Endothelial Mechanistic Target of Rapamycin Activation with Different Strains of R. rickettsii: Possible Role in Rickettsial Pathogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Infection
2.2. mTOR Inhibition
2.3. Western Blotting
2.4. RNA Isolation and qPCR
2.5. Densitometry and Statistical Analysis
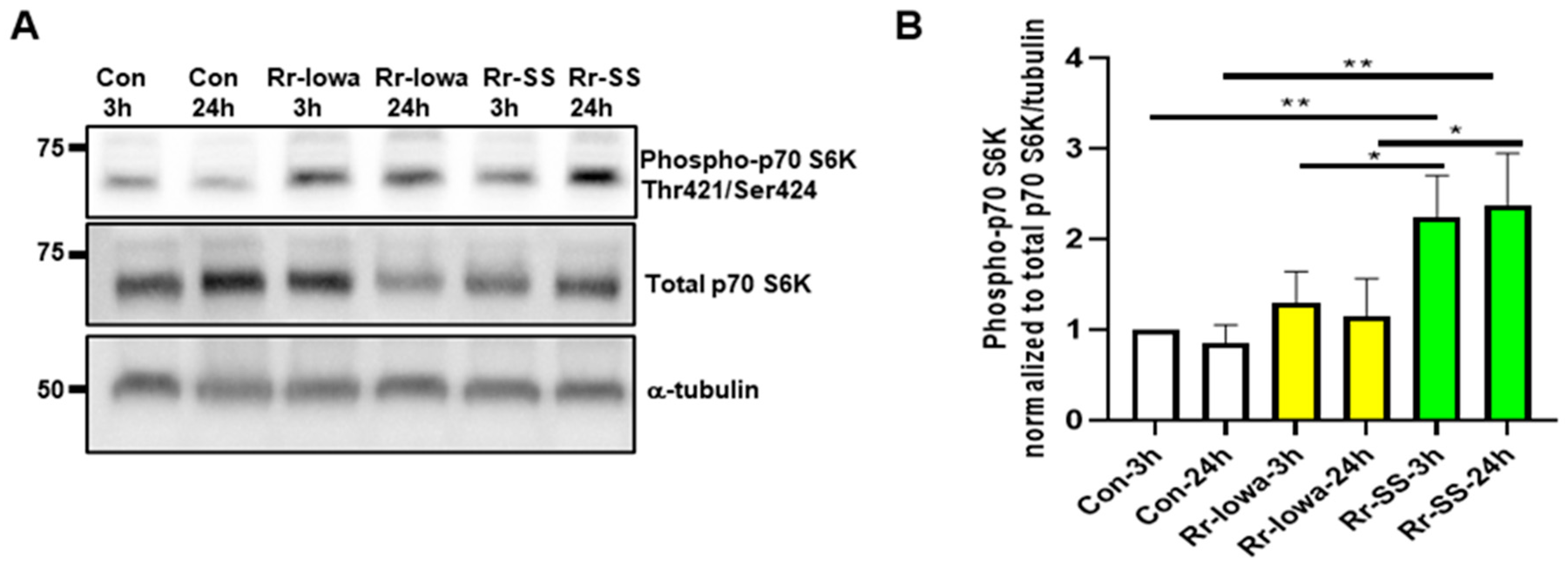
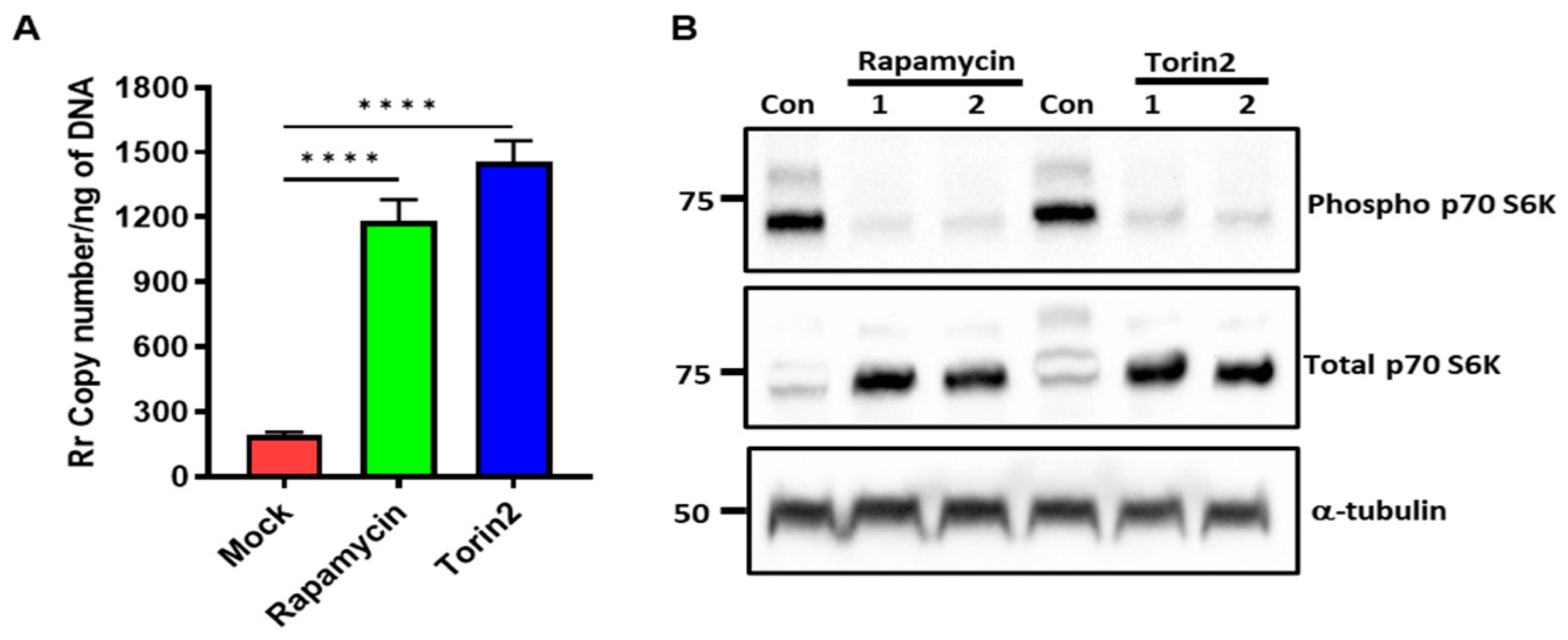
3. Result
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahni, A.; Fang, R.; Sahni, S.K.; Walker, D.H. Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection. Annu. Rev. Pathol. 2019, 14, 127–152. [Google Scholar] [CrossRef]
- Narra, H.P.; Sahni, A.; Walker, D.H.; Sahni, S.K. Recent research milestones in the pathogenesis of human rickettsioses and opportunities ahead. Future Microbiol. 2020, 15, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Narra, H.P.; Walker, D.H.; Sahni, S.K. Endothelial activation and injury: The mechanisms of rickettsial vasculitis. In Vascular Responses to Pathogens; Gavins, F., Stokes, K.Y., Eds.; Elsevier Press: Amsterdam, The Netherlands, 2016; pp. 111–122. [Google Scholar]
- Walker, D.H.; Valbuena, G.A.; Olano, J.P. Pathogenic mechanisms of diseases caused by Rickettsia. Ann. N. Y. Acad. Sci. 2003, 990, 1–11. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122 Pt 20, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Siqueira, M.d.S.; Ribeiro, R.d.M.; Travassos, L.H. Autophagy and Its Interaction With Intracellular Bacterial Pathogens. Front. Immunol. 2018, 9, 935. [Google Scholar] [CrossRef]
- Levine, B.; Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef]
- Baxt, L.A.; Garza-Mayers, A.C.; Goldberg, M.B. Bacterial Subversion of Host Innate Immune Pathways. Science 2013, 340, 697–701. [Google Scholar] [CrossRef]
- Huang, J.; Brumell, J.H. Bacteria–autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef]
- Riebisch, A.K.; Mühlen, S.; Beer, Y.Y.; Schmitz, I. Autophagy—A Story of Bacteria Interfering with the Host Cell Degradation Machinery. Pathogens 2021, 10, 110. [Google Scholar] [CrossRef]
- Sahni, A.; Narra, H.P.; Sahni, S.K. Activation of Mechanistic Target of Rapamycin (mTOR) in Human Endothelial Cells Infected with Pathogenic Spotted Fever Group Rickettsiae. Int. J. Mol. Sci. 2020, 21, 7179. [Google Scholar] [CrossRef]
- Sahni, A.; Narra, H.P.; Patel, J.; Sahni, S.K. MicroRNA-regulated rickettsial invasion into host endothelium via fibroblast growth factor 2 and its receptor FGFR1. Cells 2018, 7, 240. [Google Scholar] [CrossRef]
