Seasonal Variations of Sediment Fungal Community of a Shallow Lake in North China
Abstract
1. Introduction
2. Materials and Methods
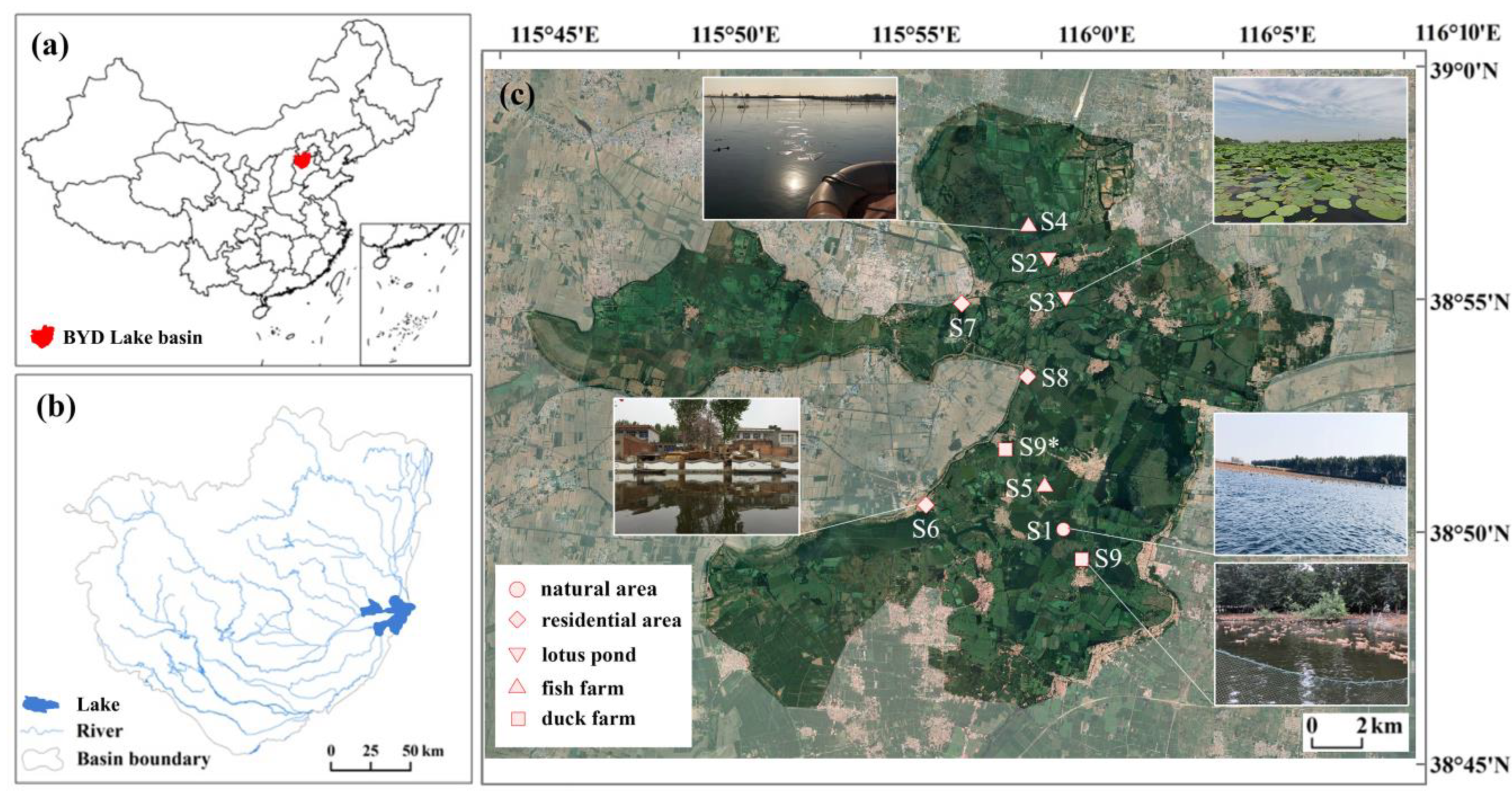
2.1. Study Area and Sampling
2.2. Determination of Physicochemical Properties and Heavy Metals
2.3. DNA Extraction, PCR Amplification and Sequencing Environmental Is Acidic
2.4. Statistical Analyses
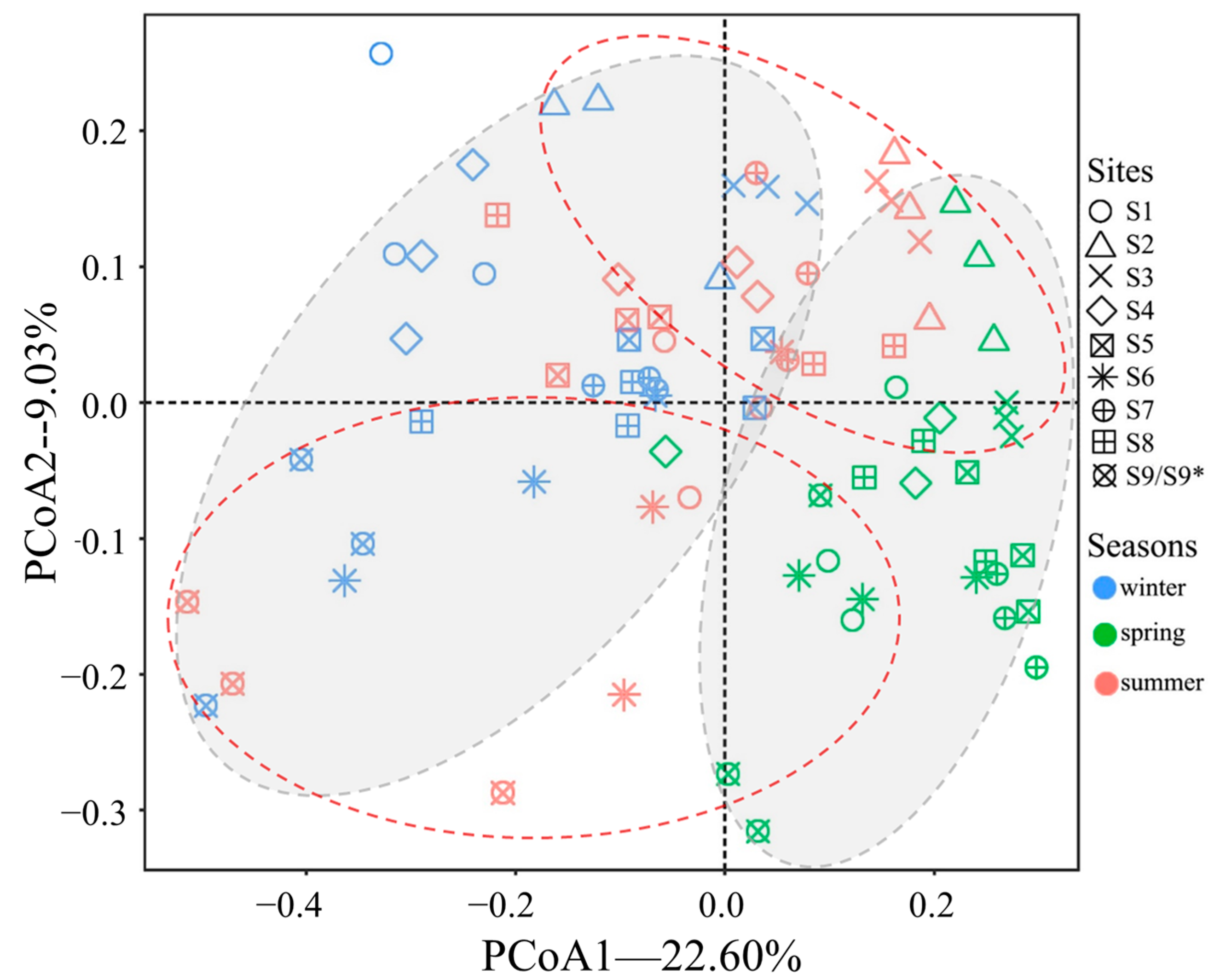
3. Results
3.1. Sediment Physical and Chemical Properties
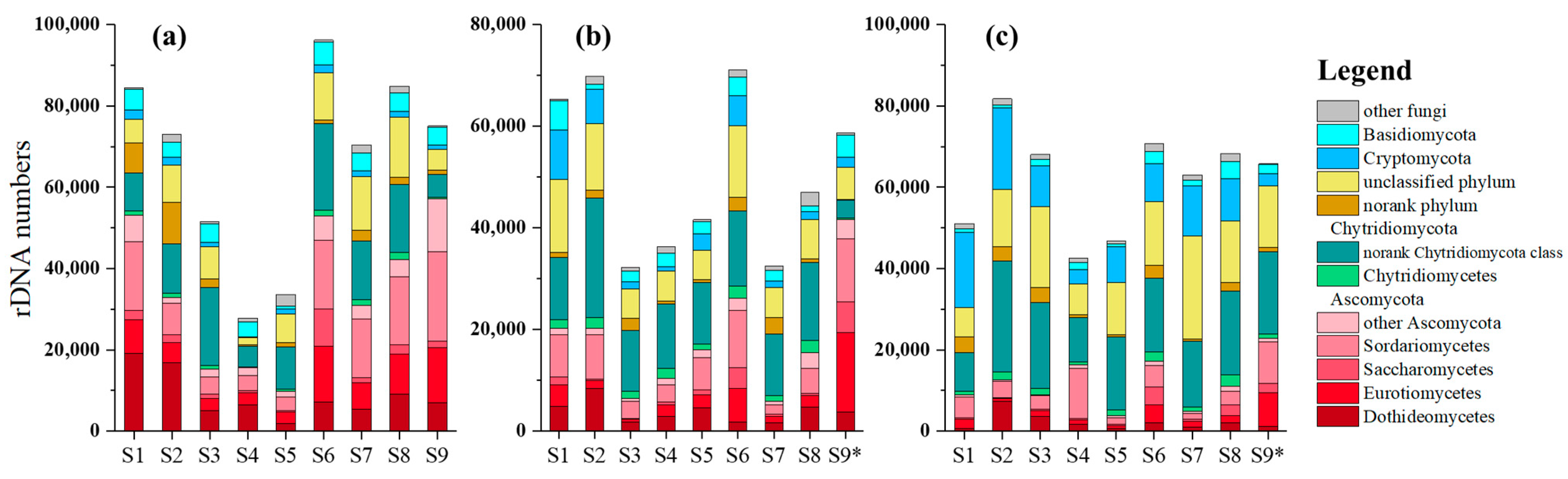
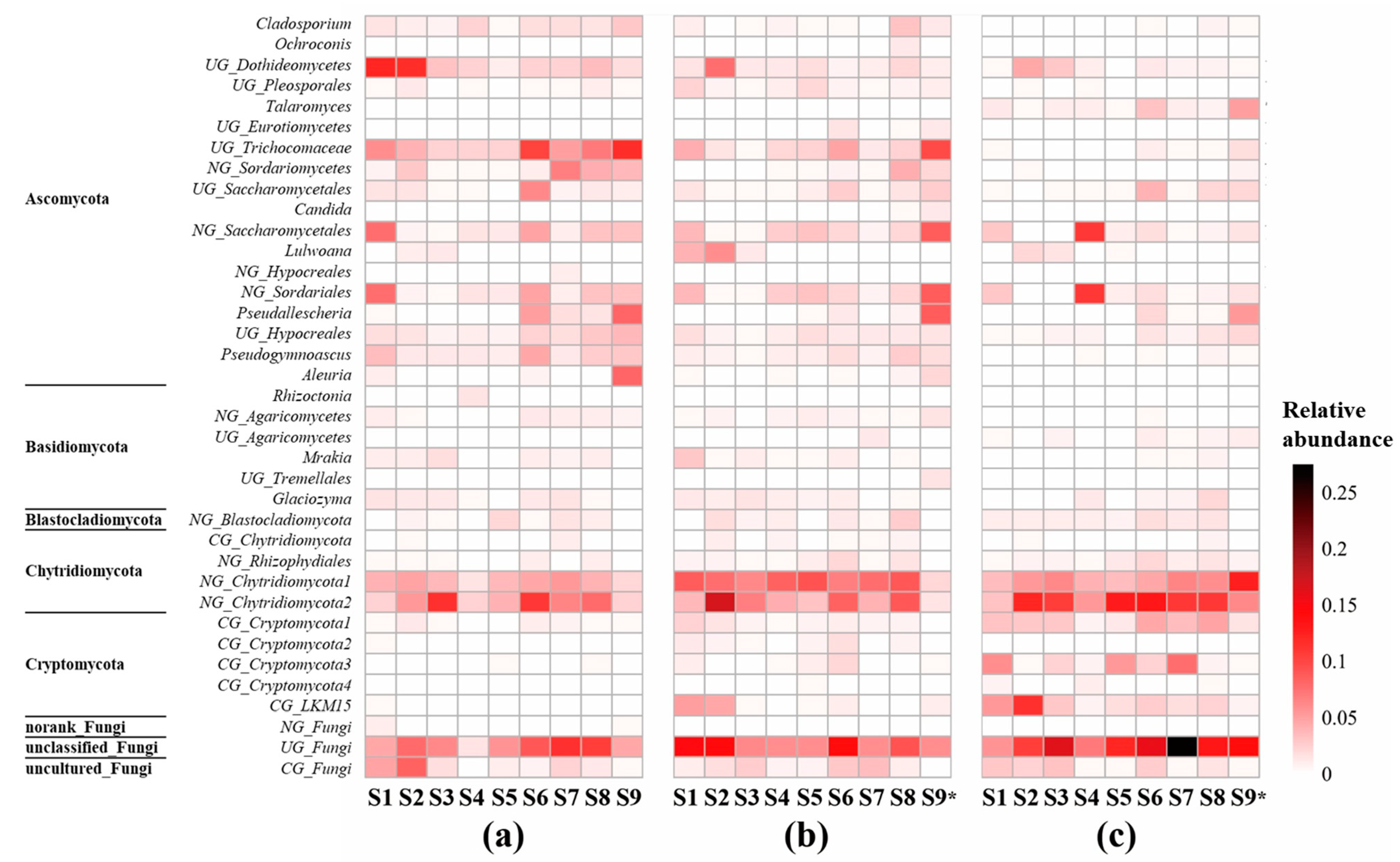
3.2. Compositions of Fungal Community in Sediments
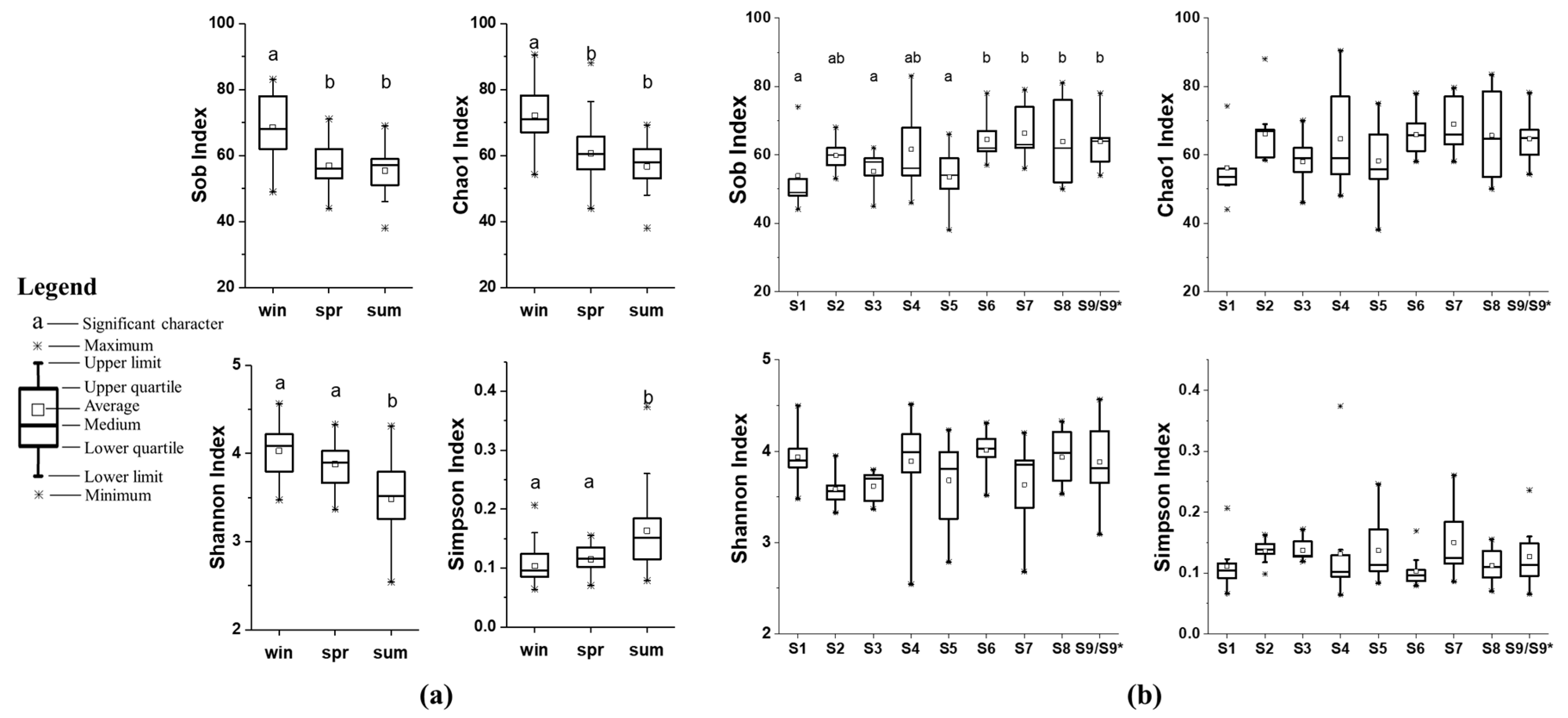
3.3. Fungal Diversity in Sediment
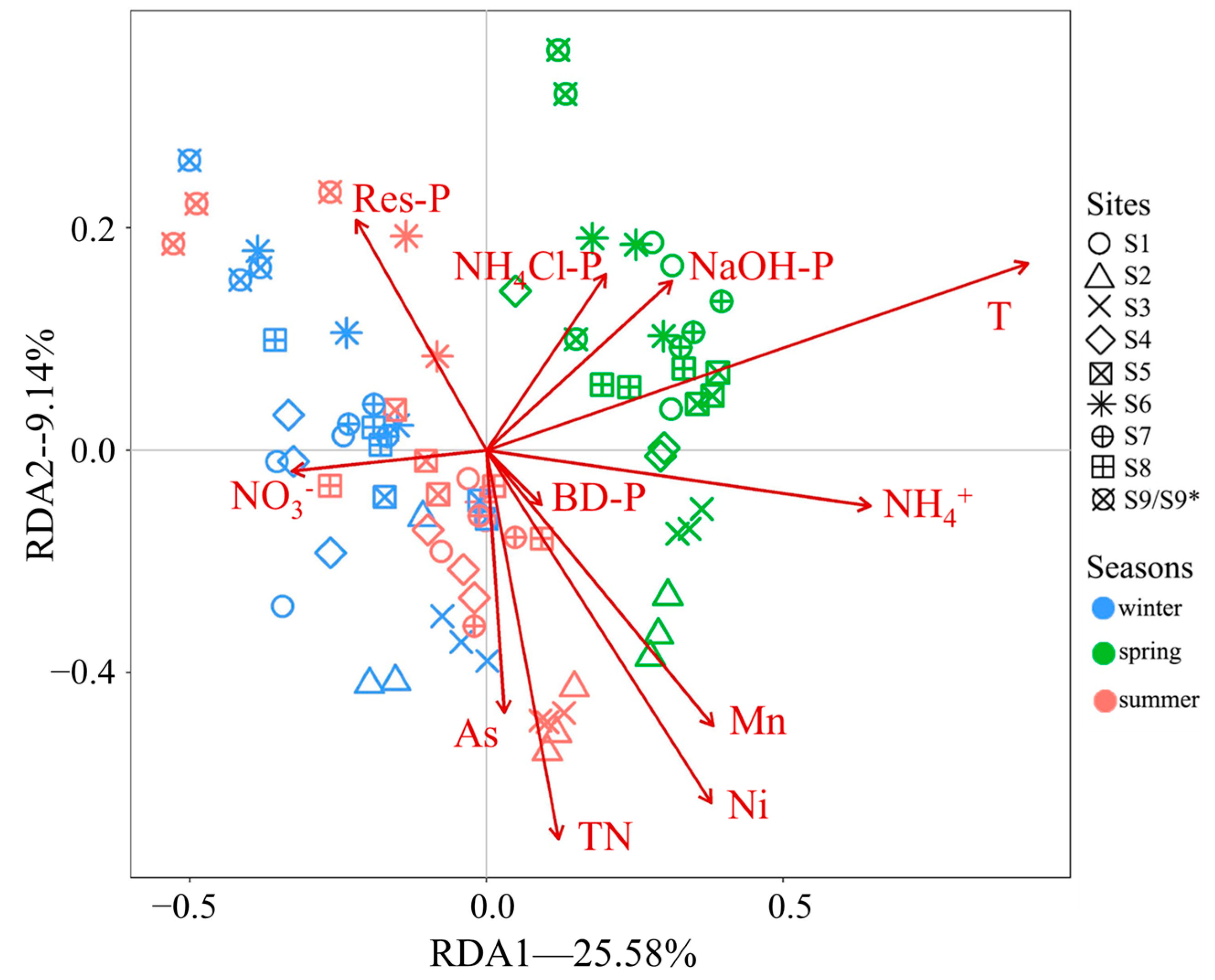
3.4. RDA of Environmental Factors and the Fungal Community
4. Discussion
4.1. The Compositions of Fungal Communities in the Sediment Environment of Shallow Lakes
4.2. The Temporal and Spatial Variations of Fungal Communities
