Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
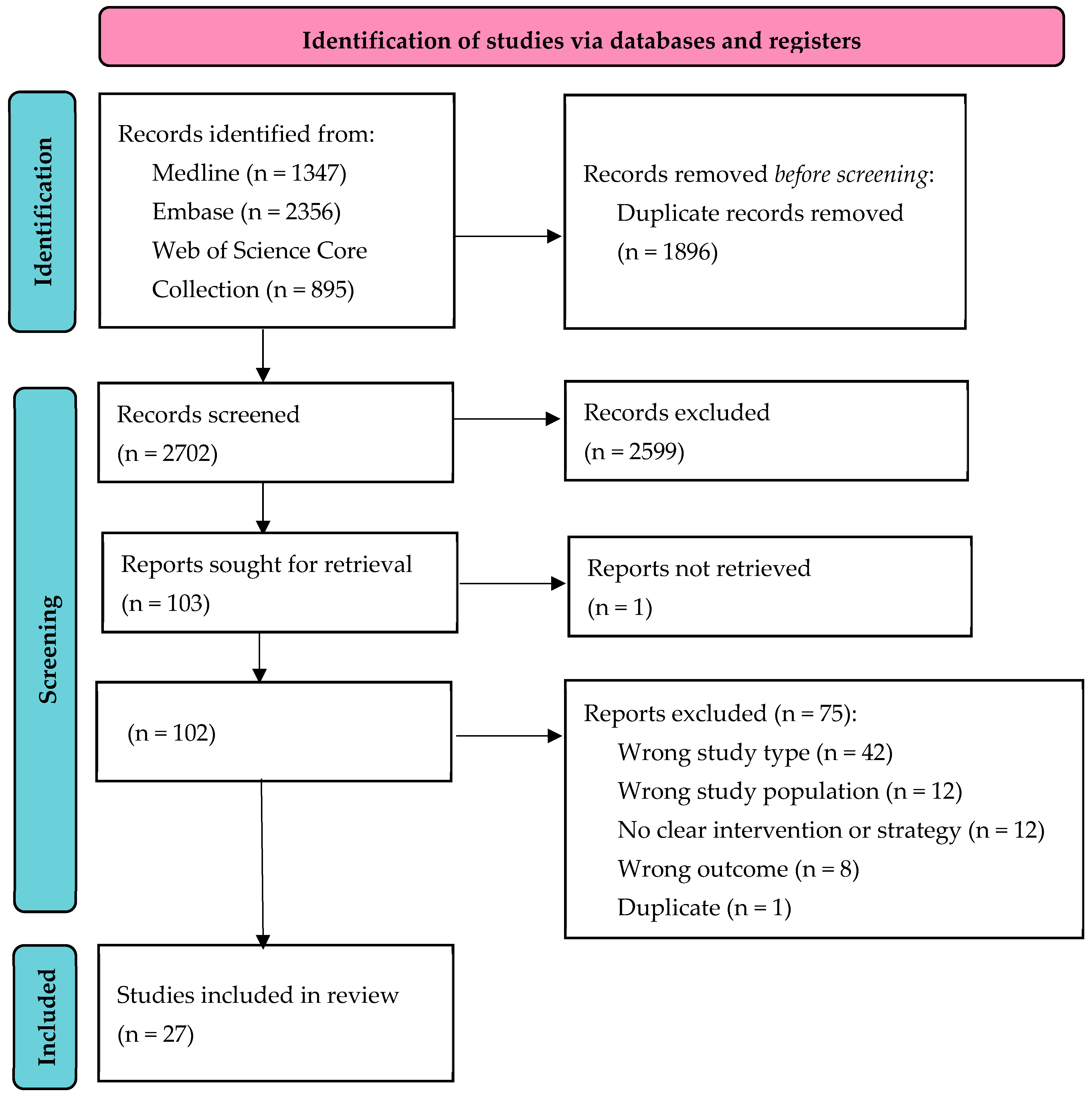
2.2. Search
2.3. Data Extraction and Presentation
2.4. Quality Assessment
3. Results
3.1. HIV Testing Strategies
3.2. Quality Assessment
3.3. Test-All Strategy
3.4. Indicator-Condition-Based Testing
3.5. Other Strategies
3.6. Late Diagnosis
4. Discussion
4.1. Test-All Strategy
4.2. Indicator-Condition-Based Testing
4.3. Consent Procedures
4.4. Late Diagnosis
4.5. Research Gaps
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- European Centre for Disease Prevention and Control (ECDC); WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2023 (2022 Data); European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/hivaids-surveillance-europe-2023-2022-data (accessed on 21 December 2023).
- European Centre for Disease Prevention and Control (ECDC); WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2022 (2021 Data); European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-joint-report-surveillance-2021-data (accessed on 21 December 2023).
- UNAIDS. UNAIDS Data 2021. 2021. Available online: https://aidsinfo.unaids.org/ (accessed on 21 December 2023).
