The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
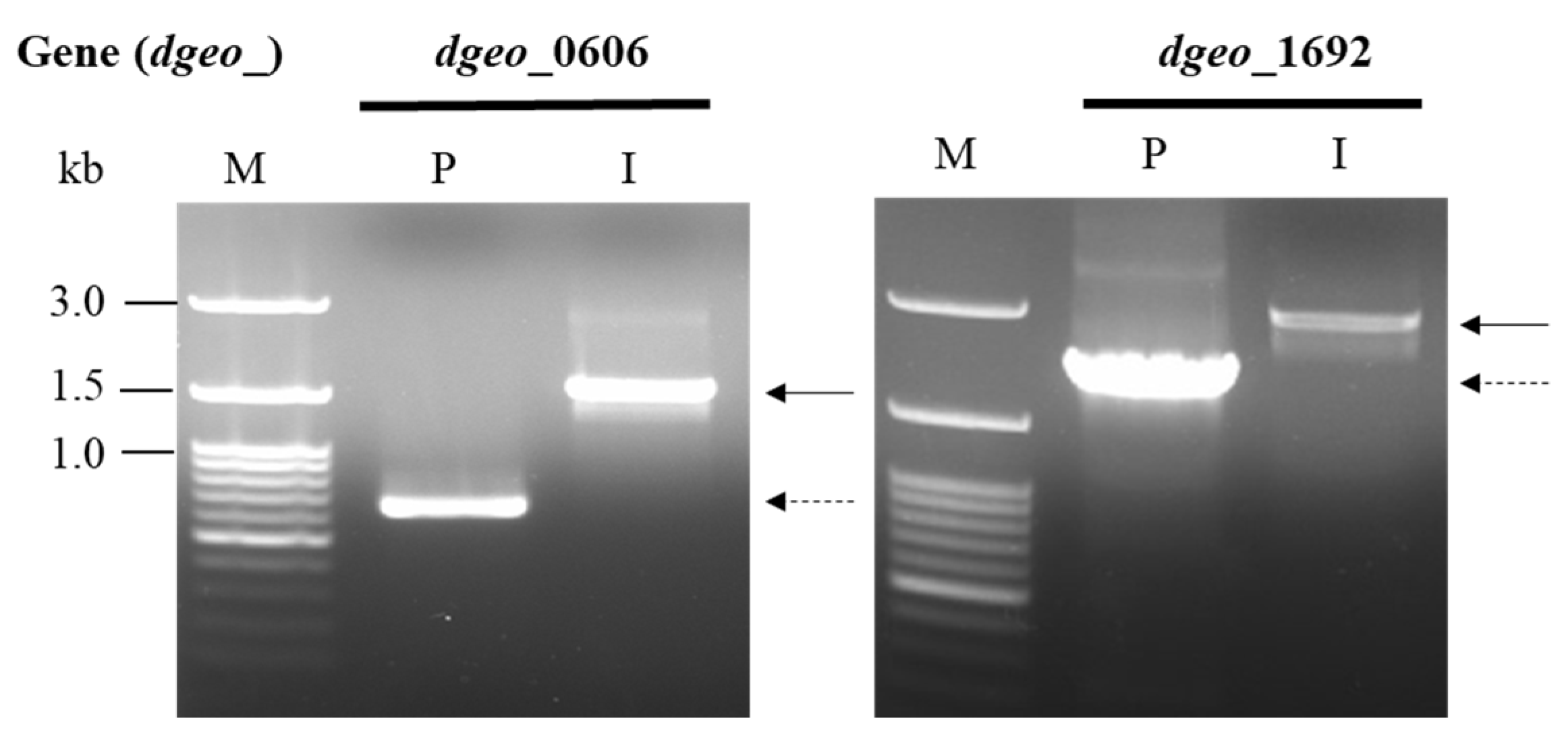
2.2. Construction of the ∆dgeo_0606 and ∆dgeo_1692 Mutant Strains
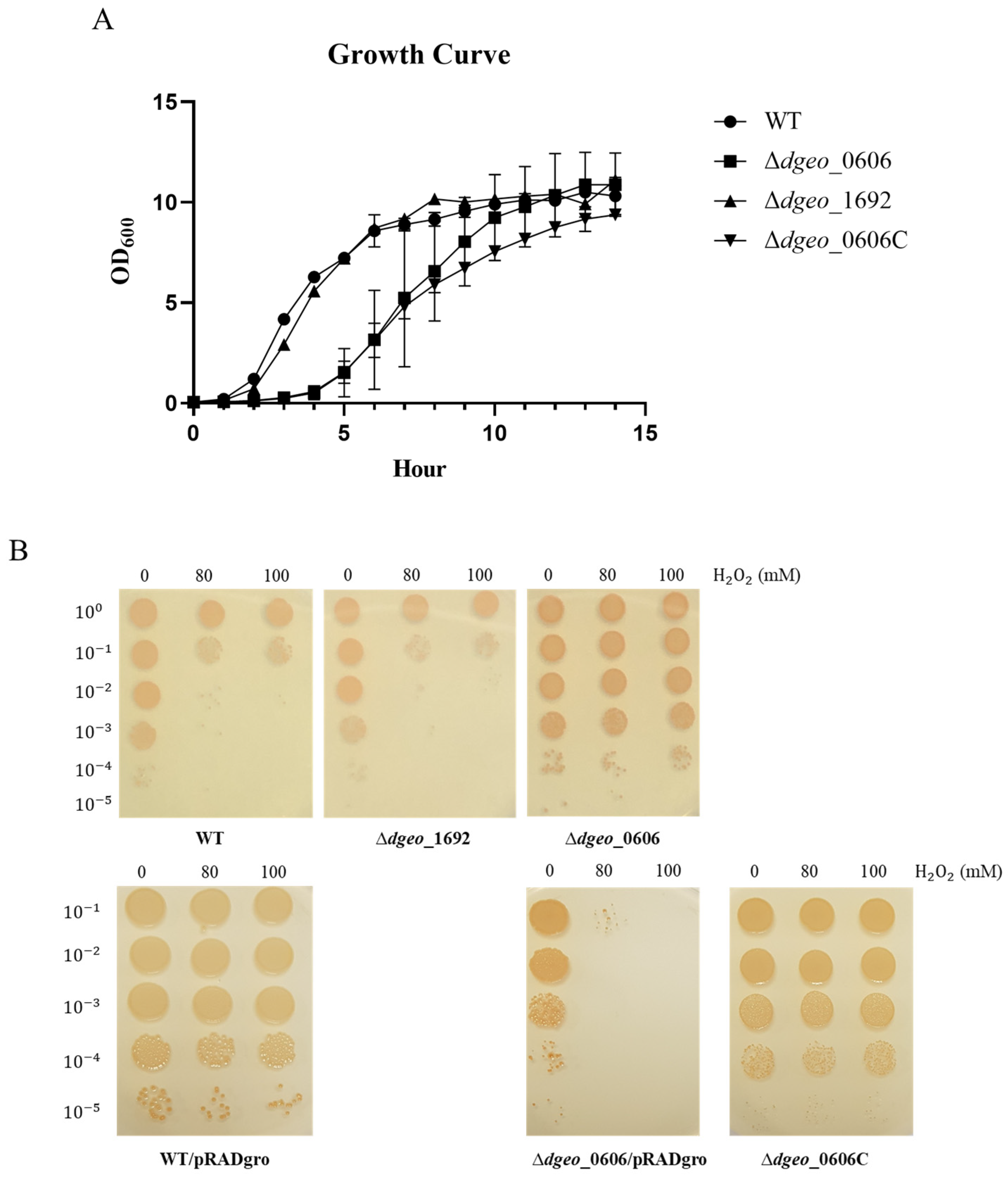
2.3. Growth Curves of Wild-Type and Mutant Strains
2.4. Viability Test in H2O2 Oxidative Stress Conditions
2.5. Gamma Radiation Treatment Conditions
2.6. Selection of Non-Pigmented Colonies and Detection of Transposition of Insertion Sequences
2.7. Analysis of IS Types in Transposition Events
2.8. Quantitative RT-PCR Analysis for Measurement of Gene Expression Level
3. Results
3.1. Construction of the ∆dgeo_0606 and ∆dgeo_1692 Mutants of D. geothermalis
3.2. Selection of Non-Pigmented Clones by Gamma-Irradiation from WT and Mutant Strains
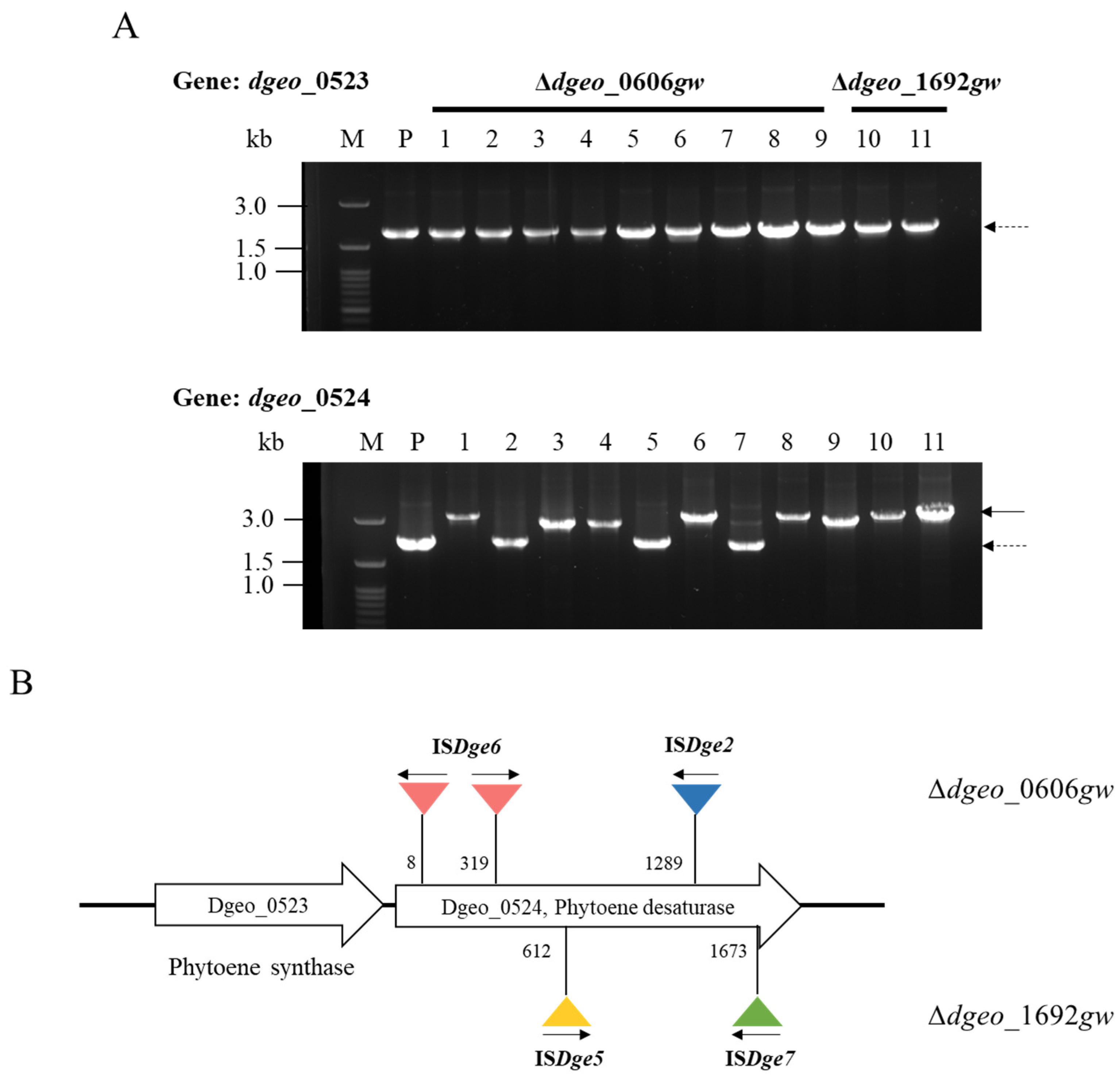
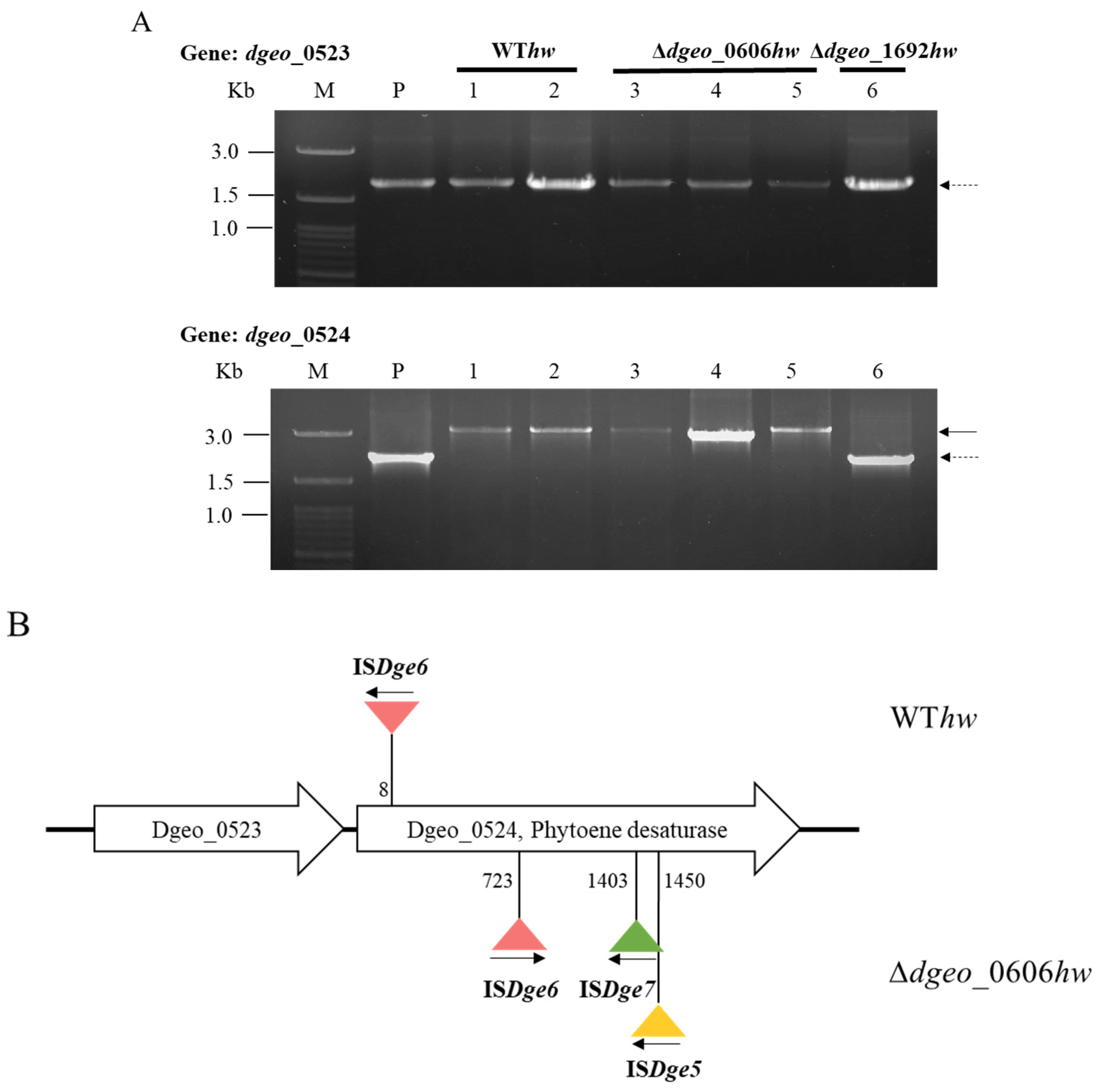
3.3. Detection of IS Transposition Events in Non-Pigmented Strains
3.4. Determination of IS Type and Transposition Loci under Gamma-Irradiation
3.5. The Effect of H2O2 Treatment on WT, ∆dgeo_0606, and ∆dgeo_1692
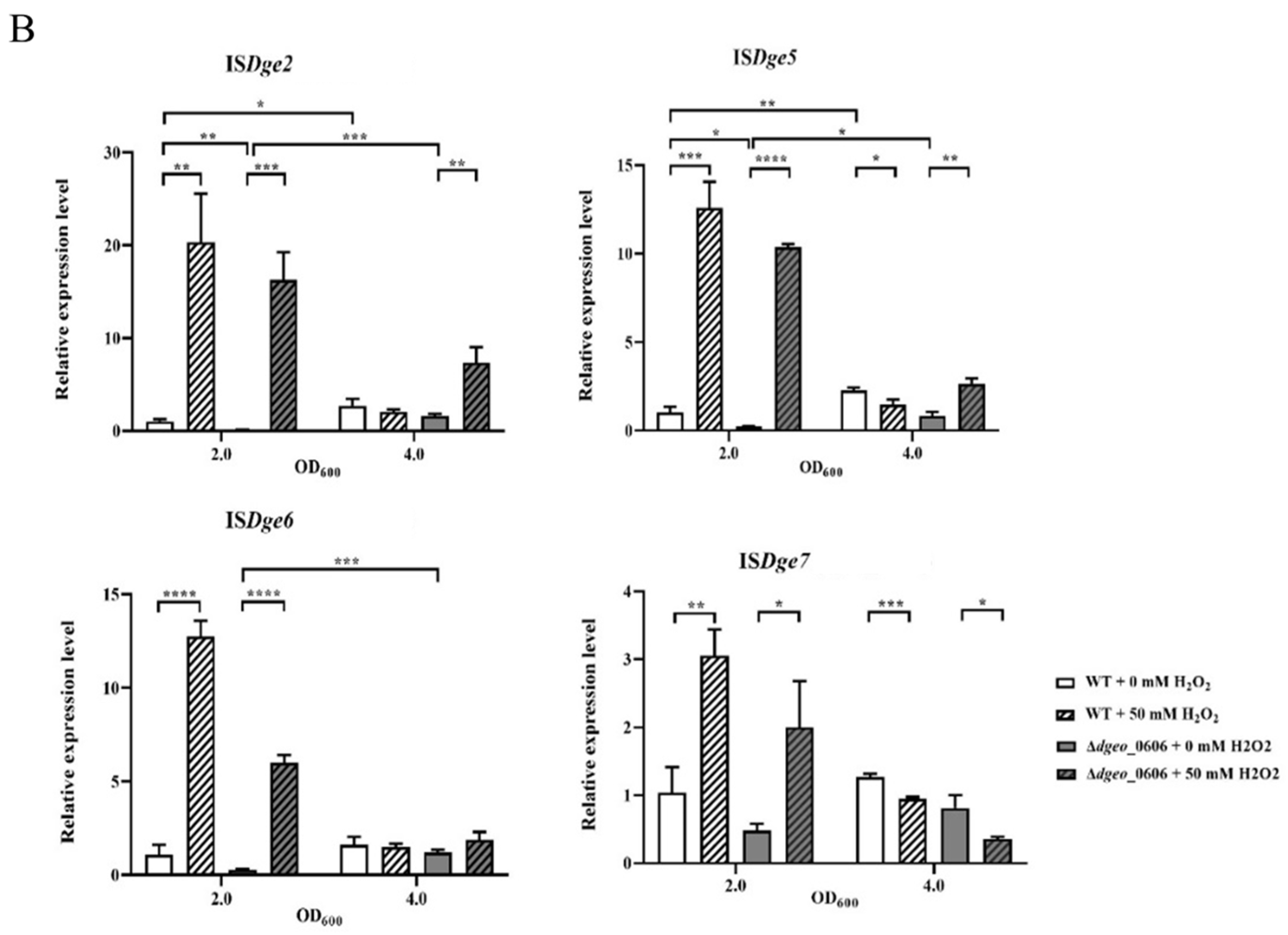
3.6. Gene Expression Levels of Catalase, Cystine Importers, and Transposases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—The consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Battista, J.R.; Earl, A.M.; Park, M.J. Why is Deinococcus radiodurans so resistant to ionizing radiation? Trend Microbiol. 1999, 7, 362–365. [Google Scholar] [CrossRef]
