Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Methods
2.1. Population
2.2. DNA Extraction and Whole Genome Sequencing
2.3. Bioinformatics Analysis and Drug Resistance Prediction
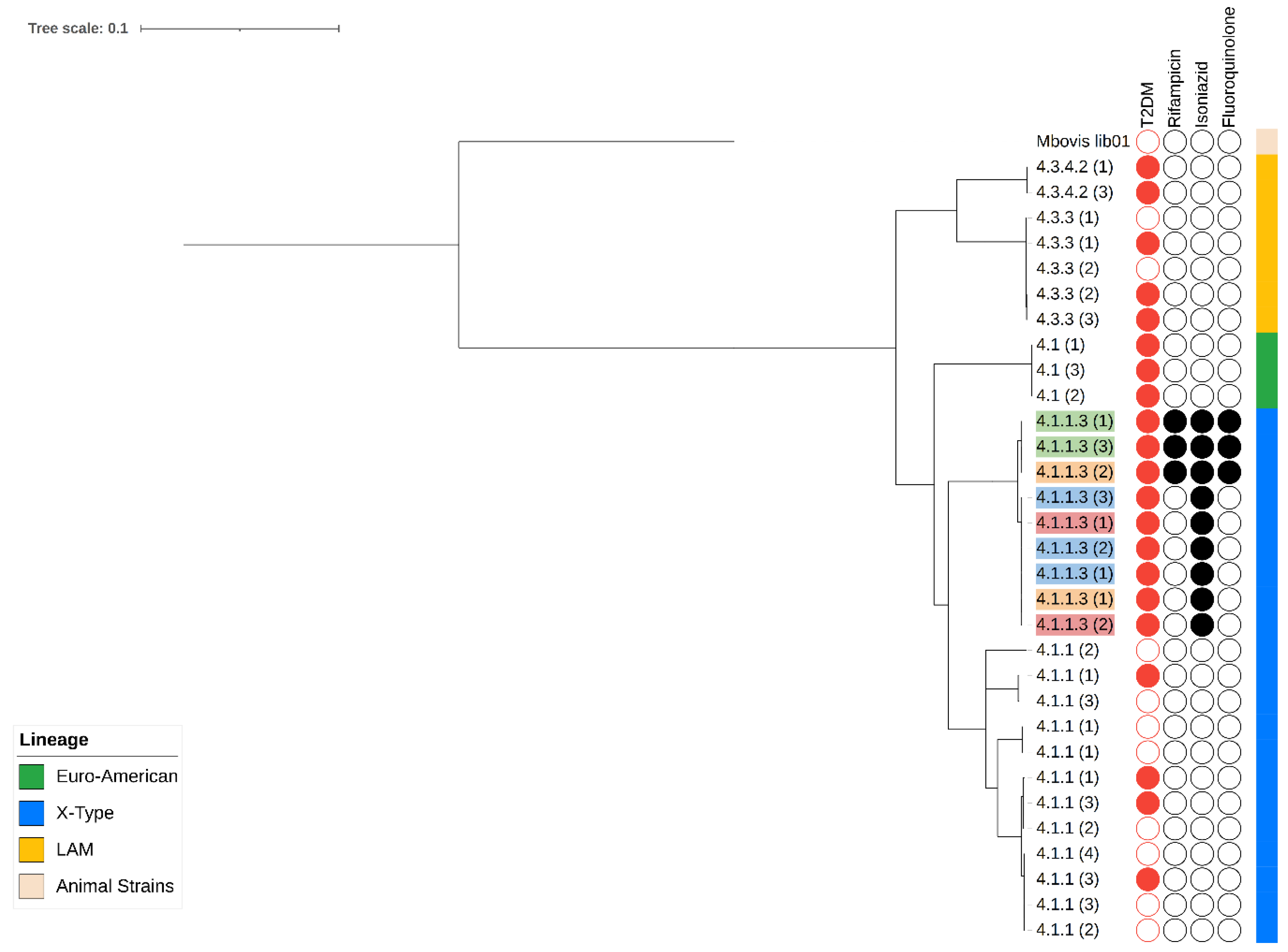
2.4. Phylogenetic Analysis and Cluster Identification
2.5. Allelic Frequency, Mutation Rate, and Statistical Analysis
3. Results
3.1. Population Characteristics and Genotypic Resistance
3.2. Characterization of Variants Associated with Resistance and Phylogeny
3.3. Characterization of Serial Isolates: Mutations in Canonical Resistance Genes and Phylogeny
3.4. Total Number of Polymorphisms and Quantification of the Mutation Rate of Serial Isolates
3.5. Phylogenetic Relationship and Mutation Rates of Serial Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Annual report of tuberculosis. In Annual Global TB Report of WHO; WHO: Geneva, Switzerland, 2022; Volume 8, ISBN 978-92-4-006172-9. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022#:~:text=context of global...-,Download,-Read More%0Ahtt (accessed on 22 October 2023).