- Rydkina, E.; Sahni, S.K.; Santucci, L.A.; Turpin, L.C.; Baggs, R.B.; Silverman, D.J. Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. Microb. Pathog. 2004, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Narra, H.P.; Patel, J.; Sahni, S.K. MicroRNA Signature of Human Microvascular Endothelium Infected with Rickettsia rickettsii. Int. J. Mol. Sci. 2017, 18, 1471. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, M.E.; Dasch, G.A.; Silverman, D.J. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J. Clin. Microbiol. 2003, 41, 5466–5472. [Google Scholar] [CrossRef]
- Weichhart, T.; Hengstschläger, M.; Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Ellison, D.W.; Clark, T.R.; Sturdevant, D.E.; Virtaneva, K.; Porcella, S.F.; Hackstadt, T. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infect. Immun. 2008, 76, 542–550. [Google Scholar] [CrossRef]
- Liko, D.; Hall, M.N. mTOR in health and in sickness. J. Mol. Med. 2015, 93, 1061–1073. [Google Scholar] [CrossRef]
- Bhalla, M.; Law, D.; Dowd, G.C.; Ireton, K. Host Serine/Threonine Kinases mTOR and Protein Kinase C-α Promote InlB-Mediated Entry of Listeria monocytogenes. Infect. Immun. 2017, 85, e00087-17. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Hos, N.J.; Gutierrez, S.; Fischer, J.; Stepek, J.M.; Daglidu, E.; Krönke, M.; Robinson, N. Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog. 2017, 13, e1006227. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.; Andersson, B.; Lorell, C.; Raffetseder, J.; Larsson, M.; Blomgran, R. Autophagy induction targeting mTORC1 enhances Mycobacterium tuberculosis replication in HIV co-infected human macrophages. Sci. Rep. 2016, 6, 28171. [Google Scholar] [CrossRef]
- Edwards, M.W.; Aultman, J.A.; Harber, G.; Bhatt, J.M.; Sztul, E.; Xu, Q.; Zhang, P.; Michalek, S.M.; Katz, J. Role of mTOR downstream effector signaling molecules in Francisella tularensis internalization by murine macrophages. PLoS ONE 2013, 8, e83226. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Herrera, B.B.; Eshleman, H.D.; Fu, Y.; Bloom, A.; Li, Z.; Sacks, D.B.; Goldberg, M.B. Shigella Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation. PLoS Pathog. 2015, 11, e1005200. [Google Scholar] [CrossRef]