- Agapov, A.A.; Kulbachinskiy, A.V. Mechanisms of stress resistance and gene regulation in the radioresistant bacterium Deinococcus radiodurans. Biochemistry 2015, 80, 1201–1216. [Google Scholar] [CrossRef]
- Loprasert, S.; Vattanaviboon, P.; Pratuan, W.; Chamnongpol, S.; Mongkolsuk, S. Regulation of the oxidative stress protective enzymes, catalase and superoxide dismutase in Xanthomonas—A review. Gene 1996, 179, 33–37. [Google Scholar] [CrossRef]
- Hua, Y.; Narumi, I.; Gao, G.; Tian, B.; Satoh, K.; Kitayama, S. PprI: A general switch responsible for extreme radioresistance of Deinococcus radiodurans. Biochem. Biophys. 2003, 306, 354–360. [Google Scholar] [CrossRef]
- Makarova, K.S.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE 2007, 9, e955. [Google Scholar] [CrossRef]
- Blasius, M.; Hubscher, U.; Sommer, S. Deinococcus radiodurans: What belongs to the survival kit? Crit. Rev. Biochem. Mol. Biol. 2008, 43, 221–238. [Google Scholar] [CrossRef]
- Daly, M.J. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef]
- Helmann, J.D. Bacillithiol, a new player in bacterial redox homeostasis. Antioxid. Redox Signal. 2011, 15, 123–133. [Google Scholar] [CrossRef]
- Krisko, A.; Radman, M. Biology of extreme radiation resistance: The way of Deinococcus radiodurans. Cold Spring Harb. Perspect. Biol. 2013, 5, a012765. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.; Lim, S.; Sim, J.; Rhie, H.G.; Lee, S.J. Oxidative stress response of Deinococcus geothermalis via a cystine importer. J. Microbiol. 2017, 55, 137–146. [Google Scholar] [CrossRef]