4.3. The Factors Affect Sediment Fungal Community Compositions
4.4. Deficiencies of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postel, S.; Carpenter, S. Freshwater ecosystem services. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997; pp. 195–214. [Google Scholar]
- Zedler, J.B. Progress in wetland restoration ecology. Trends Ecol. Evol. 2000, 15, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Brönmark, C.; Hansson, L.A. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–307. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Nõges, T.; Nõges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Webster, J.; Ridgway, I. The application of the equilibrium partitioning approach for establishing sediment quality criteria at two UK sea disposal and outfall sites. Mar. Pollut. Bull. 1994, 28, 653–661. [Google Scholar] [CrossRef]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Wurzbacher, C.; Bärlocher, F.; Grossart, H.P. Fungi in lake ecosystems. Aquat. Microb. Ecol. 2010, 59, 125–149. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Rojas-Jimenez, K. Aquatic fungi: Targeting the forgotten in microbial ecology. Curr. Opin. Microbiol. 2016, 31, 140–145. [Google Scholar] [CrossRef]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.-F.; Darling, A.; Malfatti, S.; Swan, B.K.; Gies, E.A.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef]
- Uesaka, K.; Oka, H.; Kato, R.; Kanie, K.; Kojima, T.; Tsugawa, H.; Toda, Y.; Horinouchi, T. Bioinformatics in bioscience and bioengineering: Recent advances, applications, and perspectives. J. Biosci. Bioeng. 2022, 134, 363–373. [Google Scholar] [CrossRef]
- Grossart, H.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lin, C.; Tang, C. Hydrology, environment and ecological evolution of Lake Baiyangdian since 1960s. J. Lake Sci. 2020, 32, 1333–1347. (In Chinese) [Google Scholar]
