Performance Evaluation of NovaplexTM Multiplex Real-Time PCR Assay for Detection of Streptococcus agalactiae Serotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
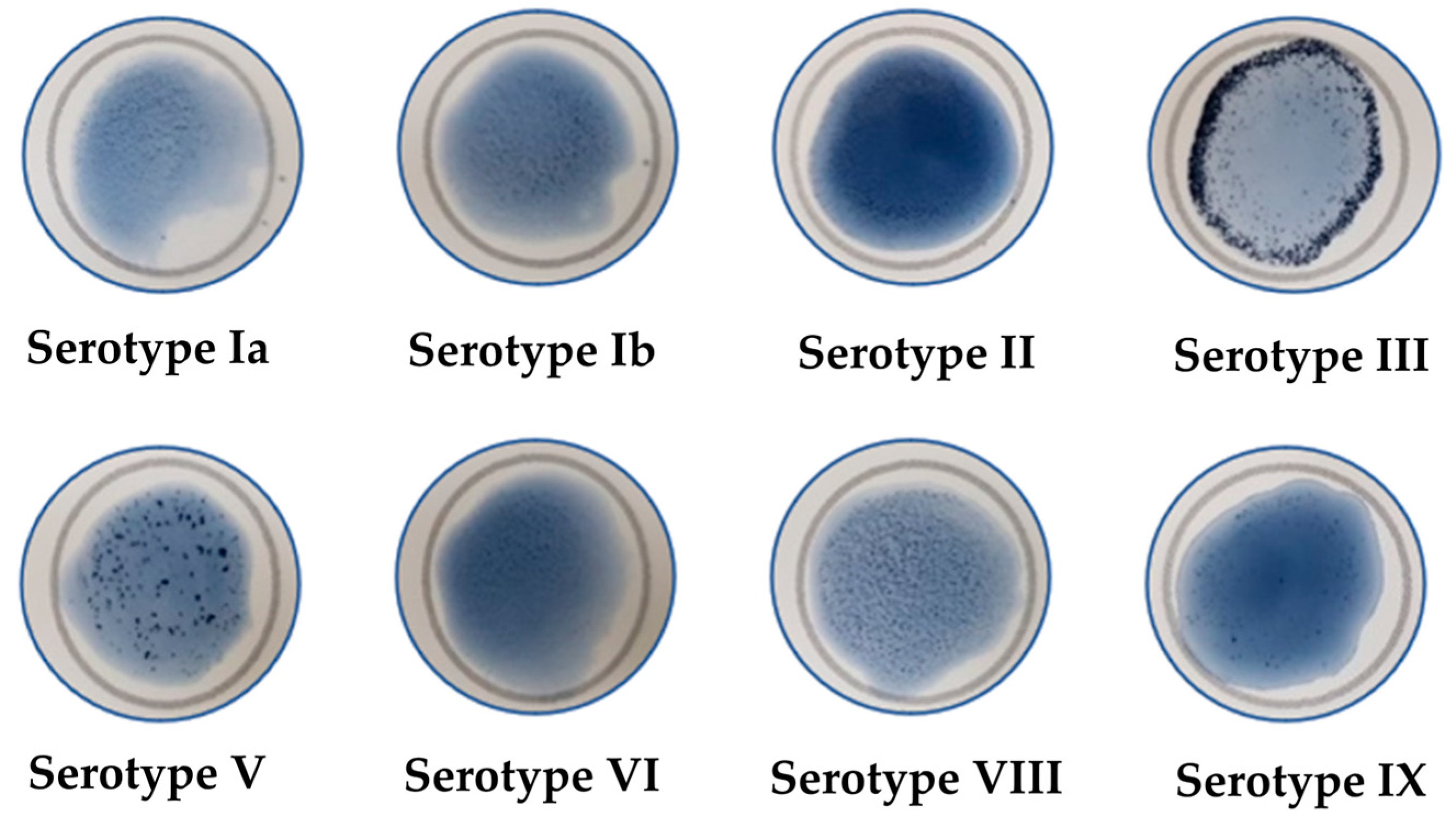
2.2. Serotyping bt Latex Agglutination (LA)
2.3. Extraction of Nucleic Acid and Multiplex Polymerase Chain Reaction (mPCR)
2.4. Multiplex Real-Time PCR
2.5. Sensitivity and Specificity of NovaPCR
2.6. Interpretation and Statistical Analysis
3. Results
3.1. Serotype Distribution by LA Test
3.2. Serotype Distribution by Conventional mPCR
3.3. Serotype Identification by NovaPCR
3.4. Sensitivity and Specificity of NovaPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farley, M.M. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 2001, 33, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.S.; Schonheyder, H.C.; Knudsen, J.D.; Lyytikainen, O.; Dryden, M.; Kennedy, K.J.; Valiquette, L.; Pinholt, M.; Jacobsson, G.; Laupland, K.B.; et al. The changing epidemiology of group B streptococcus bloodstream infection: A multi-national population-based assessment. Infect. Dis. 2016, 48, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Brigtsen, A.K.; Dedi, L.; Melby, K.K.; Holberg-Petersen, M.; Radtke, A.; Lyng, R.V.; Andresen, L.L.; Jacobsen, A.F.; Fugelseth, D.; Whitelaw, A. Comparison of PCR and serotyping of Group B Streptococcus in pregnant women: The Oslo GBS-study. J. Microbiol. Methods 2015, 108, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Ang, I.; Fung, K.; Liyanapathirana, V.; Luo, M.J.; Lai, R. Hypervirulent Clone of Group B Streptococcus Serotype III Sequence Type 283, Hong Kong, 1993–2012. Emerg. Infect. Dis. 2016, 22, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Rothen, J.; Sapugahawatte, D.N.; Li, C.; Lo, N.; Vogel, G.; Foucault, F.; Pfluger, V.; Pothier, J.F.; Blom, J.; Daubenberger, C.; et al. A simple, rapid typing method for Streptococcus agalactiae based on ribosomal subunit proteins by MALDI-TOF MS. Sci. Rep. 2020, 10, 8788. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Heath, P.T. An overview of global GBS epidemiology. Vaccine 2013, 31 (Suppl. 4), D7–D12. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Lu, J.-J.; Lin, C.; Chu, S.-M.; Lin, L.-C.; Lai, M.-Y.; Huang, H.-R.; Chiang, M.-C.; Tsai, M.-H. Clustered regularly interspaced short palindromic repeat analysis of clonal complex 17 serotype iii group b streptococcus strains causing neonatal invasive diseases. Int. J. Mol. Sci. 2021, 22, 11626. [Google Scholar] [CrossRef]
- Miselli, F.; Frabboni, I.; Di Martino, M.; Zinani, I.; Buttera, M.; Insalaco, A.; Stefanelli, F.; Lugli, L.; Berardi, A. Transmission of Group B Streptococcus in late-onset neonatal disease: A narrative review of current evidence. Ther. Adv. Infect. Dis. 2022, 9, 20499361221142732. [Google Scholar] [CrossRef]
- Edwards, M.S.; Baker, C.J. Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 2005, 41, 839–847. [Google Scholar] [CrossRef]