- Lim, S.; Jung, J.H.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. 2019, 43, 19–52. [Google Scholar] [CrossRef]
- de Groot, A.; Blanchard, L.; Rouhier, N.; Rey, P. Thiol reductases in Deinococcus bacteria and roles in stress tolerance. Antioxidants 2022, 11, 561. [Google Scholar] [CrossRef]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, 32–36. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. 2014, 38, 865–891. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Varani, A.; Ton-Hoang, B.; Chandler, M. Everyman’s guide to bacterial insertion sequences. Microbiol. Spectr. 2015, 3, 555–590. [Google Scholar] [CrossRef]
- Mahillon, J.; Chandler, M. Insertion sequences. Microbiol. Mol. Biol. Rev. 1998, 62, 725–774. [Google Scholar] [CrossRef]
- Narumi, I.; Cherdchu, K.; Kitayama, S.; Watanabe, H. The Deinococcus radiodurans uvrA gene: Identification of mutation sites in two mitomycin-sensitive strains and the first discovery of insertion sequence element from deinobacteria. Gene 1997, 198, 115–126. [Google Scholar] [CrossRef]
- Ohtsubo, Y.; Genka, H.; Komatsu, H.; Nagata, Y.; Tsuda, M. High-temperature-induced transposition of insertion elements in Burkholderia multivorans. Appl. Environ. Microbiol. 2005, 71, 1822–1828. [Google Scholar] [CrossRef][Green Version]