- Tominski, D.; Katchanov, J.; Driesch, D.; Daley, M.B.; Liedtke, A.; Schneider, A.; Slevogt, H.; Arastéh, K.; Stocker, H. The late-presenting HIV-infected patient 30 years after the introduction of HIV testing: Spectrum of opportunistic diseases and missed opportunities for early diagnosis. HIV Med. 2017, 18, 125–132. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef]
- May, M.; Gompels, M.; Delpech, V.; Porter, K.; Post, F.; Johnson, M.; Dunn, D.; Palfreeman, A.; Gilson, R.; Gazzard, B.; et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ 2011, 343, d6016. [Google Scholar] [CrossRef]
- Nakagawa, F.; Lodwick, R.K.; Smith, C.J.; Smith, R.; Cambiano, V.; Lundgren, J.D.; Delpech, V.; Phillips, A.N. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012, 26, 335–343. [Google Scholar] [CrossRef]
- Popping, S.; Versteegh, L.; Nichols, B.E.; van de Vijver, D.A.M.C.; van Sighem, A.; Reiss, P.; Geerlings, S.; Boucher, C.A.B.; Verbon, A. Characteristics and short- and long-term direct medical costs among adults with timely and delayed presentation for HIV care in the Netherlands. PLoS ONE 2023, 18, e0280877. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, R.F.; Irvine, M.A.; Leber, W.; Cambiano, V.; Figueroa, J.; McMullen, H.; Anderson, J.; Santos, A.C.; Terris-Prestholt, F.; Miners, A.; et al. Cost-effectiveness of screening for HIV in primary care: A health economics modelling analysis. Lancet HIV 2017, 4, e465–e474. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. HIV Testing in Europe and Central Asia. Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2022 Progress Report; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-testing-europe-and-central-asia (accessed on 21 December 2023).
- Desai, S.; Tavoschi, L.; Sullivan, A.K.; Combs, L.; Raben, D.; Delpech, V.; Jakobsen, S.F.; Amato-Gauci, A.J.; Croxford, S. HIV testing strategies employed in health care settings in the European Union/European Economic Area (EU/EEA): Evidence from a systematic review. HIV Med. 2020, 21, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Stichting HIV Monitoring (SHM). HIV Monitoring Report; Stichting HIV Monitoring (SHM): Amsterdam, The Netherlands, 2023; Available online: https://www.hiv-monitoring.nl/application/files/5717/0066/1002/NL_HIV_MONITORING_REPORT_2023.pdf (accessed on 21 December 2023).
- EuroTEST. HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings; EuroTEST: Brussels, Belgium, 2014; Available online: https://eurotest.org/projects-collaborations/indicator-condition-guided-hiv-testinghides/guidance-hiv-indicator-conditions (accessed on 21 December 2023).
- Bogers, S.J.; Hulstein, S.H.; Schim van der Loeff, M.F.; de Bree, G.J.; Reiss, P.; van Bergen, J.; Geerlings, S.E.; HIV Transmission Elimination AMsterdam (H-TEAM) Consortium. Current evidence on the adoption of indicator condition guided testing for HIV in western countries: A systematic review and meta-analysis. eClinicalMedicine 2021, 35, 100877. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Public Health Guidance on HIV, Hepatitis B and C Testing in the EU/EEA; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-hiv-hepatitis-b-and-c-testing-eueea (accessed on 21 December 2023).
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 21 December 2023).
- British HIV Association. Adult HIV Testing Guidelines 2020; British HIV Association/British Association for Sexual Health and HIV/British Infection Association: London, UK, 2020; Available online: https://www.bhiva.org/HIV-testing-guidelines (accessed on 21 December 2023).
- Elmahdi, R.; Gerver, S.M.; Gomez Guillen, G.; Fidler, S.; Cooke, G.; Ward, H. Low levels of HIV test coverage in clinical settings in the UK: A systematic review of adherence to 2008 guidelines. Sex Transm. Infect. 2014, 90, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; De Wit, J. HIV Testing Services: Analysis of Guidelines and Perceptions of Practice across the WHO European Region. Summary Report; Centre for Social Research in Health, UNSW Australia: Kensington, Australia, 2016. [Google Scholar]
- Jordans, C.C.E.; Vasylyev, M.; Rae, C.; Jakobsen, M.L.; Vassilenko, A.; Dauby, N.; Grevsen, A.L.; Jakobsen, S.F.; Raahauge, A.; Champenois, K.; et al. National medical specialty guidelines of HIV indicator conditions in Europe lack adequate HIV testing recommendations: A systematic guideline review. Eurosurveillance 2022, 27, 2200338. [Google Scholar] [CrossRef]
- Sullivan, A.K.; Raben, D.; Reekie, J.; Rayment, M.; Mocroft, A.; Esser, S.; Leon, A.; Begovac, J.; Brinkman, K.; Zangerle, R.; et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: Results from HIDES I (HIV indicator diseases across Europe study). PLoS ONE 2013, 8, e52845. [Google Scholar] [CrossRef]
- Raben, D.; Sullivan, A.K.; Mocroft, A.; Kutsyna, G.; Hadžiosmanović, V.; Vassilenko, A.; Chkhartisvili, N.; Mitsura, V.; Pedersen, C.; Anderson, J.; et al. Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study—2012–2015. PLoS ONE 2019, 14, e0220108. [Google Scholar] [CrossRef]
- Raben, D.; Sullivan, A. Implementation of indicator condition guided HIV testing still lagging behind the evidence. eClinicalMedicine 2021, 36, 100918. [Google Scholar] [CrossRef]
- World Health Organization. Countries. Available online: https://www.who.int/countries (accessed on 1 December 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- ROBINS-I Tool. Available online: https://www.riskofbias.info/welcome/home/current-version-of-robins-i (accessed on 1 December 2023).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 1 December 2023).
