Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Isolation of Fungi
2.2. Macro- and Micromorphology of Fusarium Isolates
2.3. DNA Extraction and PCR
2.4. Phylogenetic Analysis
2.5. Pathogenicity Test
- 0: The absence of symptoms.
- 1: The taproot is slightly brown and the adventitious roots and vegetative part show no symptoms of the lesion.
- 2: The taproot is brown, the adventitious roots have visible damage, the growth of the vegetative part is limited, and there is a slight wilting.
- 3: The taproot is completely brown, the appendages are poorly developed, the growth of the stem is 50% behind the control, and there is significant wilting.
- 4: The taproot is dark brown, completely necrotic or may not have formed at all, there are no adventitious roots, and the plants are completely necrotic or withered.
- Weakly aggressive (WA): no more than 10% of the studied varieties could be characterized as being in stages 3–4 of the four-point scale.
- Moderately aggressive (MA): 25–50% of the studied varieties could be characterized as being in stages 3–4 of the four-point scale.
- Highly aggressive (HA): 75–100% of the studied varieties could be characterized as being in stages 3–4 of the four-point scale.
2.6. Statistical Processing
3. Results
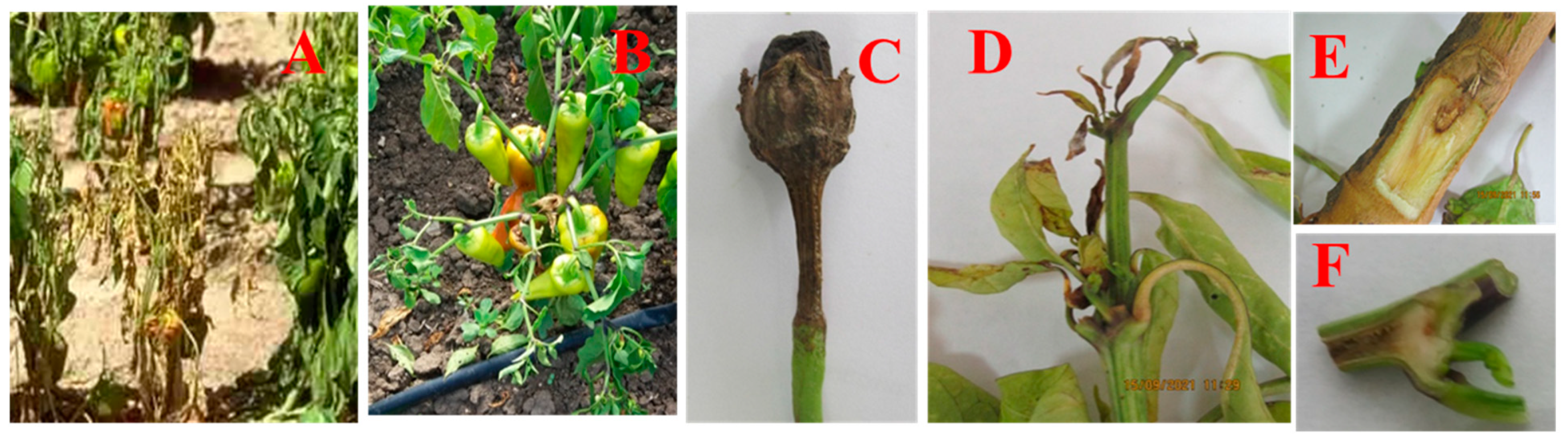
3.1. Phytopathological Monitoring and Symptoms
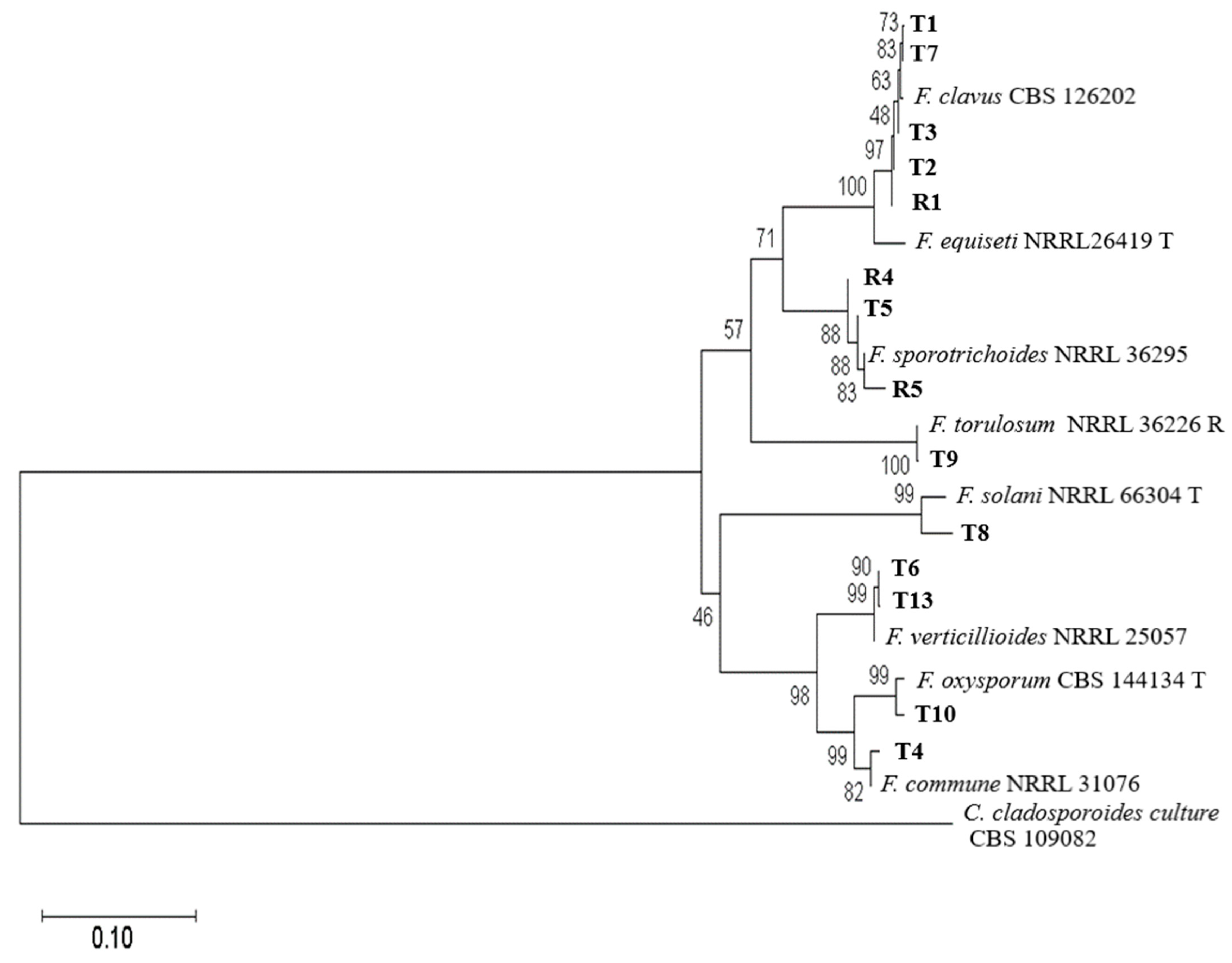
3.2. Phylogenetic Analysis of Fungi Fusarium
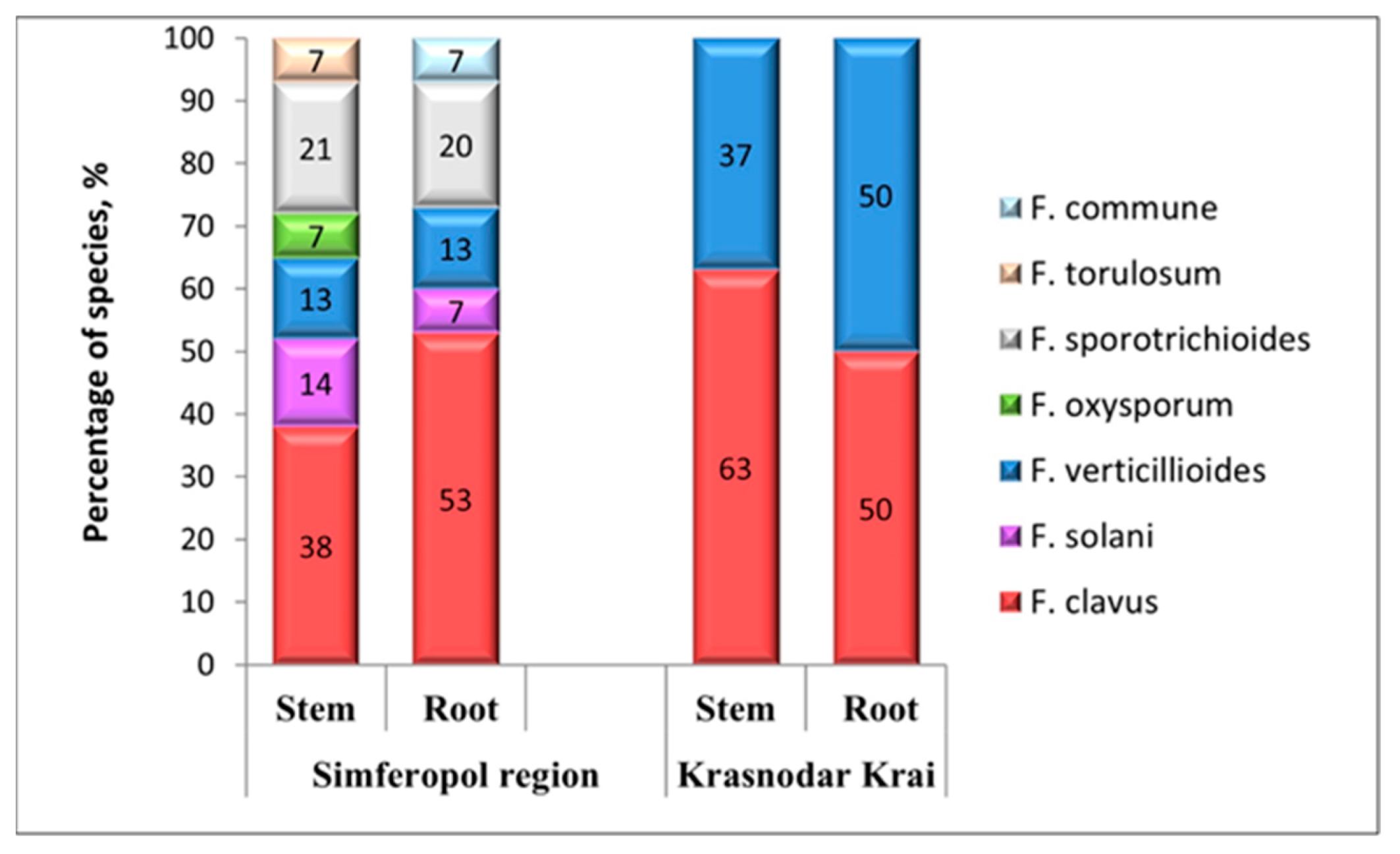
3.3. Geographical Distribution of Different Fusarium Species in the Southern Regions
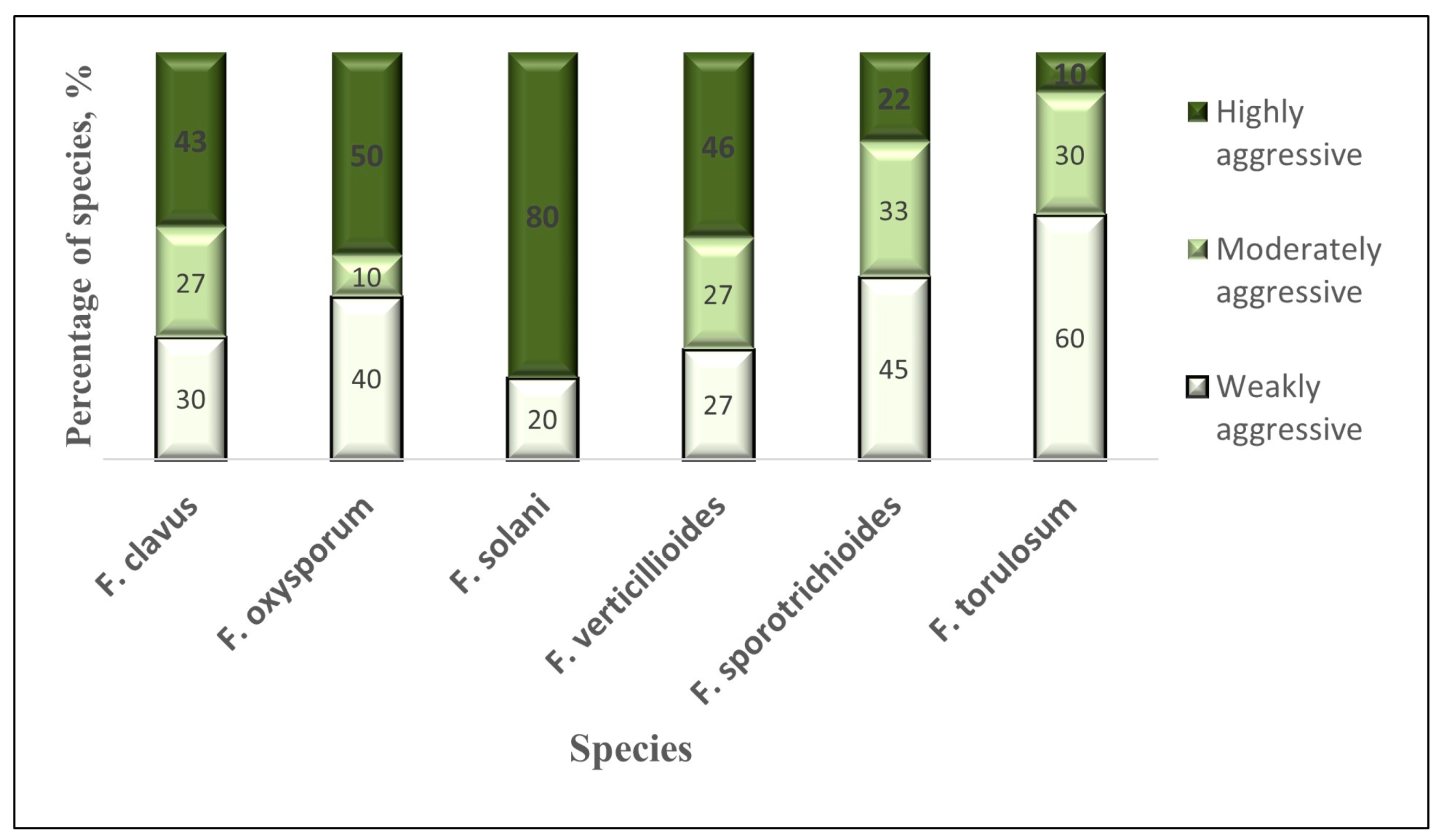
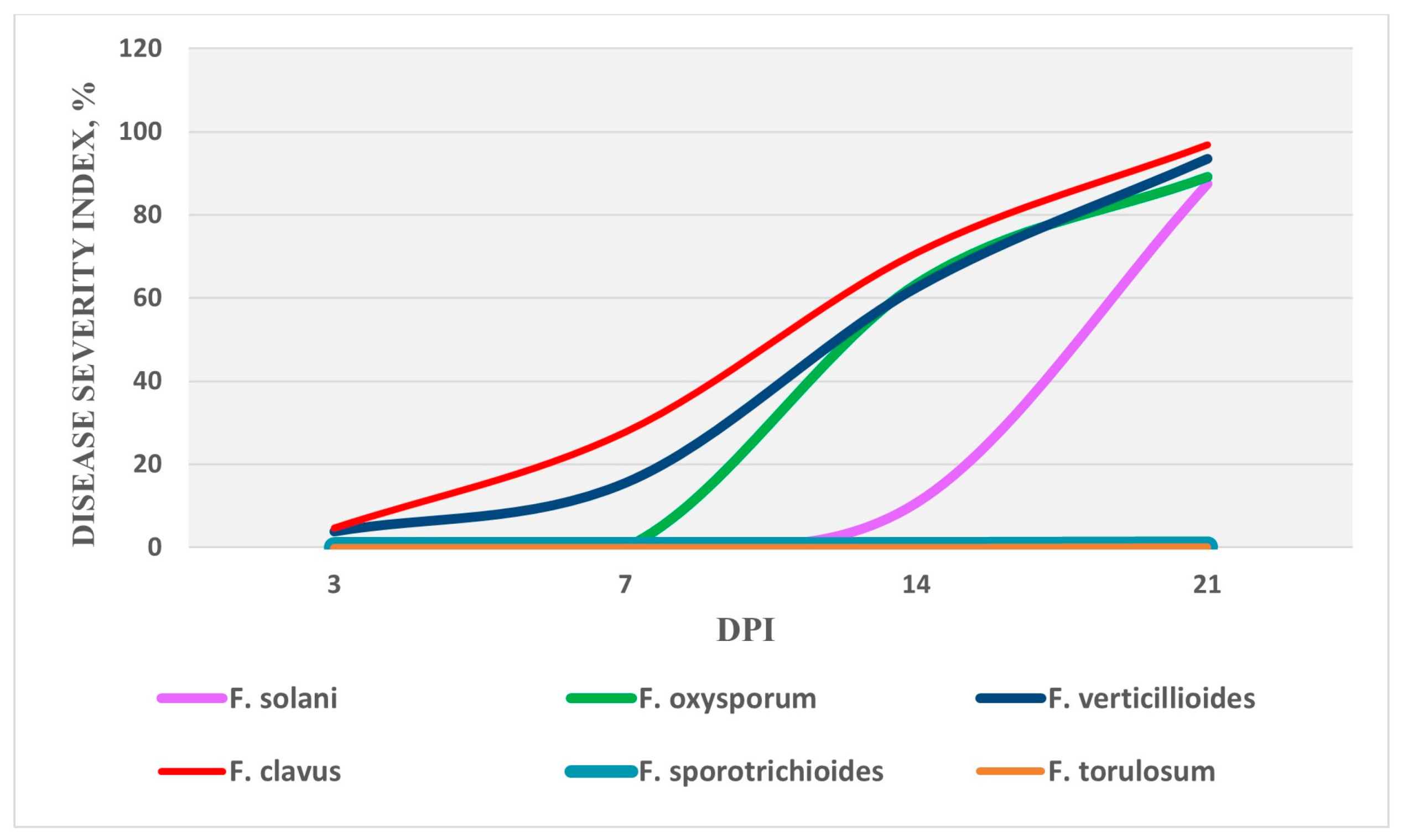
3.4. Study of Pathogenic Properties of Fusarium Fungi
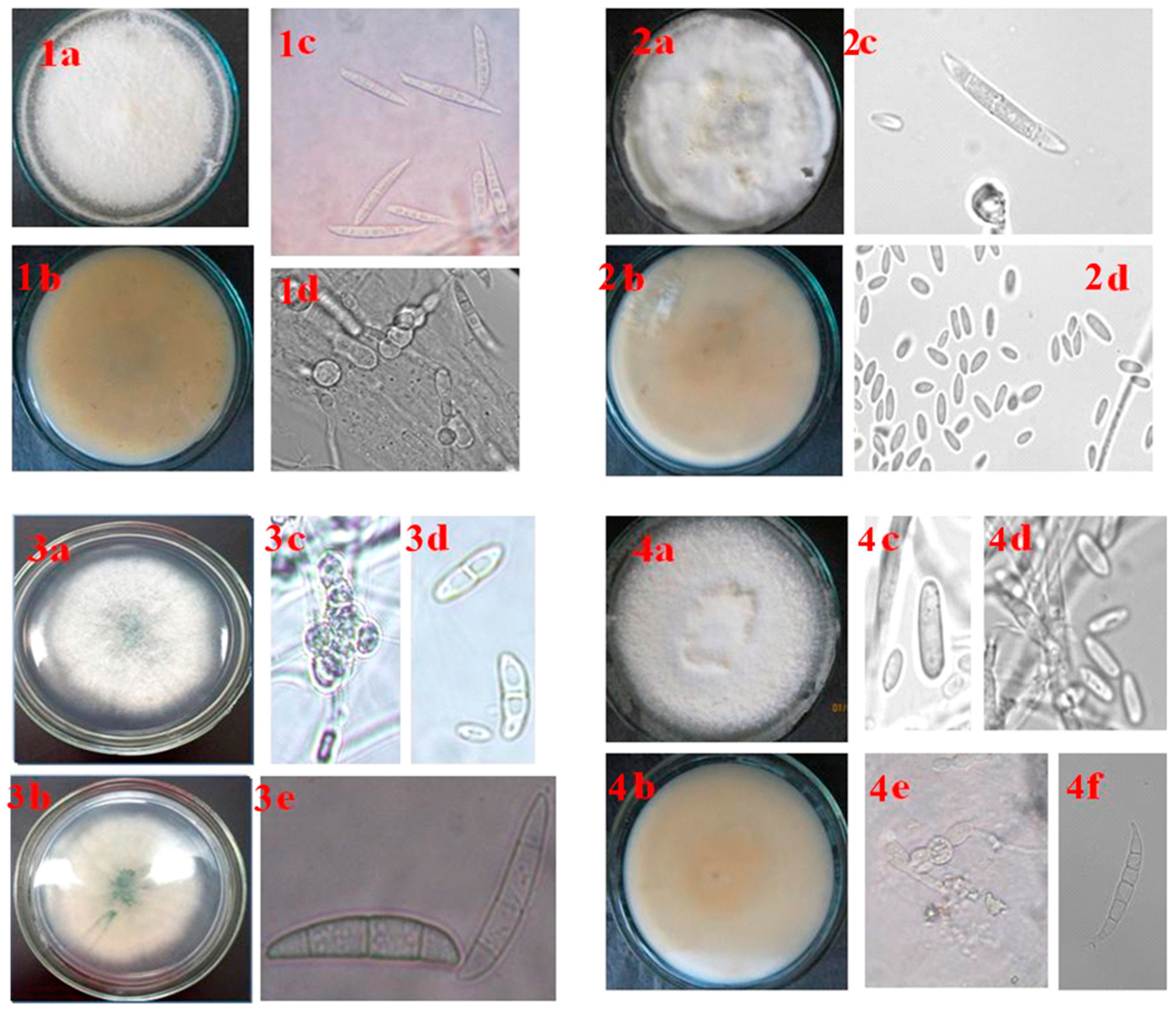
3.5. Morphological Description of Fusarium Species Associated with Pepper wilt in the Southern Regions of the Russian Federation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dzhos, E.A.; Shumilina, D.V.; Pyshnaya, O.N.; Mamedov, M.I.; Baikov, A.A.; Matyukina, A.A. Creation of an Interspecific Hybrid of Capsicum annuum L. and C. frutescens L. Using Biotechnological Approaches. Veg. Crops Russ. 2021, 27–33. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Wahba, N.M.; Ahmed, A.S.; Ebraheim, Z.Z. Antimicrobial Effects of Pepper, Parsley, and Dill and Their Roles in the Microbiological Quality Enhancement of Traditional Egyptian Kareish Cheese. Foodborne Pathog. Dis. 2010, 7, 411–418. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/ (accessed on 23 December 2023).