- Pasternak, C.; Ton-Hoang, B.; Coste, G.; Bailone, A.; Chandler, M.; Sommer, S. Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. PLoS Genet. 2010, 6, e1000799. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 1997, 47, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Bae, M.K.; Choi, N.; Lee, S.J. Genome plasticity by insertion sequences learned from a case of radiation-resistant bacterium Deinococcus geothermalis. Bioinform. Biol. Insights 2021, 15, 11779322211037437. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, N.; Bae, M.K.; Choo, K.; Lee, S.J. Transposition of insertion sequences was triggered by oxidative stress in radiation-resistant bacterium Deinococcus geothermalis. Microorganisms 2019, 7, 446. [Google Scholar] [CrossRef]
- Lee, C.; Choo, K.; Lee, S.J. Active transposition of insertion sequences by oxidative stress in Deinococcus geothermalis. Front. Microbiol. 2020, 11, 558747. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lee, C.; Shin, E.; Lee, S.J. Influence of redox imbalances on the transposition of insertion sequences in Deinococcus geothermalis. Antioxidants 2021, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Shin, E.; Lee, C.; Choi, N.; Kim, Y.; Yoon, K.S.; Lee, S.J. Transposition of insertion sequences by dielectric barrier discharge plasma and gamma irradiation in the radiation-resistant bacterium Deinococcus geothermalis. J. Microbiol. Methods 2022, 196, 106473. [Google Scholar] [CrossRef]
- Borukhov, S.; Nudler, E. RNA polymerase holoenzyme: Structure, function and biological implications. Curr. Opin. Microbiol. 2003, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, M.J.; Wiedmann, M.; Boor, K.J. Alternative sigma factors and their roles in bacterial virulence. Microbiol. Mol. Biol. Rev. 2005, 69, 527–543. [Google Scholar] [CrossRef]