- Gómez-Ayerbe, C.; Martínez-Sanz, J.; Muriel, A.; Pérez Elías, P.; Moreno, A.; Barea, R.; Polo, L.; Cano, A.; Uranga, A.; Santos, C.; et al. Impact of a structured HIV testing program in a hospital emergency department and a primary care center. PLoS ONE 2019, 14, e0220375. [Google Scholar] [CrossRef] [PubMed]
- Bogers, S.J.; Schim van der Loeff, M.F.; Boyd, A.; Davidovich, U.; van der Valk, M.; Brinkman, K.; Sigaloff, K.; Branger, J.; Bokhizzou, N.; de Bree, G.J.; et al. Improving indicator-condition guided testing for HIV in the hospital setting (PROTEST 2·0): A multicenter, interrupted time-series analysis. Lancet Reg. Health Eur. 2022, 23, 100515. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Patterson, A.; Dragovic, B.; Kelly, B.; Hamzah, L.; Hempling, M. High-level compliance to opt-out HIV testing in the emergency department (ED) of a large teaching hospital using the biochemistry sample as the sample type for HIV screening. HIV Med. 2022, 23, 1214–1218. [Google Scholar] [CrossRef]
- Orkin, C.; Flanagan, S.; Wallis, E.; Ireland, G.; Dhairyawan, R.; Fox, J.; Nandwani, R.; O’Connell, R.; Lascar, M.; Bulman, J.; et al. Incorporating HIV/hepatitis B virus/hepatitis C virus combined testing into routine blood tests in nine UK Emergency Departments: The “Going Viral” campaign. HIV Med. 2016, 17, 222–230. [Google Scholar] [CrossRef]
- Bath, R.; O’Connell, R.; Lascar, M.; Ferrand, R.; Strachan, S.; Matin, N.; Bassnet, I.; Orkin, C. TestMeEast: A campaign to increase HIV testing in hospitals and to reduce late diagnosis. AIDS Care 2016, 28, 608–611. [Google Scholar] [CrossRef]
- Herbert, R.; Ashraf, A.N.; Yates, T.A.; Spriggs, K.; Malinnag, M.; Durward-Brown, E.; Phillips, D.; Mewse, E.; Daniel, A.; Armstrong, M.; et al. Nurse-delivered universal point-of-care testing for HIV in an open-access returning traveller clinic. HIV Med. 2012, 13, 499–504. [Google Scholar] [CrossRef]
- Burns, F.; Edwards, S.G.; Woods, J.; Haidari, G.; Calderon, Y.; Leider, J.; Morris, S.; Tobin, R.; Cartledge, J.; Brown, M. Acceptability, feasibility and costs of universal offer of rapid point of care testing for HIV in an acute admissions unit: Results of the RAPID project. HIV Med. 2013, 14 (Suppl. 3), 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hill-Tout, R.; Cormack, I.; Elgalib, A. Routine HIV testing in acute medical admissions in a high prevalence area reduces morbidity and mortality of HIV: A full cycle audit. Int. J. STD AIDS 2016, 27, 591–594. [Google Scholar] [CrossRef]
- Palfreeman, A.; Nyatsanza, F.; Farn, H.; McKinnon, G.; Schober, P.; McNally, P. HIV testing for acute medical admissions: Evaluation of a pilot study in Leicester, England. Sex Transm. Infect. 2013, 89, 308–310. [Google Scholar] [CrossRef]
- Fox, M.; Pettit, R.; Mutengesa, E.; Harper, A.; Nakhoul, M.; Mitra, A. Improving detection of undiagnosed HIV through routine screening in a central London emergency department. BMJ Open Qual. 2022, 11, e001799. [Google Scholar] [CrossRef] [PubMed]
- Rayment, M.; Rae, C.; Ghooloo, F.; Doku, E.; Hardie, J.; Finlay, S.; Gidwani, S.; Atkins, M.; Roberts, P.; Sullivan, A.K. Routine HIV testing in the emergency department: Tough lessons in sustainability. HIV Med. 2013, 14 (Suppl. 3), 6–9. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.S.; Manavi, K. The prevalence of HIV among women with high-grade cervical smear abnormalities in Birmingham, United Kingdom: A prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.; Sanghera, T.; Bexley, A.; Hayes, M.; Perry, N.; Dosekun, O.; Perera, S. HIV testing in patients presenting with indicator conditions in outpatient settings: Offer and uptake rates, and educational and active interventions. Int. J. STD AIDS 2018, 29, 1289–1294. [Google Scholar] [CrossRef]
- Sharvill, R.; Fernandes, A.; Allen, K.; Astin, J. Adopting universal testing for HIV in intensive care for patients admitted with severe pneumonia: Results from our change in practice. Int. J. STD AIDS 2017, 28, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Sokhi, D.S.; Oxenham, C.; Coates, R.; Forbes, M.; Gupta, N.K.; Blackburn, D.J. Four-Stage Audit Demonstrating Increased Uptake of HIV Testing in Acute Neurology Admissions Using Staged Practical Interventions. PLoS ONE 2015, 10, e0134574. [Google Scholar] [CrossRef] [PubMed]
- Freer, J.; Lascar, M.; Phiri, E. Tailoring HIV testing in a setting of late HIV diagnosis: Is the tide turning? Br. J. Hosp. Med. Lond. 2015, 76, 592–595. [Google Scholar]
- Casalino, E.; Bernot, B.; Bouchaud, O.; Alloui, C.; Choquet, C.; Bouvet, E.; Damond, F.; Firmin, S.; Delobelle, A.; Nkoumazok, B.E.; et al. Twelve months of routine HIV screening in 6 emergency departments in the Paris area: Results from the ANRS URDEP study. PLoS ONE 2012, 7, e46437. [Google Scholar] [CrossRef]