- Jeon, C.Y.; Murray, M.B. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Med. 2008, 5, 1091–1101. [Google Scholar] [CrossRef]
- Ponce-De-Leon, A.; de Lourdes Garcia-Garcia, M.; Garcia-Sancho, M.C.; Gomez-Perez, F.J.; Valdespino-Gomez, J.L.; Olaiz-Fernandez, G.; Rojas, R.; Ferreyra-Reyes, L.; Cano-Arellano, B.; Bobadilla, M.; et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004, 27, 1584–1590. [Google Scholar] [CrossRef]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.E.; Goonesekera, S.D.; Murray, M.B. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Adane, H.T.; Howe, R.C.; Wassie, L.; Magee, M.J. Diabetes mellitus is associated with an increased risk of unsuccessful treatment outcomes among drug-susceptible tuberculosis patients in Ethiopia: A prospective health facility-based study. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 31, 100368. [Google Scholar] [CrossRef]
- Pérez-Navarro, L.M.; Fuentes-Domínguez, F.J.; Zenteno-Cuevas, R. Type 2 diabetes mellitus and its influence in the development of multidrug resistance tuberculosis in patients from southeastern Mexico. J. Diabetes Complicat. 2015, 29, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Jiang, G.; Du, J.; Li, L.; Yue, W.; Harries, A.D.; Gudmund Hinderaker, S.; Lin, Y. Is resistance to anti-tuberculosis drugs associated with type 2 diabetes mellitus? A register review in Beijing, China. Glob. Health Action 2014, 7, 24022. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Shin, J.W.; Kim, J.Y.; Park, I.W.; Choi, B.W.; Choi, J.C.; Kim, Y.S. The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1305–1310. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khattak, M.; Mushtaq, U.; Latif, M.; Ahmad, I.; Rasool, M.F.; Shakeel, S.; Hayat, K.; Hussain, R.; Alhazmi, G.A.; et al. The impact of diabetes mellitus on the emergence of multi-drug resistant tuberculosis and treatment failure in TB-diabetes comorbid patients: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1244450. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P.; Jamwal, S.V.; Saquib, N.; Sinha, N.; Siddiqui, Z.; Manivel, V.; Chatterjee, S.; Rao, K.V.S. Pathogenicity of Mycobacterium tuberculosis Is Expressed by Regulating Metabolic Thresholds of the Host Macrophage. PLoS Pathog. 2014, 10, e1004265. [Google Scholar] [CrossRef]
- Meng, F.; Lan, L.; Wu, G.; Ren, X.; Yuan, X.; Yang, M.; Chen, Q.; Peng, X.; Liu, D. Impact of diabetes itself and glycemic control status on tuberculosis. Front. Endocrinol. 2023, 14, 1250001. [Google Scholar] [CrossRef]
- Ruslami, R.; Nijland, H.M.J.J.; Adhiarta, I.G.N.; Kariadi, S.H.K.S.K.S.; Alisjahbana, B.; Aarnoutse, R.E.; Van Crevel, R. Pharmacokinetics of Antituberculosis Drugs in Pulmonary Tuberculosis Patients with Type 2 Diabetes. Antimicrob. Agents Chemother. 2010, 54, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Nijland, H.M.J.; Ruslami, R.; Stalenhoef, J.E.; Nelwan, E.J.; Alisjahbana, B.; Nelwan, R.H.H.; van der Ven, A.J.A.M.; Danusantoso, H.; Aarnoutse, R.E.; van Crevel, R. Exposure to Rifampicin Is Strongly Reduced in Patients with Tuberculosis and Type 2 Diabetes. Clin. Infect. Dis. 2006, 43, 848–854. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.A.; Metwally, A.S.; Galal, A.A.A. Impact of diabetes mellitus on rifampicin’s plasma concentration and bioavailability in patients with tuberculosis: A systematic review and meta-analysis study. Therapies 2023, 78, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 63ra159. [Google Scholar] [CrossRef] [PubMed]