- Lange, R.; Hengge-Aronis, R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 1991, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C.; Libby, S.J.; Buchmeier, N.A.; Loewen, P.C.; Switala, J.; Harwood, J.; Guiney, D.G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 11978–11982. [Google Scholar] [CrossRef]
- Yildiz, F.H.; Schoolnik, G.K. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 1998, 180, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, F.; Bally, M.; Chapon-Herve, V.; Michel, G.; Lazdunski, A.; Williams, P.; Stewart, G.S. RpoS-dependent stress tolerance in Pseudomonas aeruginosa. Microbiology 1999, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.F.; Bono, J.L.; Carroll, J.A.; Stewart, P.; Tilly, K.; Rosa, P. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J. Bacteriol. 2000, 182, 2909–2918. [Google Scholar] [CrossRef]
- Choo, K.; Kim, M.; Abdi Nansa, S.; Bae, M.K.; Lee, C.; Lee, S.J. Redox potential change by the cystine importer affected on enzymatic antioxidant protection in Deinococcus geothermalis. Antonie Leeuwenhoek 2020, 113, 779–790. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Carotenoid biosynthesis in extremeophilic Deinococcus-Thermus bacteria. Trend Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.Z.; Zhang, Z.; Butcher, A.M.; Moshayedi, A.; Saier, M.H., Jr. Hopping into a