- d’Almeida, K.W.; Kierzek, G.; de Truchis, P.; Le Vu, S.; Pateron, D.; Renaud, B.; Semaille, C.; Bousquet, V.; Simon, F.; Guillemot, D.; et al. Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch. Intern. Med. 2012, 172, 12–20. [Google Scholar] [CrossRef]
- Leblanc, J.; Hejblum, G.; Costagliola, D.; Durand-Zaleski, I.; Lert, F.; de Truchis, P.; Verbeke, G.; Rousseau, A.; Piquet, H.; Simon, F.; et al. Targeted HIV Screening in Eight Emergency Departments: The DICI-VIH Cluster-Randomized Two-Period Crossover Trial. Ann. Emerg. Med. 2018, 72, 41–53.e9. [Google Scholar] [CrossRef]
- Aparicio, C.; Mourez, T.; Simoneau, G.; Magnier, J.D.; Galichon, B.; Plaisance, P.; Bergmann, J.F.; Sellier, P. Proposal of HIV, HBV and HCV targeted screening: Short period feasibility study in a free-access outpatient medical structure. Presse Med. 2012, 41, e517–e523. [Google Scholar] [CrossRef]
- Barbanotti, D.; Tincati, C.; Tavelli, A.; Santoro, A.; Sala, M.; Bini, T.; De Bona, A.; d’Arminio Monforte, A.; Marchetti, G.C. HIV-Indicator Condition Guided Testing in a Hospital Setting. Life 2023, 13, 1014. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Mameli, M.S.; Bagella, P.; Fiore, V.; Fozza, C.; Montesu, M.A.; Fois, A.G.; Filigheddu, F.; Manzoni, N.; et al. HIV Infection Indicator Disease-Based Active Case Finding in a University Hospital: Results from the SHOT Project. Infect. Dis. Rep. 2023, 15, 94–101. [Google Scholar] [CrossRef]
- González Del Castillo, J.; Fuentes Ferrer, M.E.; Fernández Pérez, C.; Molina Romera, G.; Núñez Orantos, M.J.; Estrada Pérez, V. Efficiency of screening for human immunodeficiency virus infection in emergency departments: A systematic review and meta-analysis. Emergencias 2022, 34, 204–212. [Google Scholar]
- Luiken, G.P.M.; Joore, I.K.; Taselaar, A.; Schuit, S.C.E.; Geerlings, S.E.; Govers, A.; Rood, P.P.M.; Prins, J.M.; Nichols, B.E.; Verbon, A.; et al. Non-targeted HIV screening in emergency departments in the Netherlands. Neth. J. Med. 2017, 75, 386–393. [Google Scholar]
- Grant, C.; O’Connell, S.; Lillis, D.; Moriarty, A.; Fitzgerald, I.; Dalby, L.; Bannan, C.; Tuite, H.; Crowley, B.; Plunkett, P.; et al. Opt-out screening for HIV, hepatitis B and hepatitis C: Observational study of screening acceptance, yield and treatment outcomes. Emerg. Med. J. 2020, 37, 102–105. [Google Scholar] [CrossRef]
- O’Connell, S.; Lillis, D.; Cotter, A.; O’Dea, S.; Tuite, H.; Fleming, C.; Crowley, B.; Fitzgerald, I.; Dalby, L.; Barry, H.; et al. Opt-Out Panel Testing for HIV, Hepatitis B and Hepatitis C in an Urban Emergency Department: A Pilot Study. PLoS ONE 2016, 11, e0150546. [Google Scholar] [CrossRef]
- Cholewińska, G.; Siewaszewicz, E.; Robak, J.; Dawid, E.; Smykiewicz, J.; Skowron, W. Impact of a structured medical staff education program on the effect of HIV testing in four multi-specialist hospitals of the mazowieckie voivodeship as a part of the “Stop late presenters” project. Przegl. Epidemiol. 2020, 74, 133–146. [Google Scholar]
- Vaz-Pinto, I.; Gorgulho, A.; Esteves, C.; Guimarães, M.; Castro, V.; Carrodeguas, A.; Medina, D. Increasing HIV early diagnosis by implementing an automated screening strategy in emergency departments. HIV Med. 2022, 23, 1153–1162. [Google Scholar] [CrossRef]
- Gillet, C.; Darling, K.E.A.; Senn, N.; Cavassini, M.; Hugli, O. Targeted versus non-targeted HIV testing offered via electronic questionnaire in a Swiss emergency department: A randomized controlled study. PLoS ONE 2018, 13, e0190767. [Google Scholar] [CrossRef]
- Sanders, G.D.; Bayoumi, A.M.; Sundaram, V.; Bilir, S.P.; Neukermans, C.P.; Rydzak, C.E.; Douglass, L.R.; Lazzeroni, L.C.; Holodniy, M.; Owens, D.K. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N. Engl. J. Med. 2005, 352, 570–585. [Google Scholar] [CrossRef]
- Yazdanpanah, Y.; Sloan, C.E.; Charlois-Ou, C.; Le Vu, S.; Semaille, C.; Costagliola, D.; Pillonel, J.; Poullié, A.-I.; Scemama, O.; Deuffic-Burban, S.; et al. Routine HIV screening in France: Clinical impact and cost-effectiveness. PLoS ONE 2010, 5, e13132. [Google Scholar] [CrossRef]
- Yazdanpanah, Y.; Perelman, J.; DiLorenzo, M.A.; Alves, J.; Barros, H.; Mateus, C.; Pereira, J.; Mansinho, K.; Robine, M.; Park, J.-E.; et al. Routine HIV screening in Portugal: Clinical impact and cost-effectiveness. PLoS ONE 2013, 8, e84173. [Google Scholar] [CrossRef]
- Eurotest Survey on Blood Borne Virus (HIV, Hepatitis B and C) Testing Consent Requirements. Available online: https://eurotest.org/projects-collaborations/testing-consent-survey/ (accessed on 21 December 2023).