- Jafar, N.; Edriss, H.; Nugent, K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Meena, L.S. Factors Affecting Susceptibility to Mycobacterium tuberculosis: A Close View of Immunological Defence Mechanism. Appl. Biochem. Biotechnol. 2014, 174, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, B.I.; Schlesinger, L.S. Impact of diabetes on the natural history of tuberculosis. Diabetes Res. Clin. Pract. 2014, 106, 191–199. [Google Scholar] [CrossRef]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Pérol, L.; Lindner, J.M.; Caudana, P.; Nunez, N.G.; Baeyens, A.; Valle, A.; Sedlik, C.; Loirat, D.; Boyer, O.; Créange, A.; et al. Loss of immune tolerance to IL-2 in type 1 diabetes. Nat. Commun. 2016, 7, 13027. [Google Scholar] [CrossRef]
- Chen, H.; Su, L.; Bao, J.; Zhang, K.; Li, Y.; Mao, E. The impact of pulmonary tuberculosis on immunological and metabolic features of diabetic patients. Front. Immunol. 2022, 13, 973991. [Google Scholar] [CrossRef]
- Harishankar, M.; Selvaraj, P.; Bethunaickan, R. Influence of genetic polymorphism towards pulmonary tuberculosis susceptibility. Front. Med. 2018, 5, 213. [Google Scholar] [CrossRef]
- Kaushik, G.; Vashishtha, R.; Tripathi, H.; Yadav, R. Genetic polymorphism of toll-like receptors in HIV-I infected patients with and without tuberculosis co-infection. Int. J. Mycobacteriol. 2022, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sunder, S.R.; Hanumanth, S.R.; Gaddam, S.; Jonnalagada, S.; Valluri, V.L. Association of TAP 1 and 2 gene polymorphisms with human immunodeficiency virus-tuberculosis co-infection. Hum. Immunol. 2011, 72, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Godfroid, M.; Dagan, T.; Merker, M.; Kohl, T.A.; Diel, R.; Maurer, F.P.; Niemann, S.; Kupczok, A. Insertion and deletion evolution reflects antibiotics selection pressure in a mycobacterium tuberculosis outbreak. PLoS Pathog. 2020, 16, e1008357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, J.; Li, Y.; Wang, M.; Su, J.; Lu, Y.; López, M.G.; Qian, X.; Zhu, Z.; Wang, H.; et al. Mycobacterium tuberculosis clinical isolates carry mutational signatures of host immune environments. Sci. Adv. 2020, 6, eaba4901. [Google Scholar] [CrossRef]
- Nimmo, C.; Brien, K.; Millard, J.; Grant, A.D.; Padayatchi, N.; Pym, A.S.; O’Donnell, M.; Goldstein, R.; Breuer, J.; Balloux, F. Dynamics of within-host Mycobacterium tuberculosis diversity and heteroresistance during treatment. EBioMedicine 2020, 55, 102747. [Google Scholar] [CrossRef]
- Loiseau, C.; Brites, D.; Reinhard, M.; Zürcher, K.; Borrell, S.; Ballif, M.; Fenner, L.; Cox, H.; Rutaihwa, L.K.; Wilkinson, R.J.; et al. HIV Coinfection Is Associated with Low-Fitness rpoB Variants in Rifampicin-Resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2020, 64, e00782-20. [Google Scholar] [CrossRef]
- Pérez-Martínez, D.E.; Bermúdez-Hernández, G.A.; Madrazo-Moya, C.F.; Cancino-Muñoz, I.; Montero, H.; Licona-Cassani, C.; Muñiz-Salazar, R.; Comas, I.; Zenteno-Cuevas, R. SNPs in Genes Related to DNA Damage Repair in Mycobacterium Tuberculosis: Their Association with Type 2 Diabetes Mellitus and Drug Resistance. Genes 2022, 13, 609. [Google Scholar] [CrossRef]
- Bermudez-Hernández, G.A.; Pérez-Martínez, D.E.; Madrazo-Moya, C.F.; Cancino-Muñoz, I.; Comas, I.; Zenteno-Cuevas, R. Whole genome sequencing analysis to evaluate the influence of T2DM on polymorphisms associated with drug resistance in M. tuberculosis. BMC Genom. 2022, 23, 465. [Google Scholar] [CrossRef]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef]