- Zhang, X.; Yi, Y.; Yang, Y.; Liu, H.; Yang, Z. Modelling phosphorus loading to the largest shallow lake in northern china in different shared socioeconomic pathways. J. Clean. Prod. 2021, 297, 126537. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Meng, L.; Li, H.; Fu, H.; Wang, H. Simultaneous determination of total nitrogen and organic carbon in soil with an elemental analyzer. Chin. J. Anal. Lab. 2013, 32, 41–45. (In Chinese) [Google Scholar]
- Norman, R.J.; Edberg, J.C.; Stucki, J.W. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araújo, F.; Noyma, N.P.; Becker, V. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619, 1022–1029. [Google Scholar] [CrossRef]
- Liu, X.; Dong, L.; Cheng, D.; Dai, G.; Liang, G.; Liu, G. Typical Pollutant Migration Mechanism and Risk Assessment in Baiyangdian; Science Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Borneman, J.; Hartin, R.J. PCR primers that amplify fungal rRNA genes from environmental samples. Appl. Environ. Microbiol. 2000, 66, 4356–4360. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.; Westcott, S.; Ryabin, T.; Hall, J.; Hartmann, M.; Hollister, E.; Lesniewski, R.; Oakley, B.; Parks, D.; Robinson, C.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- The Biodiversity Committee of Chinese Academy of Sciences. Catalogue of Life China: 2020 Annual Checklist; The Biodiversity Committee of Chinese Academy of Sciences: Beijing, China, 2020. [Google Scholar]
- Roskov, Y.; Ower, G.; Orrell, T.; Nicolson, D.; Bailly, N.; Kirk, P.M.; Bourgoin, T.; DeWalt, R.E.; Decock, W.; Nieukerken, E.; et al. (Eds.) Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Species 2000; Naturalis: Leiden, The Netherlands, 2019; ISSN 2405-884X. Available online: www.catalogueoflife.org/annual-checklist/2019 (accessed on 10 March 2021).