- Lysko, I.A.; Radko, D.P. Black Bacterial Pepper Spotting (Review). Rice Farming 2023, 79–83. [Google Scholar] [CrossRef]
- Fominykh, T.; Bogoutdinov, D.; Belykh, E.; Ivanova, G.; Utkina, V. Recommendations for Tomato and Pepper Protection against Viral and Phytoplasma Diseases; Ministry of Agriculture of the Astrakhan Oblast: Astrakhan, Russia, 2010. [Google Scholar]
- Sanogo, S. Chile Pepper and The Threat of Wilt Diseases. Plant Health Prog. 2003, 4, 23. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, M.; Kumar, A.; Mehta, N. Screening of Chili Cultivars against Fusarium Wilt of Chilli (Capsicum annuum L.). Int. J. Agric. Sci. Res. 2017, 7, 235–240. [Google Scholar]
- Hami, A.; Rasool, R.S.; Khan, N.A.; Mansoor, S.; Mir, M.A.; Ahmed, N.; Masoodi, K.Z. Morpho-Molecular Identification and First Report of Fusarium Equiseti in Causing Chilli Wilt from Kashmir (Northern Himalayas). Sci. Rep. 2021, 11, 3610. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In Vitro Chili Pepper Biotechnology. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Morid, B.; Hajmansoor, S.; Kakvan, N. Screening of Resistance Genes to Fusarium Root Rot and Fusarium Wilt Diseases in Tomato (Lycopersicon esculentum) Cultivars Using RAPD and CAPs Markers. Eur. J. Exp. Biol. 2012, 2, 931–939. [Google Scholar]
- Loganathan, M.; Venkataravanappa, V.; Saha, S.; Sharma, B.K.; Tirupathi, S.; Verma, M.K. Morphological, cultural and molecular characterizations of Fusarium wilt infecting tomato and chilli. In Proceedings of the National Symposium on Abiotic and Biotic Stress Management in Vegetable Crops. Indian Society of Vegetable Science, IIVR, Varanasi, India, 12–14 April 2013; pp. 12–14. [Google Scholar]
- Naik, M.; Rani, G.; Madhukar, H. Identification of Resistant Sources against Wilt of Chilli (Capsicum annuum L.) Caused by Fusarium solani (Mart.) Sacc. J. Mycopathol. Res. 2008, 46, 93–96. [Google Scholar]
- Summerell, B.A.; Leslie, J.F. Fifty Years of Fusarium: How Could Nine Species Have Ever Been Enough? Fungal Divers. 2011, 50, 135–144. [Google Scholar] [CrossRef]
- O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Robert, V.A.R.G.; Crous, P.W.; Johnston, P.R.; Aoki, T.; Rooney, A.P.; et al. Phylogenetic Diversity of Insecticolous Fusaria Inferred from Multilocus DNA Sequence Data and Their Molecular Identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; Al-Hatmi, A.M.S.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.; Blomquist, C.L.; Bowden, R.L.; et al. Phylogenomic Analysis of a 55.1-Kb 19-Gene Dataset Resolves a Monophyletic Fusarium That Includes the Fusarium solani Species Complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef]
- Kristensen, R.; Torp, M.; Kosiak, B.; Holst-Jensen, A. Phylogeny and Toxigenic Potential Is Correlated in Fusarium Species as Revealed by Partial Translation Elongation Factor 1 Alpha Gene Sequences. Mycol. Res. 2005, 109, 173–186. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA Sequence-Based Identification of Fusarium: Current Status and Future Directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Loza-Reyes, E.; Atkins, S.L.; Fraaije, B.A. The CYP51C Gene, a Reliable Marker to Resolve Interspecific Phylogenetic Relationships within the Fusarium Species Complex and a Novel Target for Species-Specific PCR. Int. J. Food Microbiol. 2010, 144, 301–309. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium Incarnatum-Equiseti Complex from China. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Joshi, M.; Srivastava, R.; Sharma, A.K.; Prakash, A. Screening of Resistant Varieties and Antagonistic Fusarium oxysporum for Biocontrol of Fusarium Wilt of Chilli. J. Plant Pathol. Microbiol. 2012, 3, 5. [Google Scholar] [CrossRef]
- Wongpia, A.; Lomthaisong, K. Changes in the 2DE Protein Profiles of Chilli Pepper (Capsicum annuum) Leaves in Response to Fusarium oxysporum Infection. ScienceAsia 2010, 36, 259–270. [Google Scholar] [CrossRef]
- Tembhurne, B.V.; Belabadevi, B.; Kisan, B.; Tilak, I.S.; Ashwathanarayana, D.S.; Suvarna; Nidagundi; Naik, M.K. Molecular Characterization and Screening for Fusarium (Fusarium solani) Resistance in Chilli (Capsicum annuum L.) Genotypes. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1585–1597. [Google Scholar] [CrossRef][Green Version]