host seat: Role of DNA structural features on IS5-mediated gene activation and inactivation under stress. PLoS ONE 2017, 12, e0180156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yen, M.R.; Saier, M.H., Jr. Precise excision of IS5 from the intergenic region between the fucPIK and the fucAO operons and mutational control of fucPIK operon expression in Escherichia coli. J. Bacteriol. 2010, 192, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kukita, C.; Humayun, M.Z.; Saier, M.H., Jr. Environmental-directed activation of the Escherichia coli flhDC operon by transposons. Microbiology 2017, 163, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, C.; Dulermo, R.; Ton-Hoang, B.; Debuchy, R.; Siguier, P.; Coste, G.; Chandler, M.; Sommer, S. ISDra2 transposition in Deinococcus radiodurans is downregulated by TnpB. Mol. Microbiol. 2013, 88, 443–455. [Google Scholar] [CrossRef]
- He, S.; Corneloup, A.; Guynet, C.; Lavatine, L.; Caumont-Sarcos, A. The IS200/IS605 family and “peel and paste” single-strand transposition mechanism. Microbiol. Spectr. 2015, 3, 609–630. [Google Scholar] [CrossRef]
- Shin, E.; Ye, Q.; Lee, S.J. Active transposition of insertion sequences in prokaryotes: Insights from the response of Deinococcus geothermalis to oxidative stress. Antioxidants 2022, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 1993, 47, 597–626. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Oyston, P.C.F. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.K.; Lidstrom, M.E. Involvement of two putative sigma factors in stress response of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 2002, 184, 6182–6189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paget, M.S. Bacterial sigma factors and anti-sigma factors: Structure, function and distribution. Biomolecules 2015, 5, 1245–1265. [Google Scholar] [CrossRef]
- de Avila e Silva, S.; Echeverrigaray, S.; Gerhardt, G.J. BacPP: Bacterial promoter prediction—A tool for accurate sigma-factor specific assignment in enterobacteria. J. Theor. Biol. 2011, 287, 92–99. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lee, S.; Shin, E.; Abdi Nansa, S.; Lee, S.-J. The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis. Microorganisms 2024, 12, 328. https://doi.org/10.3390/microorganisms12020328
Park JH, Lee S, Shin E, Abdi Nansa S, Lee S-J. The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis. Microorganisms. 2024; 12(2):328. https://doi.org/10.3390/microorganisms12020328
Chicago/Turabian StylePark, Ji Hyun, Sohee Lee, Eunjung Shin, Sama Abdi Nansa, and Sung-Jae Lee. 2024. "The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis" Microorganisms 12, no. 2: 328. https://doi.org/10.3390/microorganisms12020328
APA StylePark, J. H., Lee, S., Shin, E., Abdi Nansa, S., & Lee, S.-J. (2024). The Transposition of Insertion Sequences in Sigma-Factor- and LysR-Deficient Mutants of Deinococcus geothermalis. Microorganisms, 12(2), 328. https://doi.org/10.3390/microorganisms12020328