- Lee, N.Y.; Yan, J.J.; Wu, J.J.; Lee, H.C.; Liu, K.H.; Ko, W.C. Group B streptococcal soft tissue infections in non-pregnant adults. Clin. Microbiol. Infect. 2005, 11, 577–579. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Jose, L.; Koen, A.; Govender, N.; Wittke, F.; Olugbosi, M.; Meulen, A.S.; Baker, S.; Dull, P.M.; et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: A randomised phase 1b/2 trial. Lancet Infect. Dis. 2016, 16, 923–934. [Google Scholar] [CrossRef]
- Jin, Z.; Li, J.; Zhou, H.; Wang, Z.; Yi, L.; Liu, N.; Du, J.; Chang, C.Y.; Ji, W. Serotype Distribution, Virulence Determinants and Antimicrobial Susceptibility of Streptococcus agalactiae Isolated from Young Infants. Pathogens 2022, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J. Group B Streptococcus: Virulence Factors and Pathogenic Mechanism. Microorganisms 2022, 10, 2483. [Google Scholar] [CrossRef]
- Schindler, Y.; Rahav, G.; Nissan, I.; Treygerman, O.; Prajgrod, G.; Attia, B.Z.; Raz, R.; Valenci, G.Z.; Tekes-Manova, D.; Maor, Y. Group B streptococcus virulence factors associated with different clinical syndromes: Asymptomatic carriage in pregnant women and early-onset disease in the newborn. Front. Microbiol. 2023, 14, 1093288. [Google Scholar] [CrossRef]
- Slotved, H.-C.; Elliott, J.; Thompson, T.; Konradsen, H.B. Latex assay for serotyping of group B Streptococcus isolates. J. Clin. Microbiol. 2003, 41, 4445–4447. [Google Scholar] [CrossRef]
- Slotved, H.C.; Sauer, S.; Konradsen, H.B. False-negative results in typing of group B streptococci by the standard lancefield antigen extraction method. J. Clin. Microbiol. 2002, 40, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Campisi, E.; De Chiara, M.; Tettelin, H.; Rinaudo, D.; Toniolo, C.; Metruccio, M.; Guidotti, S.; Sorensen, U.B.; Kilian, M.; et al. Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS ONE 2015, 10, e0125985. [Google Scholar] [CrossRef]
- Sheppard, A.E.; Vaughan, A.; Jones, N.; Turner, P.; Turner, C.; Efstratiou, A.; Patel, D.; Modernising Medical Microbiology Informatics, G.; Walker, A.S.; Berkley, J.A.; et al. Capsular Typing Method for Streptococcus agalactiae Using Whole-Genome Sequence Data. J. Clin. Microbiol. 2016, 54, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Streptococcus pneumoniae Detection and Serotyping Using PCR; CDC Publication: New York, NY, USA, 2024. [Google Scholar]
- Ivanov, I.G.; Bachvarov, D.R. Determination of plasmid copy number by the “boiling” method. Anal. Biochem. 1987, 165, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Imperi, M.; Pataracchia, M.; Alfarone, G.; Baldassarri, L.; Orefici, G.; Creti, R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J. Microbiol. Methods 2010, 80, 212–214. [Google Scholar] [CrossRef]