- Dudka, I.A. Fungi as Components of Freshwater Biocenoses. Mikol. Fitopatol. 1974, 8, 444–449. [Google Scholar]
- Wijayawardene, N.; Hyde, K.; Dai, D.; Sánchez-García, M.; Goto, B.; Saxena, R.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa–2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Lepère, C.; Boucher, D.; Jardillier, L.; Domaizon, I.; Debroas, D. Succession and regulation factors of small eukaryote community composition in a lacustrine ecosystem (Lake Pavin). Appl. Environ. Microbiol. 2006, 72, 2971–2981. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, D.; Wang, J.; Wu, B.; Hussain, M.; Liu, X. Environmental factors driving fungal distribution in freshwater lake sediments across the Headwater Region of the Yellow River, China. Sci. Rep. 2018, 8, 3768. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Xie, Y.; Wang, J. Sedimentary DNA reveals over 150 years of ecosystem change by human activities in Lake Chao, China. Environ. Int. 2019, 133, 105214. [Google Scholar] [CrossRef]
- de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; de Oliveira, F.S.; Rosa, C.A.; Câmara, P.E.A.S.; Rosa, L.H. Diversity, distribution and ecology of fungal communities present in Antarctic lake sediments uncovered by DNA metabarcoding. Sci. Rep. 2022, 12, 8407. [Google Scholar] [CrossRef]
- Medina, E.M.; Buchler, N.E. Chytrid fungi. Curr. Biol. 2020, 30, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Miyauchi, S.; San Clemente, H.; Chen, E.C.H.; Pelin, A.; de la Providencia, I.; Ndikumana, S.; Beaudet, D.; Hainaut, M.; Drula, E.; et al. Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina. New Phytol. 2019, 222, 1584–1598. [Google Scholar] [CrossRef]
- Shi, X.; Li, S.; Zhang, M.; Liu, C.; Wu, Q. Temperature mainly determines the temporal succession of the photosynthetic picoeukaryote community in Lake Chaohu, a highly eutrophic shallow lake. Sci. Total. Environ. 2020, 702, 134803. [Google Scholar] [CrossRef]
- Barr, D.J.S. Chytridiomycota. Systematics and Evolution; Springer: Berlin/Heidelberg, Germany, 2001; pp. 93–112. [Google Scholar]
- Voronin, L.V. Zoosporic fungi in freshwater ecosystems. Inland Water Biol. 2008, 1, 341–346. [Google Scholar] [CrossRef]
- Tang, C.; Yi, Y.; Zhou, Y. Planktonic indicators of trophic states for a shallow lake (BYD Lake, China). Limnologica 2019, 78, 125712. [Google Scholar] [CrossRef]
- Gleason, F.H.; Carney, L.T.; Lilje, O.; Glockling, S.L. Ecological potentials of species of Rozella cryptomycota. Fung. Ecol. 2012, 5, 651–656. [Google Scholar] [CrossRef]
- Volk, T.J. Fungi. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 141–163. [Google Scholar]
- Redecker, D.; Raab, P. Phylogeny of the glomeromycota (arbuscular mycorrhizal fungi): Recent developments and new gene markers. Mycologia 2006, 98, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, J.; He, X. Symbiotic characteristics of arbuscular mycorrhizal fungi and dark septate endophytes in roots of Baiyang Lake. China Sci. Pap. 2016, 11, 1762–1768. (In Chinese) [Google Scholar]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frey, N.F.D.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Kobayashi, Y.; Kameoka, H.; Okuma, N.; Takeda, N.; Yamaguchi, K.; Bino, T.; Shigenobu, S.; Kawaguchi, M. Evidence of non-tandemly repeated rDNAs and their intragenomic heterogeneity in Rhizophagus irregularis. Commun. Biol. 2018, 1, 87. [Google Scholar] [CrossRef]
- Monchy, S.; Sanciu, G.; Jobard, M.; Rasconi, S.; Gerphagnon, M.; Chabé, M.; Cian, A.; Meloni, D.; Niquil, N.; Christaki, U.; et al. Exploring and quantifying fungal diversity in freshwater lake ecosystems using rDNA cloning/sequencing and SSU tag pyrosequencing. Environ. Microbiol. 2011, 13, 1433–1453. [Google Scholar] [CrossRef]