- Van Soolingen, D.; Hermans, P.W.M.; De Haas, P.E.W.; Soll, D.R.; Van Embden, J.D.A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: Evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 1991, 29, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.A.; Utpatel, C.; Schleusener, V.; De Filippo, M.R.; Beckert, P.; Cirillo, D.M.; Niemann, S. MTBseq: A comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ 2018, 2018, e5895. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Ngo, T.M.; Teo, Y.Y. Genomic prediction of tuberculosis drug-resistance: Benchmarking existing databases and prediction algorithms. BMC Bioinform. 2019, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Coll, F.; McNerney, R.; Guerra-Assunção, J.A.; Glynn, J.R.; Perdigão, J.; Viveiros, M.; Portugal, I.; Pain, A.; Martin, N.; Clark, T.G.; et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat. Commun. 2014, 5, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.B.; Lin, P.L.; Chase, M.R.; Shah, R.R.; Iartchouk, O.; Galagan, J.; Mohaideen, N.; Ioerger, T.R.; Sacchettini, J.C.; Lipsitch, M.; et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat. Genet. 2011, 43, 482–488. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2012. [Google Scholar]
- Lempens, P.; Meehan, C.J.; Vandelannoote, K.; Fissette, K.; De Rijk, P.; Van Deun, A.; Rigouts, L.; De Jong, B.C. Isoniazid resistance levels of Mycobacterium tuberculosis can largely be predicted by high-confidence resistance-conferring mutations. Sci. Rep. 2018, 8, 3246. [Google Scholar] [CrossRef]
- Charoenpak, R.; Santimaleeworagun, W.; Suwanpimolkul, G.; Manosuthi, W.; Kongsanan, P.; Petsong, S.; Puttilerpong, C. Association Between the Phenotype and Genotype of Isoniazid Resistance Among Mycobacterium tuberculosis Isolates in Thailand. Infect. Drug Resist. 2020, 13, 627–634. [Google Scholar] [CrossRef]
- Mejía-Ponce, P.M.; Ramos-González, E.J.; Ramos-García, A.A.; Lara-Ramírez, E.E.; Soriano-Herrera, A.R.; Medellín-Luna, M.F.; Valdez-Salazar, F.; Castro-Garay, C.Y.; Núñez-Contreras, J.J.; De Donato-Capote, M.; et al. Genomic epidemiology analysis of drug-resistant Mycobacterium tuberculosis distributed in Mexico. PLoS ONE 2023, 18, e0292965. [Google Scholar] [CrossRef]
- Manson, A.L.; Cohen, K.A.; Abeel, T.; Desjardins, C.A.; Armstrong, D.T.; Barry, C.E., 3rd; Brand, J.; Chapman, S.B.; Cho, S.N.; Gabrielian, A.; et al. Genomic analysis of globally diverse Mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat. Genet. 2017, 49, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, P.P.; Becerra, M.C.; Zur Wiesch, P.A.; Hinkley, T.; Kaur, D.; Sloutsky, A.; Cohen, T. Fitness Costs of Drug Resistance Mutations in Multidrug-Resistant Mycobacterium tuberculosis: A Household-Based Case-Control Study. J. Infect. Dis. 2016, 213, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Cuevas, R.; Mendoza-Damián, F.; Muñoz, I.C.; Enciso-Moreno, L.; Pérez-Navarro, L.M.; Ramírez-Hernández, M.D.; Vázquez-Medina, K.; Widrobo-García, L.; Lauzardo, M.; Enciso-Moreno, J.A. Description of the population structure and genetic diversity of tuberculosis in Estado de México, a low prevalence setting from Mexico. APMIS 2015, 123, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruano, A.C.; Madrazo-Moya, C.F.; Cancino-Muñoz, I.; Mejía-Ponce, P.M.; Licona-Cassani, C.; Comas, I.; Muñiz-Salazar, R.; Zenteno-Cuevas, R. Whole genomic sequencing based genotyping reveals a specific X3 sublineage restricted to Mexico and related with multidrug resistance. Sci. Rep. 2021, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Morales, E.A.; Bermudez, G.; Montero, H.; Luzania-Valerio, M.; Zenteno-Cuevas, R. Whole genome characterization, and geographical distribution of M. tuberculosis in central region of Veracruz, Mexico. Braz. J. Infect. Dis. 2022, 26, 102357. [Google Scholar] [CrossRef]