- Skoff, T.H.; Farley, M.M.; Petit, S.; Craig, A.S.; Schaffner, W.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Mohle-Boetani, J.; Zansky, S.J. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 2009, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Phares, C.R.; Lynfield, R.; Farley, M.M.; Mohle-Boetani, J.; Harrison, L.H.; Petit, S.; Craig, A.S.; Schaffner, W.; Zansky, S.M.; Gershman, K.; et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. Jama 2008, 299, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; Nizet, V.; Maldonado, Y.; Remington, J.S.; Klein, J.O. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant; Elsevier: Amsterdam, The Netherlands, 2014; pp. 411–456. [Google Scholar]
- Edmond, K.M.; Kortsalioudaki, C.; Scott, S.; Schrag, S.J.; Zaidi, A.K.; Cousens, S.; Heath, P.T. Group B streptococcal disease in infants aged younger than 3 months: Systematic review and meta-analysis. Lancet 2012, 379, 547–556. [Google Scholar] [CrossRef]
- Lo, C.W.; Liu, H.C.; Lee, C.C.; Lin, C.L.; Chen, C.L.; Jeng, M.J.; Chiu, C.H. Serotype distribution and clinical correlation of Streptococcus agalactiae causing invasive disease in infants and children in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 578–584. [Google Scholar] [CrossRef]
- Ali, M.M.; Asrat, D.; Fenta, D.A.; Chaka, T.E.; Woldeamanuel, Y. Group B Streptococcus colonization rate and serotype distribution among pregnant women and their newborns at Adama Hospital Medical College, Ethiopia. Sci. Rep. 2020, 10, 9301. [Google Scholar] [CrossRef]
- Madzivhandila, M.; Adrian, P.V.; Cutland, C.L.; Kuwanda, L.; Schrag, S.J.; Madhi, S.A. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS ONE 2011, 6, e17861. [Google Scholar] [CrossRef]
- van Kassel, M.N.; de Boer, G.; Teeri, S.A.; Jamrozy, D.; Bentley, S.D.; Brouwer, M.C.; van der Ende, A.; van de Beek, D.; Bijlsma, M.W. Molecular epidemiology and mortality of group B streptococcal meningitis and infant sepsis in the Netherlands: A 30-year nationwide surveillance study. Lancet Microbe 2021, 2, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Liu, H. Neonatal Late-Onset Meningitis Caused by Serotype III CC17 Group B Streptococci Aggregating in Two Families from Southern China. Infect. Drug Resist. 2023, 16, 3417–3424. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, D.H.; Yang, J.H.; Kim, M.Y.; Shin, S.M.; Kim, E.C.; Park, S.S.; Seong, M.W. Usefulness of a rapid real-time PCR assay in prenatal screening for group B streptococcus colonization. Ann. Lab. Med. 2013, 33, 39–44. [Google Scholar] [CrossRef]
- Yao, K.; Poulsen, K.; Maione, D.; Rinaudo, C.D.; Baldassarri, L.; Telford, J.L.; Sorensen, U.B.; Members of the DEVANI Study Group; Kilian, M. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J. Clin. Microbiol. 2013, 51, 503–507. [Google Scholar] [CrossRef]
- Gosiewski, T.; Brzychczy-Włoch, M.; Heczko, P.B. The application of multiplex PCR to detect seven different DNA targets in group B streptococci. Folia Microbiol. 2012, 57, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Cai, Y.; Gao, Y.; Wang, G.; Miao, Y.; Gao, X. Establishment and application of a rapid visual diagnostic method for Streptococcus agalactiae based on recombinase polymerase amplification and lateral flow strips. Sci. Rep. 2024, 14, 10064. [Google Scholar] [CrossRef] [PubMed]