- Malar, C.M.; Krüger, M.; Krüger, C.; Wang, Y.; Stajich, J.E.; Keller, J.; Chen, E.C.H.; Yildirir, G.; Villeneuve-Laroche, M.; Roux, C.; et al. The genome of Geosiphon pyriformis reveals ancestral traits linked to the emergence of the arbuscular mycorrhizal symbiosis. Curr. Biol. 2021, 31, 1570–1577. [Google Scholar] [CrossRef]
- Dick, M.W. Fungi, flagella and phylogeny. Mycol. Res. 1997, 101, 385–394. [Google Scholar] [CrossRef]
- Simon, M.; López-García, P.; Deschamps, P.; Moreira, D.; Restoux, G.; Bertolino, P.; Jardillier, L. Marked seasonality and high spatial variability of protist communities in shallow freshwater systems. ISME J. 2015, 9, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Kantarcioglu, A.S.; Horré, R.; Rodriguez-Tudela, J.L.; Estrella, M.C.; Berenguer, J.; De Hoog, G.S. Scedosporium apiospermum: Changing clinical spectrum of a therapy-refractory opportunist*. Med. Mycol. 2006, 44, 295–327. [Google Scholar] [CrossRef] [PubMed]
- Glockling, S.L.; Marshall, W.L.; Gleason, F.H. Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea). Fungal Ecol. 2013, 6, 237–247. [Google Scholar] [CrossRef]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef]
- Hassan, N.; Rafiq, M.; Hayat, M.; Shah, A.A.; Hasan, F. Psychrophilic and psychrotrophic fungi: A comprehensive review. Rev. Environ. Sci. Bio/Technol. 2016, 15, 147–172. [Google Scholar] [CrossRef]
- Kauffman, C.A. 33-Fungal Pneumonias, Opal, Infectious Diseases, 4th ed.; Jonathan, C., Steven, M.O., William, G.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 292–299. [Google Scholar]
- Huang, J.; Hu, B.; Qi, K.; Chen, W.; Pang, X.; Bao, W.; Tian, G. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, J.; Gao, G.; Bartlam, M.G. Distribution and diversity of fungi in freshwater sediments on a river catchment scale. Front. Microbiol. 2015, 6, 329. [Google Scholar] [CrossRef]
- Su, J.; Zhang, L.; Yu, M.; Sun, T.; Wang, B.; Zhao, J.; Wang, Z.; Sun, Y.; Zhou, S. Characteristics and distribution of nitrogen forms insediments of Baiyangdian lake in summer and autumn. Environ. Eng. 2022, 40, 53–58+153. (In Chinese) [Google Scholar]
- Talbot, J.; Treseder, K. Controls over mycorrhizal uptake of organic nitrogen. Pedobiologia 2010, 53, 169–179. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Hawkins, B.J.; Jones, M.D.; Robbins, S.; Dyer, T.; Li, T. Species turnover (β-diversity) in ectomycorrhizal fungi linked to NH4+ uptake capacity. Mol. Ecol. 2015, 24, 5992–6005. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Lu, F.; Huang, P.; Tang, M.; Xu, F.; Zhang, W.; Zhou, J.; Zhao, P.; Jia, Y.; Dai, C. Root endophyte differentially regulates plant response to NO3− and NH4+ nutrition by modulating N fluxes at the plant–fungal interface. Plant Cell Environ. 2022, 45, 1813–1828. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Bünemann, E., Oberson, A.F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. [Google Scholar]
- Wang, F.; Yao, J.; Si, Y.; Chen, H.; Russel, M.; Chen, K.; Qian, Y.; Zaray, G.; Bramanti, E. Short-time effect of heavy metals upon microbial community activity. J. Hazard. Mater. 2010, 173, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, J.; Min, N.; Sunahara, G.; Liu, J.; Li, M.; Liu, B.; Pang, W.; Cao, Y.; Li, R.; et al. Microbial metabolic activity in metal(loid)s contaminated sites impacted by different non-ferrous metal activities. J. Hazard. Mater. 2023, 459, 132005. [Google Scholar] [CrossRef]
- Priyanka; Dwivedi, S.K. Fungi mediated detoxification of heavy metals: Insights on mechanisms, influencing factors and recent developments. J. Water Process. Eng. 2023, 53, 103800. [Google Scholar]