- Martinez, J.J.; Cossart, P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J. Cell Sci. 2004, 117 Pt 21, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Gomez, M.A.; Larsson, O.; Shio, M.T.; Topisirovic, I.; Contreras, I.; Luxenburg, R.; Rosenfeld, A.; Colina, R.; McMaster, R.W.; et al. Leishmania Repression of Host Translation through mTOR Cleavage Is Required for Parasite Survival and Infection. Cell Host Microbe 2011, 9, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.R.; Noriea, N.F.; Bublitz, D.C.; Ellison, D.W.; Martens, C.; Lutter, E.I.; Hackstadt, T. Comparative genome sequencing of Rickettsia rickettsii strains that differ in virulence. Infect. Immun. 2015, 83, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.S.; Noriea, N.F.; Aistleitner, K.; Clark, T.R.; Dooley, C.A.; Nair, V.; Kaur, S.J.; Rahman, M.S.; Gillespie, J.J.; Azad, A.F.; et al. The Rickettsial Ankyrin Repeat Protein 2 Is a Type IV Secreted Effector That Associates with the Endoplasmic Reticulum. mBio 2018, 9, e00975-18. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12 (Suppl. S2), 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Paulus, G.L.; Xavier, R.J. Autophagy and checkpoints for intracellular pathogen defense. Curr. Opin. Gastroenterol. 2015, 31, 14–23. [Google Scholar] [CrossRef]
- Bechelli, J.; Rumfield, C.S.; Walker, D.H.; Widen, S.; Khanipov, K.; Fang, R. Subversion of Host Innate Immunity by Rickettsia australis via a Modified Autophagic Response in Macrophages. Front. Immunol. 2021, 12, 638469. [Google Scholar] [CrossRef]
- Bechelli, J.; Vergara, L.; Smalley, C.; Buzhdygan, T.P.; Bender, S.; Zhang, W.; Liu, Y.; Popov, V.L.; Wang, J.; Garg, N.; et al. Atg5 Supports Rickettsia australis Infection in Macrophages In Vitro and In Vivo. Infect. Immun. 2019, 87, e00651-18. [Google Scholar] [CrossRef]
- Engström, P.; Burke, T.P.; Mitchell, G.; Ingabire, N.; Mark, K.G.; Golovkine, G.; Iavarone, A.T.; Rape, M.; Cox, J.S.; Welch, M.D. Evasion of autophagy mediated by Rickettsia surface protein OmpB is critical for virulence. Nat. Microbiol. 2019, 4, 2538–2551. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, K.; Zhou, L.; Minhaowu; Wu, Y.; Zhu, M.; Lai, X.; Chen, T.; Feng, L.; Li, M.; et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013, 9, e1003697. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Smith, A.C.; Bakowski, M.A.; Yoshimori, T.; Brumell, J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006, 281, 11374–11383. [Google Scholar] [CrossRef]
- Yuan, K.; Huang, C.; Fox, J.; Laturnus, D.; Carlson, E.; Zhang, B.; Yin, Q.; Gao, H.; Wu, M. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J. Cell Sci. 2012, 125 Pt 2, 507–515. [Google Scholar] [CrossRef]