- Breeding, K.M.; Ragipani, B.; Lee, K.D.; Malik, M.; Randis, T.M.; Ratner, A.J. Real-time PCR-based serotyping of Streptococcus agalactiae. Sci. Rep. 2016, 6, 38523. [Google Scholar] [CrossRef]
- Eichinger, A.; Hagen, A.; Meyer-Bühn, M.; Huebner, J. Clinical benefits of introducing real-time multiplex PCR for cerebrospinal fluid as routine diagnostic at a tertiary care pediatric center. Infection 2019, 47, 51–58. [Google Scholar] [CrossRef]
- Le Bars, H.; Madany, N.; Lamoureux, C.; Beauruelle, C.; Vallet, S.; Payan, C.; Pilorgé, L. Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections. Diagnostics 2023, 13, 1110. [Google Scholar] [CrossRef]
- Oeser, C.; Pond, M.; Butcher, P.; Bedford Russell, A.; Henneke, P.; Laing, K.; Planche, T.; Heath, P.T.; Harris, K. PCR for the detection of pathogens in neonatal early onset sepsis. PLoS ONE 2020, 15, e0226817. [Google Scholar] [CrossRef]
- Otaguiri, E.S.; Morguette, A.E.B.; Morey, A.T.; Tavares, E.R.; Kerbauy, G.; de Almeida Torres, R.S.; Chaves Júnior, M.; Tognim, M.C.B.; Góes, V.M.; Krieger, M.A.; et al. Development of a melting-curve based multiplex real-time PCR assay for simultaneous detection of Streptococcus agalactiae and genes encoding resistance to macrolides and lincosamides. BMC Pregnancy Childbirth 2018, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Khan, M.A.; Faiz, A.; Ahmad, J.; Khidir, E.B.; Basalamah, M.A.; Aslam, A. Group B Streptococcus Colonization, Antibiotic Susceptibility, and Serotype Distribution among Saudi Pregnant Women. Infect. Chemother. 2020, 52, 70–81. [Google Scholar] [CrossRef]
- Wang, P.; Tong, J.J.; Ma, X.H.; Song, F.L.; Fan, L.; Guo, C.M.; Shi, W.; Yu, S.J.; Yao, K.H.; Yang, Y.H. Serotypes, antibiotic susceptibilities, and multi-locus sequence type profiles of Streptococcus agalactiae isolates circulating in Beijing, China. PLoS ONE 2015, 10, e0120035. [Google Scholar] [CrossRef]
- ACOG. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obs. Gynecol. 2020, 135, e51–e72. [Google Scholar] [CrossRef]
- Lohrmann, F.; Hufnagel, M.; Kunze, M.; Afshar, B.; Creti, R.; Detcheva, A.; Kozakova, J.; Rodriguez-Granger, J.; Sørensen, U.B.S.; Margarit, I.; et al. Neonatal invasive disease caused by Streptococcus agalactiae in Europe: The DEVANI multi-center study. Infection 2023, 51, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Snoek, L.; van Kassel, M.N.; Krommenhoek, J.F.; Achten, N.B.; Plotz, F.B.; van Sorge, N.M.; Brouwer, M.C.; van de Beek, D.; Bijlsma, M.W.; the NOGBS study group. Neonatal early-onset infections: Comparing the sensitivity of the neonatal early-onset sepsis calculator to the Dutch and the updated NICE guidelines in an observational cohort of culture-positive cases. eClinicalMedicine 2022, 44, 101270. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B streptococcus infection during pregnancy and infancy: Estimates of regional and global burden. Lancet Glob. Health 2022, 10, e807–e819. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, T.H.; Kim, E.T.; Kim, Y.R.; Lee, H. Molecular epidemiology and virulence factors of group B Streptococcus in South Korea according to the invasiveness. BMC Infect. Dis. 2024, 24, 740. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sense (5′-3′) |