- Yimer, S.A.; Kalayou, S.; Homberset, H.; Birhanu, A.G.; Riaz, T.; Zegeye, E.D.; Lutter, T.; Abebe, M.; Holm-Hansen, C.; Aseffa, A.; et al. Lineage-Specific Proteomic Signatures in the Mycobacterium tuberculosis Complex Reveal Differential Abundance of Proteins Involved in Virulence, DNA Repair, CRISPR-Cas, Bioenergetics and Lipid Metabolism. Front. Microbiol. 2020, 11, 550760. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Malaga, W.; Constant, P.; Caws, M.; Thi Hoang Chau, T.; Salmons, J.; Thi Ngoc Lan, N.; Bang, N.D.; Daffé, M.; Young, D.B.; et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS ONE 2011, 6, e23870. [Google Scholar] [CrossRef]
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef]
- Ronacher, K.; van Crevel, R.; Critchley, J.A.; Bremer, A.A.; Schlesinger, L.S.; Kapur, A.; Basaraba, R.; Kornfeld, H.; Restrepo, B.I. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest 2017, 152, 174–180. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef]
| Drug/Group of Isolates | TB 8 (%) | T2DM-TB 11 (%) |
|---|---|---|
| Isoniazid | 5 (63) | 10 (91) |
| Rifampicin | 1 (13) | 4 (26) |
| Pyrazinamide | 1 (13) | 2 (18) |
| Streptomycin | 3 (38) | 1 (9) |
| Ethionamide | 3 (38) | 1 (9) |
| Aminoglycosides | 2 (25) | 1 (9) |
| Fluoroquinolone | 1 (13) | 2 (18) |
| Patient | T0 (0 Day) | T1 (30th Day) | T2 (60th Day) |
|---|---|---|---|
| 2021-011 | katG S315T | katG S315T | katG S315T |
| 2021-025 | katG S315T | katG S315T | -- |
| rpoB S450L | |||
| gyrA A90G | |||
| gyrA D94G | |||
| 2021-031 | katG S315T | katG S315T | katG S315T |
| rpoB S450L | rpoB S450L | rpoB S450L | |
| gyrA A90G | gyrA A90G | gyrA A90G | |
| gyrA D94G | gyrA D94G | gyrA D94G | |
| 2021-043 | katG S315T | katG S315T | katG S315T |
| Sublineage | Classification | Mean Mutation Rate | Mutation Rate in DM2-TB | Mutation Rate in TB |
|---|---|---|---|---|
| Euro-Americano | 4.1 | 1.26 × 10−2 | 1.26 × 10−2 | 0 |
| X-type | 4.1.1 | 1.37 × 10−2 | 1.61 × 10−2 | 1.33 × 10−2 |
| X-type | 4.1.1.3 | 1.11 × 10−2 | 1.11 × 10−2 | 0 |
| LAM | 4.3.3 | 1.08 × 10−2 | 1.20 × 10−2 | 7.23 × 10−3 |
| LAM | 4.3.4.2 | 1.39 × 10−2 | 1.39 × 10−2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermúdez-Hernández, G.A.; Pérez-Martínez, D.; Ortiz-León, M.C.; Muñiz-Salazar, R.; Licona-Cassani, C.; Zenteno-Cuevas, R. Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus. Microorganisms 2024, 12, 324. https://doi.org/10.3390/microorganisms12020324
Bermúdez-Hernández GA, Pérez-Martínez D, Ortiz-León MC, Muñiz-Salazar R, Licona-Cassani C, Zenteno-Cuevas R. Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus. Microorganisms. 2024; 12(2):324. https://doi.org/10.3390/microorganisms12020324
Chicago/Turabian StyleBermúdez-Hernández, Gustavo A., Damián Pérez-Martínez, Maria Cristina Ortiz-León, Raquel Muñiz-Salazar, Cuauhtemoc Licona-Cassani, and Roberto Zenteno-Cuevas. 2024. "Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus" Microorganisms 12, no. 2: 324. https://doi.org/10.3390/microorganisms12020324
APA StyleBermúdez-Hernández, G. A., Pérez-Martínez, D., Ortiz-León, M. C., Muñiz-Salazar, R., Licona-Cassani, C., & Zenteno-Cuevas, R. (2024). Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus. Microorganisms, 12(2), 324. https://doi.org/10.3390/microorganisms12020324