- Singh, J.K.; Kumar, M.; Kumar, S.; Kumar, A.; Mehta, N. Inhibitory Effect of Botanicals on Growth and Sporulation of Fusarium oxysporum Inciting Wilt of Chilli (Capsicum annuum L.). J. Pharmacogn. Phytochem. 2017, 6, 2199–2204. [Google Scholar]
- Naik, M. Wilt of Chilli with Special Reference to Cultural, Morphological, Molecular Characterization and Pathogenic Variability of Fusarium Isolates of India; Indian Institute of Vegetable Research: Varanasi, India, 2006. [Google Scholar]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 385–614. ISBN 978-0-12-044565-3. [Google Scholar]
- Shaheen, N.; Khan, U.M.; Azhar, M.T.; Tan, D.K.Y.; Atif, R.M.; Israr, M.; Yang, S.-H.; Chung, G.; Rana, I.A. Genetics and Genomics of Fusarium Wilt of Chilies: A Review. Agronomy 2021, 11, 2162. [Google Scholar] [CrossRef]
- Prasannath, K.; Mahendran, S.; Karunakaran, S. Control of Fusarium Wilt of Chilli (Capsicum annuum L.) by Crude Plant Extracts. In Proceedings of the Tenth Annual Research Session, Chenkaladi, Sri Lanka, 1–2 December 2011; Volume 16. [Google Scholar]
- Sandoval-Denis, M.; Crous, P.W. Removing Chaos from Confusion: Assigning Names to Common Human and Animal Pathogens in Neocosmospora. Persooni-Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Mejía-Bautista, M.A.; Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Reyes-Ramírez, A.; Mejía-Bautista, M.A.; Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Reyes-Ramírez, A. Actividad in Vitro de Bacillus spp. En La Inhibición de Crecimiento Micelial de Fusarium Equiseti Y Fusarium solani Aislado de Chile Habanero (Capsicum chinense Jacq.). Agrociencia 2016, 50, 1123–1135. [Google Scholar]
- Mushtaq, M.; Hashmi, M.H. Fungi Associated with Wilt Disease of Capsicum in Sindh, Pakistan. Pak. J. Bot. 1997, 29, 217–222. [Google Scholar]
- Gagkaeva, T.; Gavrilova, O.; Orina, A. First Detection of Fusarium globosum in Small Grain Cereals on Ural and Siberian Territory. Plant Prot. News 2019, 10–18. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen Population Genetics, Evolutionary Potential, and Durable Resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, W.; Nirenberg, H.; Eckart, I.; Rummland, I.; Schwarz, R. The Genus Fusarium: A Pictorial Atlas; Kommissionsverlag P. Parey: Berlin, Germany, 1982; Volume 209. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0-470-27646-0. [Google Scholar]
- Gawel, N.; Jarret, R. A Modified CTAB DNA Extraction Procedure for Musa and Ipomoea. Plant Mol. Biol. Report. 1991, 9, 262–266. [Google Scholar] [CrossRef]
- Aaij, C.; Borst, P. The Gel Electrophoresis of DNA. Biochim. Biophys. Acta-Nucleic Acids Protein Synth. 1972, 269, 192–200. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple Sequence Alignment Using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Townsend, G. Methods for Estimating Losses Caused by Diseases in Fungicide Experiments. Plant Dis. Report. 1943, 27, 340–343. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Engalycheva, I.A.; Kozar, E.G.; Kameneva, A.V.; Kornilova, M.S.; Cucumis Melo, L. Pathocomplex Micromycetes Composition and Aggressiveness in Dry Farming Land in Volgograd Region. Biosfera 2022, 14, 311–315. [Google Scholar] [CrossRef]
- Ramdial, H.; Latchoo, R.K.; Hosein, F.N.; Rampersad, S.N. Phylogeny and Haplotype Analysis of Fungi Within the Fusarium Incarnatum-Equiseti Species Complex. Phytopathology 2017, 107, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to Names—Restyling the Fusarium incarnatum-equiseti Species Complex. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Laraba, I.; McCormick, S.P.; Vaughan, M.M.; Geiser, D.M.; O’Donnell, K. Phylogenetic Diversity, Trichothecene Potential, and Pathogenicity within Fusarium sambucinum Species Complex. PLoS ONE 2021, 16, e0245037. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, T.B.; Gao, A.; Ingram, T.; Louws, F.J. Pathogenomics Characterization of an Emerging Fungal Pathogen, Fusarium oxysporum f. sp. lycopersici in Greenhouse Tomato Production Systems. Front. Microbiol. 2020, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Armenta, Q.A.; Tovar-Pedraza, J.M.; Apodaca-Sánchez, M.A.; Correia, K.C.; Sauceda-Acosta, C.P.; Camacho-Tapia, M.; Beltrán-Peña, H. Phylogeny and Pathogenicity of Soilborne Fungi Associated with Wilt Disease Complex of Tomatillo (Physalis ixocarpa) in Northern Sinaloa, Mexico. Eur. J. Plant Pathol. 2020, 157, 733–749. [Google Scholar] [CrossRef]