- Ehrenkranz, P.D.; Pagán, J.A.; Begier, E.M.; Linas, B.P.; Madison, K.; Armstrong, K. Written informed-consent statutes and HIV testing. Am. J. Prev. Med. 2009, 37, 57–63. [Google Scholar] [CrossRef]
- Wing, C. Effects of written informed consent requirements on HIV testing rates: Evidence from a natural experiment. Am. J. Public Health 2009, 99, 1087–1092. [Google Scholar] [CrossRef]
- British HIV Association. Rapid Guidance on Assumed Consent for Opt-Out Blood-Borne Virus Testing in Emergency Departments; BHIVA, BIA and BASHH: London, UK, 2023; Available online: https://www.bhiva.org/rapid-guidance-on-assumed-consent-for-opt-out-blood-borne-virus-testing (accessed on 21 December 2023).
- European Centre for Disease Prevention and Control (ECDC). ECDC Guidance HIV Testing: Increasing Uptake and Effectiveness in the European Union; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2010. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/101129_GUI_HIV_testing.pdf (accessed on 21 December 2023).
- Croxford, S.; Stengaard, A.R.; Brännström, J.; Combs, L.; Dedes, N.; Girardi, E.; Grabar, S.; Kirk, O.; Kuchukhidze, G.; Lazarus, J.V.; et al. Late diagnosis of HIV: An updated consensus definition. HIV Med. 2022, 23, 1202–1208. [Google Scholar] [CrossRef]
| ECDC (2018) [16] | Offer integrated HIV/HBV/HCV testing to any person attending a hospital if they:
|
| Universal testing can be considered in geographical areas where the local diagnosed seroprevalence of an infection is high. | |
| The ECDC underlines that testing in hospital settings as well as routine testing in the emergency department is an acceptable strategy for patients and staff. | |
| WHO (2021) [17] | In low-HIV-burden settings, offer HIV testing to:
The WHO states that significant opportunities exist for integrating HIV testing into many clinical services, but that strategies should be guided by local epidemiology and HIV test coverage gaps. |
| BHIVA/ BASHH/ BIA (2020) [18] | Offer HIV testing to:
|
| Study | Setting | HIV Testing Strategy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Country, City | Setting + Sites | Population | Time Period | Intervention Category | Consent Mode * | Intervention Specification ** | Study Design + Control Group |
| Test-all: Comprehensive testing approach aiming to screen all individuals presenting in a given setting, universal testing (ordered by: ED followed by OPD and IPD) | |||||||||
| Casalino [48] | 2012 | France, Paris | 6 EDs | Adults (18–70 years) | 12 months | Test-all POCT | Opt-in (WIC) | ANRS URDEP study: Routine HIV screening, using a rapid test on capillary blood. Training for testing and counselling as well as posters. | Cross-sectional Control: NA |
| d’Almeida [49] | 2012 | France, Paris | 31 EDs | Adults (18–64 years) | 16 months | Test-all POCT | Opt-in (WIC) | ED team training session (lecture, rapid test, practice of test, and counselling). Information sheet for patients. POCT by triage nurses and research assistant. | Cross-sectional Control: NA |
| Gómez- Ayerbe [32] | 2019 | Spain, Madrid | 1 ED | Adults (18–60 years) | 12 months | Test all Nurse | NS | DRIVE program. Inclusion in medical or nursing consultations. Trained nurse practitioners. Questionnaire on HIV IC and risk assessment. POCT. | Cross-sectional Historic control |
| Grant [56] | 2020 | Ireland, Dublin | 1 ED | Adults (>17 years) already receiving a blood test | 36 months | Test-all | Opt-out | Opt-out testing for HIV, HBV, and HCV | Cross-sectional Control: NA |
| Luiken [55] | 2017 | Netherlands, two cities | 3 EDs | Adults (>17 years) already receiving a blood test | 14 months | Test-all | Active (WIC) | Patients were informed by posters and flyers. HIV test with an extra blood sample. Anonymized batch testing of those not consenting. | Cross-sectional Control: NA |
| Marchant [34] | 2022 | England, London | 1 ED | Adults (18–59; later 16+ years) | 3 years | Test-all Prompt | Opt-in/opt-out | HIV testing was added to all ED blood test order sets. | Cross-sectional Control: NA |
| O’Connell [57] | 2016 | Ireland, Dublin | 1 ED | Adults (>18 years) already receiving a blood test | 10 months | Test-all | Opt-out | Opt-out testing for HIV, HBV, and HCV on an extra blood sample. Patients were informed by posters and leaflets in seven languages. Staff teaching. | Cross-sectional Control: NA |
| Orkin [35] | 2016 | England, Scotland | 9 EDs | Adults (>17) already receiving a blood test | 6 days | Test-all | Opt-out | “Going Viral” campaign. Opt-out testing for HIV, HBV, and HCV. Staff were informed by training and patients were informed by posters and leaflets. | Cross-sectional Control: NA |