- China Environmental Monitoring Center. Background Value of Soil Elements in China; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Stefanowicz, A.M.; Kapusta, P.; Szarek-Łukaszewska, G.; Grodzińska, K.; Niklińska, M.; Vogt, R.D. Soil fertility and plant diversity enhance microbial performance in metal-polluted soils. Sci. Total. Environ. 2012, 439, 211–219. [Google Scholar] [CrossRef]
| Env. Factors | Minimum~Maximum | Average ± Standard Deviation | ||||
|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Winter | Spring | Summer | |
| T (°C) | −2.61~−0.24 | 6.10~7.80 | 26.40~28.50 | −1.77 ± 0.60 | 7.06 ± 0.49 | 27.27 ± 0.41 |
| pH | 7.66~8.33 | 6.90~7.93 | 7.09~7.62 | 7.93 ± 0.20 | 7.44 ± 0.22 | 7.43 ± 0.15 |
| TP (mg/kg) | 568~1734 | 473~3101 | 496~3653 | 962.6 ± 296.5 | 960.5 ± 529.4 | 1071.9 ± 599.9 |
| Res-P (mg/kg) | 144.7~588.8 | 153.6~449.7 | 36.7~1134.8 | 304.63 ± 111.84 | 185.09 ± 68.24 | 234.13 ± 212.80 |
| HCl-P (mg/kg) | 276.5~914.5 | 235.7~2109.9 | 340.6~2175.7 | 529.25 ± 164.12 | 610.12 ± 376.78 | 642.33 ± 362.42 |
| NaOH-P (mg/kg) | 48.2~204.4 | 69.1~299.8 | 83.0~338.6 | 111.06 ± 44.49 | 96.77 ± 48.16 | 158.46 ± 54.96 |
| BD-P (mg/kg) | 1.4~18.8 | 9.8~144.1 | 0.2~80.2 | 11.33 ± 4.58 | 50.37 ± 32.17 | 24.44 ± 26.80 |
| NH4Cl-P (mg/kg) | 0.78~19.74 | 0.08~25.22 | 0.02~47.59 | 5.86 ± 5.37 | 9.28 ± 8.90 | 12.45 ± 16.63 |
| TN (g/kg) | 0.70~12.40 | 9.76~13.24 | 1.37~6.82 | 3.70 ± 2.44 | 10.49 ± 3.66 | 3.75 ± 1.73 |
| NO3− (mg/kg) | 1.13~8.93 | 0.98~3.21 | 0.80~6.77 | 4.59 ± 1.80 | 1.64 ± 0.73 | 2.13 ± 1.62 |
| NH4+ (mg/kg) | 0.84~5.26 | 2.38~6.25 | 1.78~12.21 | 2.12 ± 1.14 | 4.10 ± 1.03 | 6.56 ± 3.19 |
| TOC (g/kg) | 4.34~132.95 | 28.22~115.65 | 12.97~66.83 | 36.02 ± 26.70 | 83.30 ± 21.91 | 36.44 ± 17.63 |
| As (mg/kg) | 5.66~16.90 | 3.48~16.10 | 4.88~12.88 | 10.32 ± 2.73 | 10.06 ± 2.99 | 9.12 ± 1.81 |
| Cd (mg/kg) | 0.07~2.36 | 0.07~0.71 | 0.16~0.75 | 0.49 ± 0.47 | 0.43 ± 0.19 | 0.43 ± 0.13 |
| Cr (mg/kg) | 48.60~80.10 | 35.00~248.90 | 21.00~97.0 | 61.30 ± 8.84 | 83.85 ± 53.75 | 49.93 ± 15.83 |
| Cu (mg/kg) | 16.70~57.90 | 16.00~74.30 | 20.10~72.10 | 32.53 ± 11.58 | 38.06 ± 16.09 | 41.53 ± 15.76 |
| Pb (mg/kg) | 16.20~44.70 | 11.20~26.20 | 14.60~34.40 | 25.68 ± 6.75 | 19.60 ± 3.85 | 22.37 ± 4.86 |
| Zn (mg/kg) | 54.60~244.00 | 54.20~332.80 | 67.20~249.00 | 112.11 ± 49.15 | 115.99 ± 54.73 | 119.80 ± 41.48 |
| Co (mg/kg) | 7.39~13.70 | 3.50~12.02 | 7.83~13.22 | 11.12 ± 2.19 | 8.89 ± 1.94 | 10.10 ± 1.48 |
| Mn (mg/kg) | 448~796 | 403~915 | 420~751 | 600.46 ± 90.26 | 602.28 ± 146.86 | 622.61 ± 95.35 |
| Ni (mg/kg) | 20.00~30.30 | 19.20~131.60 | 23.60~83.50 | 30.12 ± 9.62 | 45.25 ± 29.48 | 40.70 ± 17.28 |
| Fe (g/kg) | 23.26~33.24 | 10.78~35.74 | 24.95~37.68 | 29.24 ± 4.71 | 26.12 ± 5.77 | 30.32 ± 3.60 |
| Factors | T | Res-P | NaOH-P | BD-P | NH4Cl-P | TN | NO3− | NH4+ | As | Mn | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 22.47% | 3.98% | 1.71% | 2.72% | 3.25% | 4.87% | 1.70% | 1.51% | 1.91% | 2.33% | 2.60% |
| p | 0.001 | 0.001 | 0.018 | 0.002 | 0.001 | 0.001 | 0.018 | 0.049 | 0.003 | 0.006 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Yin, S. Seasonal Variations of Sediment Fungal Community of a Shallow Lake in North China. Microorganisms 2024, 12, 2127. https://doi.org/10.3390/microorganisms12112127
Yi Y, Yin S. Seasonal Variations of Sediment Fungal Community of a Shallow Lake in North China. Microorganisms. 2024; 12(11):2127. https://doi.org/10.3390/microorganisms12112127
Chicago/Turabian StyleYi, Yujun, and Senlu Yin. 2024. "Seasonal Variations of Sediment Fungal Community of a Shallow Lake in North China" Microorganisms 12, no. 11: 2127. https://doi.org/10.3390/microorganisms12112127
APA StyleYi, Y., & Yin, S. (2024). Seasonal Variations of Sediment Fungal Community of a Shallow Lake in North China. Microorganisms, 12(11), 2127. https://doi.org/10.3390/microorganisms12112127