|---|---|
| cpsI-Ia-6-7-F | GAATTGATAACTTTTGTGGATTGCGATGA |
| cpsI-6-R | CAATTCTGTCGGACTATCCTGATG |
| cpsI-7-R | TGTCGCTTCCACACTGAGTGTTGA |
| cpsL-F | CAATCCTAAGTATTTTCGGTTCATT |
| cpsL-R | TAGGAACATGTTCATTAACATAGC |
| cpsG-F | ACATGAACAGCAGTTCAACGGT |
| cpsG-R | ATGCTCTCCAAACTGTTCTTGT |
| cpsG-2-3-6-R | TCCATCTACATCTTCAATCCAAGC |
| cpsN-5-F | ATGCAACCAAGTGATTATCATGTA |
| cpsN-5-R | CTCTTCACTCTTTAGTGTAGGTAT |
| cpsJ-8-F | TATTTGGGAGGTAATCAAGAGACA |
| cpsJ-8-R | GTTTGGAGCATTCAAGATAACTCT |
| cpsJ-2-4-F | CATTTATTGATTCAGACGATTACATTGA |
| cpsJ-2-R | CCTCTTTCTCTAAAATATTCCAACC |
| cpsJ-4-R | CCTCAGGATATTTACGAATTCTGTA |
| cpsI-7-9-F | CTGTAATTGGAGGAATGTGGATCG |
| cpsI-9-R | AATCATCTTCATAATTTATCTCCCATT |
| cpsJ-Ib-F | GCAATTCTTAACAGAATATTCAGTTG |
| cpsJ-Ib-R | GCGTTTCTTTATCACATACTCTTG |
| Serotype/Specimen | Blood | Urine | Vaginal Discharge | Pus | Others | Total |
|---|---|---|---|---|---|---|
| Ia | 2 | 4 | 4 | 4 | 6 | 20 |
| Ib | 1 | 7 | 1 | 4 | 7 | 20 |
| II | 2 | 4 | 2 | 0 | 2 | 10 |
| III | 7 | 7 | 3 | 0 | 3 | 20 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 |
| V | 4 | 7 | 2 | 2 | 6 | 21 |
| VI | 1 | 9 | 2 | 5 | 3 | 20 |
| VII | 0 | 0 | 0 | 0 | 0 | 0 |
| VIII | 8 | 6 | 3 | 3 | 0 | 20 |
| IX | 0 | 1 | 0 | 0 | 0 | 1 |
| Serotype | LA Test | mPCR | NovaPCR | Kappa Value | Agreement | |
|---|---|---|---|---|---|---|
| Observed Kappa (95% CI) | Positive (%) (95% CI) | Negative (%) (95% CI) | ||||
| Ia | 20 | 20 | 18 | 0.983 | 94 | 99 |
| Ib | 20 | 20 | 20 | 1 | 100 | 100 |
| II | 10 | 10 | 10 | 1 | 100 | 100 |
| III | 20 | 20 | 20 | 1 | 100 | 100 |
| IV | 0 | 0 | 1 | NA | NA | NA |
| V | 21 | 21 | 22 | 1 | 100 | 100 |
| VI | 20 | 20 | 20 | 1 | 100 | 100 |
| VII | 0 | 0 | 0 | NA | NA | NA |
| VIII | 20 | 20 | 20 | 1 | 100 | 100 |
| IX | 1 | 1 | 1 | 1 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handigund, M.; Lee, J. Performance Evaluation of NovaplexTM Multiplex Real-Time PCR Assay for Detection of Streptococcus agalactiae Serotypes. Microorganisms 2024, 12, 2043. https://doi.org/10.3390/microorganisms12102043
Handigund M, Lee J. Performance Evaluation of NovaplexTM Multiplex Real-Time PCR Assay for Detection of Streptococcus agalactiae Serotypes. Microorganisms. 2024; 12(10):2043. https://doi.org/10.3390/microorganisms12102043
Chicago/Turabian StyleHandigund, Mallikarjun, and Jaehyeon Lee. 2024. "Performance Evaluation of NovaplexTM Multiplex Real-Time PCR Assay for Detection of Streptococcus agalactiae Serotypes" Microorganisms 12, no. 10: 2043. https://doi.org/10.3390/microorganisms12102043
APA StyleHandigund, M., & Lee, J. (2024). Performance Evaluation of NovaplexTM Multiplex Real-Time PCR Assay for Detection of Streptococcus agalactiae Serotypes. Microorganisms, 12(10), 2043. https://doi.org/10.3390/microorganisms12102043