| Vaz-Pinto [59] | 2022 | Portugal, Cascais | 1 ED | Adults (18–64) already receiving a blood test | 3 years | Test-all Prompt | Opt-out | Automatically generated HIV test request if exclusion criteria were not met (age, no bloodwork, known HIV-positive, or tested). Nurses’ training. | Cross-sectional Historic control |
| Bath [36] | 2016 | England, London | 2 EDs/6 OPDs | Adults (>16 years) already receiving a blood test | 5 days | Test-all Project | Opt-out | TestMeEast: HIV testing during National HIV Testing Week. Student and charity volunteers, training session, social media, banners, posters, leaflets. | Cross-sectional Control: NA |
| Herbert [37] | 2012 | England, London | 1 OPD | Adults (>17) attending a returning traveler clinic | 28 months | Test-all POCT | Active choice | Targeted vs. universal. Phase 0: symptom-based testing. Phase 1: universal offer of HIV test. Phase 2: POCT (15 months) + training. | Cross-sectional Baseline control |
| Cholewińska [58] | 2020 | Poland | 4 OPDs/IPDs | Patients eligible for HIV test according to MD | 6 months | Test-all Edu | NS | Nationwide project “STOP Late Presenters”: (a) voluntary training in the form of a presentation; (b) information materials and leaflets. | Cross-sectional Historic control |
| Burns [38] | 2012 | England, London | 1 IPD | Adults (19–65 years) presenting to AMU. | 16 weeks | Test-all POCT | Active choice | RAPID: Employment of a health advisor (HA) offering POCT with the aid of an educational video available in up to four languages. | Cross-sectional Control: NA |
| Hill-Tout [39] | 2016 | England, London | 1 IPD | AMU targeted testing without vs. with screening | 19 months | Test-all | NS | Targeted versus universal. Routine HIV screening was introduced in the acute medical unit (AMU). This study audited the effects retrospectively. | Cross-sectional Historic control |
| Palfreeman [40] | 2013 | England, Leicester | 1 IPD | New admissions (15–59 years) admitted to the AMU | 24 months | Test-all | Opt-in | Routine testing in the AMU, introduced to staff by e-mail/meetings and to patients by posters/flyers. Pilot phase: weekly AMU visit. Post-pilot: no visits. | Cross-sectional Pre-intervention |
| Project: Implementation of comprehensive projects, campaigns, or plan–do–check–act (PDCA) cycles aimed at promoting HIV testing. | |||||||||
| Fox [41] | 2022 | England, London | 1 ED | Adults (16–59 years) | 29 months | Project | Opt-in/opt-out | PDCA cycle: (1) survey for barriers; (2) teaching session for ED; (3) HIV advocate nurse champion; (4) Prompts; (5) Gamified teaching; (6) HIV testing to care set. | Cross-sectional Pre-intervention |
| Rayment [42] | 2013 | England, London | 1 ED | Adults (16–65 years) Later: no age limit | 30 months | Project | Opt-in | Implementation based on HINTS study: PDCA cycle, training, nurse-based testing, champions, incentivization, information technology solutions. | Prospective Cross-sectional |
| IC (indicator-condition-guided testing): Targeted testing of individuals based on medical conditions or symptoms that indicate a potential risk for HIV infection (ED; OPD; IPD). | |||||||||
| Gonzalez Del C. [54] | 2023 | Spain | 34 EDs | People presenting with one of the six prioritized HIV ICs | 6 months | IC Edu | NS | Intensive training program “dejatuhuella”, focused on testing in six HIV ICs. a Four educational sessions in every ED, along with webinars, courses, and meetings. | Cross-sectional Pre-intervention |
| Qureshi [43] | 2017 | England, Birmingham | 1 OPD | Women with cervical dyskaryosis for colposcopy | 21 months | IC | Active choice | Offer of HIV testing as part of clinical management. An information leaflet upon arrival at the clinic. Discussion of questions about HIV testing. | Cross-sectional Control: NA |
| Youssef [44] | 2018 | England, Brighton | 3 OPDs | Patients aged > 15 years attending three specific OPDs | 12 weeks | IC Prompt | NS | Singular education program followed by either prompt (6 weeks) or no-prompt (6 weeks). Prompt identified HIV ICs before appointment. | Non-randomized Crossover trial |
| Barbanotti [52] | 2023 | Italy, Milan | 1 IPD | Admitted patients identified with an HIV IC in seven wards | 24 months | IC | Opt-in | ICEBERG study: A dedicated healthcare professional in charge of patients’ enrolment and HIV test prescription in case of an observed HIV IC b. | Cross-sectional Control: NA |
| Bogers [29] | 2022 | Netherlands Amsterdam | 5 IPDs | Adults (>18) with HIV ICs in disease billing code | 12 months | IC Edu | NS | PROTEST 2.0: HIV ICs were assessed using electronic health records. c Interventions: presentation, discussion, feedback, pocket cards, posters. | Cross-sectional Pre-intervention |