- Owen, K.A.; Meyer, C.B.; Bouton, A.H.; Casanova, J.E. Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 2014, 10, e1004159. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, F.P.; Moris, A.; Nikolic, D.S.; Lehmann, M.; Cardinaud, S.; Stalder, R.; Garcia, E.; Dinkins, C.; Leuba, F.; Wu, L.; et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 2010, 32, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.; Brunton, J.; Ziehr, B.; Taft-Benz, S.; Moorman, N.; Kawula, T. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog. 2013, 9, e1003562. [Google Scholar] [CrossRef] [PubMed]
- Rydkina, E.; Silverman, D.J.; Sahni, S.K. Similarities and differences in host cell signaling following infection with different Rickettsia species. Ann. N. Y. Acad. Sci. 2005, 1063, 203–206. [Google Scholar] [CrossRef]
- Sahni, S.K.; Kiriakidi, S.; Colonne, M.P.; Sahni, A.; Silverman, D.J. Selective activation of signal transducer and activator of transcription (STAT) proteins STAT1 and STAT3 in human endothelial cells infected with Rickettsia rickettsii. Clin. Microbiol. Infect. 2009, 15 (Suppl. 2), 303–304. [Google Scholar] [CrossRef]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.M.; Kolbe, T.; Stulnig, T.M.; Hörl, W.H.; Hengstschläger, M.; et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Saha, B.; Riley, J.L. The battle over mTOR: An emerging theatre in host-pathogen immunity. PLoS Pathog. 2012, 8, e1002894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahni, A.; Alsing, J.; Narra, H.P.; Montini, M.; Zafar, Y.; Sahni, S.K. Endothelial Mechanistic Target of Rapamycin Activation with Different Strains of R. rickettsii: Possible Role in Rickettsial Pathogenesis. Microorganisms 2024, 12, 296. https://doi.org/10.3390/microorganisms12020296
Sahni A, Alsing J, Narra HP, Montini M, Zafar Y, Sahni SK. Endothelial Mechanistic Target of Rapamycin Activation with Different Strains of R. rickettsii: Possible Role in Rickettsial Pathogenesis. Microorganisms. 2024; 12(2):296. https://doi.org/10.3390/microorganisms12020296
Chicago/Turabian StyleSahni, Abha, Jessica Alsing, Hema P. Narra, Michelle Montini, Yasim Zafar, and Sanjeev K. Sahni. 2024. "Endothelial Mechanistic Target of Rapamycin Activation with Different Strains of R. rickettsii: Possible Role in Rickettsial Pathogenesis" Microorganisms 12, no. 2: 296. https://doi.org/10.3390/microorganisms12020296
APA StyleSahni, A., Alsing, J., Narra, H. P., Montini, M., Zafar, Y., & Sahni, S. K. (2024). Endothelial Mechanistic Target of Rapamycin Activation with Different Strains of R. rickettsii: Possible Role in Rickettsial Pathogenesis. Microorganisms, 12(2), 296. https://doi.org/10.3390/microorganisms12020296