- Stefańczyk, E.; Sobkowiak, S. Isolation, Identification and Preservation of Fusarium spp. Causing Dry Rot of Potato Tubers. Plant Breed. Seed Sci. 2017, 76, 45–51. [Google Scholar] [CrossRef]
- Song, H.-H.; Lee, H.-S.; Jeong, J.-H.; Park, H.-S.; Lee, C. Diversity in Beauvericin and Enniatins H, I, and MK1688 by Fusarium oxysporum Isolated from Potato. Int. J. Food Microbiol. 2008, 122, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sunpapao, A.; Suwannarach, N.; Kumla, J.; Dumhai, R.; Riangwong, K.; Sanguansub, S.; Wanchana, S.; Arikit, S. Morphological and Molecular Identification of Plant Pathogenic Fungi Associated with Dirty Panicle Disease in Coconuts (Cocos nucifera) in Thailand. J. Fungi 2022, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, G.; Matic, S.; Guarnaccia, V.; Garibaldi, A.; Gullino, M.L. First Report of Fusarium clavum Causing Leaf Spot and Fruit Rot on Tomato in Italy. Plant Dis. 2021, 105, 2250. [Google Scholar] [CrossRef]
- Rabaaoui, A.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.O.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium Species Isolated from Sudden Decline Syndrome and Leaf Wilt Symptoms on Date Palms (Phoenix dactylifera) in Tunisia. Toxins 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Azil, N.; Stefańczyk, E.; Sobkowiak, S.; Chihat, S.; Boureghda, H.; Śliwka, J. Identification and Pathogenicity of Fusarium spp. Associated with Tuber Dry Rot and Wilt of Potato in Algeria. Eur. J. Plant Pathol. 2021, 159, 495–509. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Guarnaccia, V.; Gullino, M.L.; Garibaldi, A. Emerging Leafy Vegetable Crop Diseases Caused by the Fusarium incarnatum-equiseti Species Complex. Phytopathol. Mediterr. 2020, 59, 303–317. [Google Scholar]
- Meshram, V.; Elazar, M.; Maymon, M.; Sharma, G.; Shawahna, R.; Belausov, E.; Charuvi, D.; Freeman, S. Endophytic Fusarium Clavum Confers Growth and Salt Tolerance in Cucumis melo. Environ. Exp. Bot. 2023, 206, 105153. [Google Scholar] [CrossRef]
- Chang, K.; Hwang, S.; Conner, R.; Ahmed, H.; Zhou, Q.; Turnbull, G.; Strelkov, S.; McLaren, D.; Gossen, B. First Report of Fusarium Proliferatum Causing Root Rot in Soybean (Glycine max L.) in Canada. Crop. Prot. 2015, 67, 52–58. [Google Scholar] [CrossRef]
- Seefelder, W.; Humpf, H.-U.; Schwerdt, G.; Freudinger, R.; Gekle, M. Induction of Apoptosis in Cultured Human Proximal Tubule Cells by Fumonisins and Fumonisin Metabolites. Toxicol. Appl. Pharmacol. 2003, 192, 146–153. [Google Scholar] [CrossRef]
- Scheffer, R.P. Role of Toxins in Evolution and Ecology of Plant Pathogenic Fungi. Experientia 1991, 47, 804–811. [Google Scholar] [CrossRef]


| Strain | Fusarium Species | Origin | Substrate | Year | ID GenBank | % Similarities EF1a * | ||
|---|---|---|---|---|---|---|---|---|
| ITS1/4 | SSU | EF1-a | ||||||
| F-14-20 (T1) | F. clavus | Simferopol | stem | 2022 | PP033904 | PP033928 | PP054861 | 99.33 |
| F-38-21 (T3) | F. clavus | Simferopol | root | 2022 | PP033903 | PP033927 | PP054862 | 99.66 |
| F-56-21 (T7) | F. clavus | Simferopol | root | 2022 | PP033902 | PP033926 | PP054863 | 99.61 |
| F-53-22 (T6) | F. verticillioides | Krymsk | stem | 2021 | PP033900 | PP033924 | PP054856 | 99.54 |
| F-14-22 (T13) | F. verticillioides | Simferopol | stem | 2021 | PP033899 | PP033923 | PP054857 | 99.69 |
| F-14-20 (T8) | F. solani | Simferopol | stem | 2020 | PP033908 | PP033932 | PP054853 | 95.26 |
| F-28-19 (T10) | F. oxysporum | Simferopol | stem | 2022 | PP033907 | PP033930 | PP054859 | 98.54 |
| F-41-19 (T4) | F. commune | Simferopol | root | 2021 | PP033906 | PP033929 | PP054860 | 98.87 |
| F-23-20 (R1) | F. clavus | Krymsk | stem | 2021 | PP033913 | PP033937 | PP054850 | 99.5 |
| F-29-20 (T2) | F. clavus | Krymsk | stem | 2021 | PP033912 | PP033936 | PP054849 | 99.48 |
| F-5-21 (R4) | F. sporotrichioides | Simferopol | root | 2021 | PP033909 | PP033933 | PP054851 | 98.86 |
| F-17-21 (T5) | F. sporotrichioides | Simferopol | root | 2021 | PP033910 | PP033934 | PP054854 | 99.19 |
| F-19-21 (R5) | F. sporotrichioides | Simferopol | stem | 2021 | PP033905 | PP033931 | PP054858 | 98.22 |
| F-14-22 (T9) | F. torulosum | Simferopol | stem | 2022 | PP033911 | PP033935 | PP054855 | 99.68 |
| Primer | Sequence 5′-3′ | Product Size (bp) | Tm (°C) | A Source |
|---|---|---|---|---|
| ITS1 ITS4 | F:TCCGTAGGTGAACCTGCGG R:TCCTCCGCTTATTGATATGC | ~550 | 62* | [21] |