| De Vito [53] | 2023 | Italy, Sassari | 1 IPD | Patients identified with an HIV IC in one of six wards | 16 months | IC | Opt-in (WIC) | SHOT Project: Each ward was provided with forms to collect data from patients, included in the screening in case of an observed HIV IC d. | Cross-sectional Control: NA |
| Sharvill [45] | 2017 | England, Bath | 1 IPD | Adults admitted to the ICU with pneumonia | 1 year | IC Prompt | Opt-out | Routine HIV testing was added to the automated pneumonia screen.Prompt in case of diagnoses, pneumonia, RTI, chest infection, or chest sepsis. | Cross-sectional Pre-intervention |
| Sokhi [46] | 2015 | England, Sheffield | 1 IPD | Patients admitted as acute non-stroke neurology cases | 2 years | IC Prompt | Opt-in | Four phases: (1) Protocol disseminated to clinical staff; (2) Protocol and posters on noticeboards/offices/trolleys; (3) Prompt and education; (4) Continuation phase. | Cross-sectional Control: NA |
| Other HIV testing strategies (ED; OPD). | |||||||||
| Gillet [60] | 2018 | Switzerland, Lausanne | 1 ED | Adults (18–75 years) | 3 months | Key vs. test-all | Active choice | Targeted arm: Testing offer based on HIV testing criteria. Non-targeted arm: Active choice based on information on HIV. Crossover to the other arm. | Randomized controlled study |
| Leblanc [50] | 2018 | France, Paris | 8 EDs | Adults (18–64) | 12 months | Nurse POCT | Opt-out | DICI-VIH study: ED randomization to symptom-driven physician testing alone or additional nurse-based POCT based on risk assessment. Crossover. | Cluster randomized trial |
| Aparicio [51] | 2012 | France, Paris | 1 OPD | Adults from SS Africa, the Antilles, Réunion, and Guyana | 28 days | Key | Opt-in | Targeted testing: Patients presenting with a medical problem, wound dressing, or blood sample were offered testing by the attending doctor/nurse. | Cross-sectional Control: NA |
| Freer [47] | 2015 | England, London | 1 OPD | People deciding to visit a rapid HIV testing service | 12 months | POCT | Active choice | Universal testing: A rapid walk-in HIV testing service with oral swabs in the OPD for people attending the OPD or walk-in. Information by posters and staff. | Cross-sectional Control: NA |
| Study, Setting, and Strategy | HIV Testing Strategy | Control Group | Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year | Country, Setting | Strategy | Population | Testing Rate * | New HIV Diagnoses (HIV Detection Rate) ** | Population | Testing Rate * | New HIV Diagnoses (HIV Detection Rate) ** | (HIV Testing Rate and HIV Case-Finding) | ||
| Eligible (Total) | Tested | Eligible (Total) | Tested | ||||||||
| Test-all: Comprehensive testing approach aiming to screen all individuals presenting in a given setting (ordered as follows: ED, followed by OPD and IPD). | |||||||||||
| Casalino, 2012 [48] | France, ED | Test-all POCT | 183,957 (311,153) | 7215 | 3.9% | 40 (0.55%) | |||||
| d’Almeida, 2012 [49] | France, ED | Test-all POCT | 78,411 (138,691) | 12,754 | 16% | 18 (0.14%) | |||||
| Gómez-Ayerbe, 2019 [32] | Spain, ED | Test-all Nurse | NA (63,054) | 1635 | 2.6% | 14 (0.86%) | NA (63,054) | 966 | 0.5% | 1 (0.62%) | Testing rate: increased: 0.5% vs. 2.6%. Case-finding: increased: 3.2 vs. 22.2 per 100,000 ED visitors. |
| Grant, 2020 [56] | Ireland, ED | Test-all | 88,854 (140,500) | 41,535 | 47% | 38 (0.09%) | |||||
| Luiken, 2017 [55] | Netherlands ED | Test-all | 7577 (NA) | 3223 | 43% | 2 (0.06%) | |||||
| Marchant 2022 [34] | England, ED | Test-all | 110,683 (NA) | 78,333 | 70% | 50 (0.06%) | |||||
| O’Connell, 2016 [57] | Ireland, ED | Test-all | 18,819 (40,000) | 8839 | 47% | 7 (0.08%) | |||||
| Orkin, 2016 [35] | UK, ED | Test-all | 7807 (NA) | 2118 | 27% | 6 (0.52%) | |||||
| Vaz-Pinto, 2022 [59] | Portugal, ED | Test-all Prompt | 43,153 (252,153) | 38,357 | 89% | 69 (0.18%) | NA (282,751) | NA | NA | 37 | Testing rate: NA. Case-finding: increased: 13 vs. 27 per 100,000 ED visitors. |
| Bath, 2016 [36] | England, ED/OPD | Test-all Project | 4317 (10,386) | 2402 | 56% | 3 (0.12%) | |||||
| Herbert 1, 2012 [37] | England, OPD | Test-all POCT | NA (3623) | 1444 | 40% | 9 (0.62%) | NA (1342) | 38 | 2.8% | 0 | Testing rate: increased: Phase 0 vs. 1 vs. 2; 2.8% vs. 23% vs. 45%. Case finding: NA. |
| Cholewińska 2020 [58] | Poland, OPD/IPD | Test-all Edu | NA (NA) | 869 | NA | 4 (0.87%) | NA (112,928) | 878 | 0.8% | NA | Testing rate: NA. Denominator unknown. Case-finding: NA. Denominator unknown. |
| Burns, 2012 [38] | England, IPD | Test-all POCT | 282 (606) | 131 | 46% | 3 (2.22%) | |||||
| Test-all: Comprehensive testing approach aiming to screen all individuals presenting in a given setting (ordered as follows: ED, followed by OPD and IPD)—continued. | |||||||||||
