 7 | |
| A8 | PP176480, PP176484, PP176485, PP176487 | 5/10.6 | 6/21.4 | 1, 3, 7 | |
| mix 2 | 4/8.5 | - | 3 | ||
| Total | 47/100 | 28/100 | |||
| pnp | P1 | PP176489, PP176491 | 1/2.1 | 5/18.5 | 3, 5, 7 |
| P2 | PP176490, PP176492–PP176495 | 47/97.9 | 22/81.5 | 1, 2, 3, 4, 5, 6, 7 | |
| Total | 48/100 | 27/100 | |||
| imp | I1 | PP176496, PP176500, PP176506 | 4/8.5 | 10/35.7 | 2, 3, 5, 7 |
| I3 | PP176497, PP176501 | 1/2.1 | 1/3.6 | 3, 6 | |
| I4 | PP176498, PP176502, PP176507, PP176508 | 26/55.3 | 13/46.4 | 1, 2, 3, 5, 6, 7 | |
| I10 | PP176499, PP176503 | 5/10.6 | 4/14.3 | 3, 4, 6, 7 | |
| I13 | PP176505 | 1/2.1 | - | 3 | |
| I34 | PP176504 | 5/10.6 | - | 3 | |
| mix 2 | 5/10.6 | - | 3 | ||
| Total | 47/100 | 28/100 | |||
| secY | S1 | PP176509, PP176511 | 1/1.9 | 6/21.4 | 5, 7 |
| S2 | PP176510, PP176512, PP176514, PP176515 | 52/96.3 | 22/78.6 | 1, 2, 3, 4, 5, 6, 7 | |
| S3 | PP176513 | 1/1.9 | - | 3 | |
| Total | 54/100 | 28/100 |
| Haplotype | Plant Source (No. of Samples/%) | Insect Source (No. of Samples/%) | Sampling Site (CZ) |
|---|---|---|---|
| A3-P1-I1-S1 | 1/2.9 | 2/10 | 5, 7 |
| A3-P2-I1-S1 | - | 1/5 | 7 |
| A5-P2-I3-S2 | - | 1/5 | 6 |
| A5-P2-I4-S2 | 10/28.6 | 6/30 | 1, 2, 3, 5, 6, 7 |
| A5-P2-I34-S2 | 1/2.9 | - | 3 |
| A6-P2-I3-S2 | 1/2.9 | - | 3 |
| A6-P2-I4-S2 | 12/34.3 | 4/20 | 2, 3, 5, 7 |
| A6-P2-I10-S2 | 4/11.4 | 3/15 | 3, 6, 7 |
| A6-P2-I34-S2 | 2/5.7 | - | 3 |
| A8-P1-I13-S3 | 1/2.9 | - | 3 |
| A8-P2-I1-S2 | 3/8.6 | 3/15 | 1, 3, 7 |
| Total | 35/100 | 20/100 |
| ANOVA | Factor | F-Value | p-Value |
|---|---|---|---|
| Single factor | rootstock type | 61.022 | ˂0.001 ** |
| ‘Ca. P. prunorum’ isolate | 29.375 | ˂0.001 ** | |
| sampling year | 0.581 | 0.559 | |
| sampling season | 99.36 | ˂0.001 ** | |
| Interaction between factors | rootstock type × ‘Ca. P. prunorum’ isolate | 1.713 | 0.181 |
| rootstock type × sampling year | 0.884 | 0.473 | |
| rootstock type × sampling season | 3.998 | 0.019 * | |
| ‘Ca. P. prunorum’ isolate × sampling year | 4.634 | 0.010 * | |
| ‘Ca. P. prunorum’ isolate × sampling season | 4.663 | 0.031 * | |
| sampling year × sampling season | 2.195 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, T.; Šafářová, D.; Navrátil, M.; Nečas, T. Molecular Characterization of ‘Candidatus Phytoplasma prunorum’ in the Czech Republic and Susceptibility of Apricot Rootstocks to the Two Most Abundant Haplotypes. Microorganisms 2024, 12, 399. https://doi.org/10.3390/microorganisms12020399
Kiss T, Šafářová D, Navrátil M, Nečas T. Molecular Characterization of ‘Candidatus Phytoplasma prunorum’ in the Czech Republic and Susceptibility of Apricot Rootstocks to the Two Most Abundant Haplotypes. Microorganisms. 2024; 12(2):399. https://doi.org/10.3390/microorganisms12020399
Chicago/Turabian StyleKiss, Tomáš, Dana Šafářová, Milan Navrátil, and Tomáš Nečas. 2024. "Molecular Characterization of ‘Candidatus Phytoplasma prunorum’ in the Czech Republic and Susceptibility of Apricot Rootstocks to the Two Most Abundant Haplotypes" Microorganisms 12, no. 2: 399. https://doi.org/10.3390/microorganisms12020399
APA StyleKiss, T., Šafářová, D., Navrátil, M., & Nečas, T. (2024). Molecular Characterization of ‘Candidatus Phytoplasma prunorum’ in the Czech Republic and Susceptibility of Apricot Rootstocks to the Two Most Abundant Haplotypes. Microorganisms, 12(2), 399. https://doi.org/10.3390/microorganisms12020399