| ITS5 ITS4 | F: GGAAGTAAAAGTCGTAACAAGG R:TCCTCCGCTTATTGATATGC | ~550 | 62* | [21] |
| EF-1 EF-2 | F:ATGGGTAAGGARGACAAGAC R:GGARGTACCAGTSATCATG | ~680 | 55* | [19] |
| Strains | Type | Degree of Aggressiveness * | Withering, Score ** | |
|---|---|---|---|---|
| Range | Average | |||
| Control | 0 | 0 a | ||
| F-41-19(T4) | F. commune | WA | 0–0.5 | 0.3 a |
| F-14-22 | F. verticillioides | WA | 0–0.5 | 0.5 a |
| F-38-21 | F. clavus | WA | 0–0.5 | 0.5 a |
| F-17-21 | F. sporotrichioides | WA | 0–0.5 | 0.2 a |
| F-29-20 | F. clavus | WA | 0–0.5 | 0.2 a |
| F-31-22 | F. torulosum | WA | 0–0.5 | 0.2 a |
| F-21-20 | F. solani | WA | 0–0.5 | 0.2 a |
| F-15-22(T9) | F. torulosum | HA | 0–3.1 | 2.5 bc |
| F-5-21(R4) | F. sporotrichioides | HA | 0–3.3 | 2.6 bc |
| F-28-19(T10) | F. oxysporum | HA | 0.5–4 | 2.8 bc |
| F-23-20(R1) | F. clavus | HA | 0.5–4 | 3.0 c |
| F-53-22(T6) | F. verticillioides | HA | 0.5–4 | 3.2 cd |
| F-56-21(T7) | F. clavus | HA | 0.5–4 | 3.5 de |
| F-14-20(T8) | F. solani | HA | 0.5–4 | 4.0 e |
| Strains | Type | DSI *, % | EA **, % | Proportion of *** Susceptible Samples, % | ||
|---|---|---|---|---|---|---|
| Range | Average | Root | Stem | |||
| Control | 0 | 0a | 0 a | 0 a | 0 | |
| F-5-21(R4) | F. sporotrichioides | 0–5.4 | 0.1 a | 0 a | 0 a | 0 |
| F-15-22(T9) | F. torulosum | 0–5.4 | 0.1 a | 0 a | 0 a | 0 |
| F-14-20(T1) | F. solani | 12.5–100 | 87.5 b | −28.0 b | −24.0 b | 68.8 |
| F-28-19(T10) | F. oxysporum | 12.5–100 | 89.2 b | −24.0 b | −22.0 b | 74.5 |
| F-56-21(T7) | F. clavus | 12.5–100 | 96.8 bc | −39.0 bc | −40.0 bc | 76.0 |
| F-53-22(T6) | F. verticillioides | 12.5–100 | 93.5 bc | −41.0 bc | −44.0 bc | 76.0 |
| Characteristic | F. verticillioides, F-53-22(T6) | F. oxysporum, F-28-19(T10) | F. solani, F-14-20(T1) | F. clavus, F-56-21(T7) |
|---|---|---|---|---|
| growth rate on PDA, mm/day±SE | 13.3 ± 0.21 | 14.7 ± 0.36 | 10.5 ± 0.30 | 14.3 ± 0.27 |
| mycelium | White, dense mycelium | White, felt mycelium | White, felt mycelium | White, felt, uniform mycelium |
| type of conidiophores | monophialides | monophialides | monophialides | monophialides |
| pigment | yellowish on days 19–21 | lemon on days 9–12 | blue on days 5–14 | lilac on days 4–5 |
| Microconidia: | ||||
| size, µm | 8.3 ± 1.1 × 2.9 ± 0.7 | 7.9 ± 1.1 × 2.8 ± 0.3 | 12.1 ± 1.1 × 4.7 ± 0.6 | 4.6 ± 0.6 × 2.4 ± 0.2 |
| septation | 0 | 0–1 | 0–1 | 0–1 |
| shape | oval | oval | oval to slightly curved | oval to slightly curved |
| Macroconidia: | ||||
| size, µm | 16.5 ± 2.5 × 3.1 ± 1.1 | 15.3 ± 4.1 × 3.2 ± 0.9 | 18.5 ± 0.9 × 5.9 ± 0.5 | 15.1 ± 0.5 × 2.8 ± 0.3 |
| septation | 3–4 | 3–4 | 2–3 | 3–5 |
| shape | straight to slightly curved | falcate to almost straight | slightly curved | fusiform or slightly curved |
| Chlamydospores: | ||||
| size, µm | not formed | 8.5 ± 0.7 × 8.5 ± 0.9 | 8.6 ± 0.8 × 8.6 ± 0.9 | 10.4 ± 0.9 × 10.1 ± 1.1 |
| shape | not formed | globose | globose | globose |
| abundance | not formed | abundant, single, or in pairs | abundant, single, or in pairs | formed in large quantities of 3–4 pieces in a chain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engalycheva, I.; Kozar, E.; Frolova, S.; Vetrova, S.; Tikhonova, T.; Dzhos, E.; Engalychev, M.; Chizhik, V.; Martynov, V.; Shingaliev, A.; et al. Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity. Microorganisms 2024, 12, 343. https://doi.org/10.3390/microorganisms12020343
Engalycheva I, Kozar E, Frolova S, Vetrova S, Tikhonova T, Dzhos E, Engalychev M, Chizhik V, Martynov V, Shingaliev A, et al. Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity. Microorganisms. 2024; 12(2):343. https://doi.org/10.3390/microorganisms12020343
Chicago/Turabian StyleEngalycheva, Irina, Elena Kozar, Svetlana Frolova, Svetlana Vetrova, Tatyana Tikhonova, Elena Dzhos, Myazar Engalychev, Vera Chizhik, Viktor Martynov, Andrey Shingaliev, and et al. 2024. "Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity" Microorganisms 12, no. 2: 343. https://doi.org/10.3390/microorganisms12020343
APA StyleEngalycheva, I., Kozar, E., Frolova, S., Vetrova, S., Tikhonova, T., Dzhos, E., Engalychev, M., Chizhik, V., Martynov, V., Shingaliev, A., Dudnikova, K., Dudnikov, M., & Kostanchuk, Y. (2024). Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity. Microorganisms, 12(2), 343. https://doi.org/10.3390/microorganisms12020343