| Hill-Tout 2, 2016 [39] | England, IPD | Test-all | NA (19,110) | 4955 | 26% | 21 (0.42%) | NA (NA) | NA | NA | 88 | Testing rate: NA. Case-finding: NA. |
| Palfreeman 2013 [40] | England, IPD | Test-all | 5517 (NA) | 938 | 17% | 10 (1.07%) | 5484 (NA) | 205 | 3.7% | 4 (1.95%) | Testing rate: increased. Pre-pilot vs. pilot vs. post-pilot: 3.7% vs. 17% vs. 22.5%. Case-finding: increased. 7 vs. 18 vs. 24 per 10,000 admissions. |
| Post-pilot 6225 | 1399 | 23% | 15 (1.07%) | ||||||||
| Project: Implementation of comprehensive projects, campaigns, or plan–do–check–act (PDCA) cycles aimed at promoting HIV testing. | |||||||||||
| Fox, 2022 [41] | England, ED | Project | NA (46,375) | 9600 | 21% | 8 (0.08%) | NA (42,809) | 2825 | 6.6% | NA | Testing rate: increased. From baseline to end: 8% to 44%. Case-finding: NA. |
| Rayment, 2013 [42] | England, ED | Project | 44,582 (NA) | 4327 | 9.7% | 13 (0.30%) | Increased testing rate: months 1–22 to 22–30, 11% vs. 29%. Case-finding: NA. | ||||
| Other HIV testing strategies (ED; OPD). | |||||||||||
| Gillet 3, 2018 [60] | Switzerland ED | Key vs. test-all | 17 (80) | 8 | 10% | 0 | 80 (80) | 38 | 48% | 0 | Testing rate: no increase. Targeted versus universal approach: 10% vs. 48%. Case-finding: NA. |
| Leblanc, 2018 [50] | France, ED | Nurse POCT | 74,161 (102,240) | 2915 | 3.9% | 22 (0.54%) | 74,166 (105,582) | 92 | 0.12% | 6 (6.5%) | Testing rate: increased. Physician- vs. nurse-driven: 0.12% vs. 3.9%. Case-finding: increased. 0.8 vs. 3.0 per 10,000 ED visitors. |
| Aparicio, 2012 [51] | France, OPD | Key | 272 (NA) | 166 | 61% | 3 (1.8%) | |||||
| Freer, 2015 [47] | England, OPD | POCT | NA (NA) | 148 | NA | 3 (1.4%) | NA (NA) | 420 | NA | 0 | Testing rate: NA. Case-finding: NA. |
| IC (indicator-condition-guided testing): Targeted testing of individuals based on medical conditions or symptoms that indicate a potential risk for HIV infection (ED; OPD; IPD). | |||||||||||
| Gonzalez Del Castillo, 2023 [54] | Spain, ED | IC Edu | 16,618 (1,796,741) | 7002 | 42% | 224 (1.67%) | 15,879 (1,670,027) | 3393 | 21% | 65 (0.93%) | Testing rate: increased among ED visitors (0.42% vs. 0.75%) and among HIV ICs (21% vs. 42%). Case-finding: increased among ED visitors (3.9 vs. 12 per 100,000) and among HIV ICs (0.41% vs. 1.35%). |
| Qureshi, 2017 [43] | England, OPD | IC | 533 (3262) | 244 | 46% | 0 | |||||
| Youssef, 2018 [44] | England, OPD | IC Prompt | 215 (NA) | 74 | 34% | 0 | 252 (NA) | 8 | 3.2% | 0 | Testing rate: increased among HIV ICs without prompt vs. with prompt (3.2% vs. 34%). Case-finding: NA. |
| Barbanotti, 2023 [52] | Italy, IPD | IC | NA (NA) | 520 | NA | 20 (3.8%) | |||||
| Bogers, 2022 [29] | Netherlands IPD | IC Edu | 1256 (NA) | 590 | 47% | 1 (0.2%) | 6739 (NA) | 2478 | 37% | 17 (0.7%) | Testing rate: increased among HIV ICs (37% vs. 47%). Case-finding: reduced among HIV ICs. |
| De Vito, 2023 [53] | Italy, IPD | IC | NA (NA) | 300 | NA | 11 (3.7%) | |||||
| Sharvill, 2017 [45] | England, IPD | IC Prompt | 59 (NA) | 48 | 81% | 0 | 68 (NA) | 22 | 32% | 0 | Testing rate: increased, 32% vs. 81%. Case-finding: NA. |
| Sokhi, 2015 [46] | England, IPD | IC Prompt | 4349 (6723) | 378 | 8.7% | 0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vliegenthart-Jongbloed, K.J.; Vasylyev, M.; Jordans, C.C.E.; Bernardino, J.I.; Nozza, S.; Psomas, C.K.; Voit, F.; Barber, T.J.; Skrzat-Klapaczyńska, A.; Săndulescu, O.; et al. Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals. Microorganisms 2024, 12, 254. https://doi.org/10.3390/microorganisms12020254
Vliegenthart-Jongbloed KJ, Vasylyev M, Jordans CCE, Bernardino JI, Nozza S, Psomas CK, Voit F, Barber TJ, Skrzat-Klapaczyńska A, Săndulescu O, et al. Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals. Microorganisms. 2024; 12(2):254. https://doi.org/10.3390/microorganisms12020254
Chicago/Turabian StyleVliegenthart-Jongbloed, Klaske J., Marta Vasylyev, Carlijn C. E. Jordans, Jose I. Bernardino, Silvia Nozza, Christina K. Psomas, Florian Voit, Tristan J. Barber, Agata Skrzat-Klapaczyńska, Oana Săndulescu, and et al. 2024. "Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals" Microorganisms 12, no. 2: 254. https://doi.org/10.3390/microorganisms12020254
APA StyleVliegenthart-Jongbloed, K. J., Vasylyev, M., Jordans, C. C. E., Bernardino, J. I., Nozza, S., Psomas, C. K., Voit, F., Barber, T. J., Skrzat-Klapaczyńska, A., Săndulescu, O., & Rokx, C., on behalf of the #aware.hiv Europe Project. (2024). Systematic Review: Strategies for Improving HIV Testing and Detection Rates in European Hospitals. Microorganisms, 12(2), 254. https://doi.org/10.3390/microorganisms12020254











