Wheat Bran and Saccharomyces Cerevisiae Biomass’ Effect on Aerobic and Anaerobic Degradation Efficiency of Paper Composite
Abstract
1. Introduction
- (a)
- Biodegradable food packaging offers significant benefits to the circular economy by purifying waste streams, diverting food waste from landfills, and providing sustainable alternatives for non-recyclable packaging [4]. This approach facilitates the return of biomaterials to the soil, thereby enhancing biological recycling benefits [5,6,7,8].
- (b)
- The utilization of plant-based feedstocks, particularly those sourced from industrial waste or by-products, highlights the importance of renewable resources with lower environmental impacts [2].
- (c)
- The application of paper (both wood and non-wood fiber) in fully green food packaging remains limited due to stringent barrier property requirements. The natural components of yeast, such as proteins and glucans, have unique characteristics that could enhance fully green food packaging barrier properties.
2. Materials and Methods
2.1. Test Samples
2.2. Inoculum
2.3. Aerobic Biodegradation Tests
- M(O2) = Molecular weight of oxygen (32,000 mg/mol)
- R = Gas constant (83.144 L·hPa/(mol·K))
- T0 = Temperature (273.15 K)
- Tm = Measuring temperature (319.15 K for performed BOD)
- Vtot = Bottle volume [mL]
- V1 = Sample volume [mL]
- α = Bunsen absorption coefficient (0.03103)
- Δp(O2) = Difference in the partial oxygen pressure [hPa]
2.4. Anaerobic Biodegradation
2.5. Analytical Determination
3. Results and Discussion
3.1. Aerobic Biodegradation Results
3.2. Anaerobic Biodegradation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markevičiūtė, Z.; Varžinskas, V. Smart Material Choice: The Importance of Circular Design Strategy Applications for Bio-Based Food Packaging Preproduction and End-of-Life Life Cycle Stages. Sustainability 2022, 14, 6366. [Google Scholar] [CrossRef]
- Markevičiūtė, Z.; Varžinskas, V. Plant-Origin Feedstock Applications in Fully Green Food Packaging: The Potential for Tree-Free Paper and Plant-Origin Bio-Plastics in the Baltic Sea Region. Sustainability 2022, 14, 7393. [Google Scholar] [CrossRef]
- Markevičiūtė, Z.; Lyytikäinen, J.; Leminen, V.; Varžinskas, V. Grain by-products and Saccharomyces cerevisiae application in paper packaging material: Impact on physical–mechanical and barrier properties. Discov. Sustain. 2024, 5, 82. [Google Scholar] [CrossRef]
- Kachook, O.; Cramer, K.; Gendell, A. Understanding the Role of Compostable Packaging in North America, Sustainable Packaging Coalition. 2021. Available online: https://sustainablepackaging.org/wp-content/uploads/2023/07/UnderstandingCompostablePackagingGuide.pdf (accessed on 27 September 2024).
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Odegard, I.; Nusselder, S.; Lindgreen, E.R.; Bergsma, G.; Graaff, L. Biobased Plastics in a Circular Economy—Policy Suggestions for Biobased and Biobased Biodegradable Plastics; CE Delft: Delft, The Netherlands, 2017. [Google Scholar]
- Spierling, S.; Knüpffer, E.; Behnsena, H.; Mudersbacha, M.; Kriegb, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endresa, H.-J. Bio-based plastics—A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Delgado, J.F.; Sceni, P.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Development of innovative biodegradable films based on biomass of Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Water vapour transport in biopolymeric materials: Effects of thickness and water vapour pressure gradient on yeast biomass-based films. J. Polym. Environ. 2022, 30, 2976–2989. [Google Scholar] [CrossRef]
- Lago, A.; Delgado, J.F.; Rezzani, G.D.; Cottet, C.; Ramírez Tapias, Y.A.; Peltzer, M.A.; Salvay, A.G. Multi-Component Biodegradable Materials Based on Water Kefir Grains and Yeast Biomasses: Effect of the Mixing Ratio on the Properties of the Films. Polymers 2023, 15, 2594. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.C.; Abeywickrama, T.D.; Rahman, F.A. Role of genetically engineered yeast in plastic degradation. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Daverey, A., Dutta, K., Joshi, S., Gea, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 567–584. [Google Scholar]
- Zahmatkesh Anbarani, M.; Esmaeili Nasrabadi, A.; Bonyadi, Z. Use of Saccharomyces cerevisiae as new technique to remove polystyrene from aqueous medium: Modeling, optimization, and performance. Appl. Water Sci. 2023, 13, 166. [Google Scholar] [CrossRef]
- Rozzi, A.; Remigi, E. Methods of assessing microbial activity and inhibition under anaerobic conditions: A literature review. Rev. Environ. Sci. Bio/Technol. 2004, 3, 93–115. [Google Scholar] [CrossRef]
- Zavala, M.; Funamizu, N.; Takakuwa, T. Temperature effect on aerobic biodegradation of feces using sawdust as a matrix. Water 2004, 38, 2406–2416. [Google Scholar] [CrossRef]
- Lim, B.-R.; Huang, X.; Hu, H.-Y.; Goto, N.; Fujie, K. Effects of temperature on biodegradation characteristics of organic pollutants and microbial community in a solid phase aerobic bioreactor treating high strength organic wastewater. Water Sci. Technol. 2001, 43, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, T.; Li, T.; Lü, F.; He, P. Comparison of sludge digestion under aerobic and anaerobic conditions with a focus on the degradation of proteins at mesophilic temperature. Bioresour. Technol. 2013, 140, 131–137. [Google Scholar] [CrossRef]
- Ma, A.; As, A.; Ma, C.; Zhang, M.A.; Xu, Z.A.; As, A.; Mf, A.; Wei, W.A.; Ly, B. Ultra-high temperature aerobic fermentation pretreatment composting: Parameters optimization, mechanisms and compost quality assessment. J. Environ. Chem. Eng. 2021, 9, 105453. [Google Scholar]
- Xu, H.; Li, Y.; Hua, D.; Zhao, Y.; Chen, L.; Zhou, L.; Chen, G. Effect of microaerobic microbial pretreatment on anaerobic digestion of a lignocellulosic substrate under controlled pH conditions. Bioresour. Technol. 2021, 328, 124852. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Taherzadeh, M.J. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Subirats, J.; Sharpe, H.; Topp, E. Fate of Clostridia and other spore-forming Firmicute bacteria during feedstock anaerobic digestion and aerobic composting. J. Environ. Manag. 2022, 309, 114643. [Google Scholar] [CrossRef]
- Liu, R.; Gong, H.; Xu, Y.; Cai, C.; Hua, Y.; Li, L.; Dai, L.; Dai, X. The transition temperature (42 °C) from mesophilic to thermophilic microorganisms enhances biomethane potential of corn stover. Sci. Total Environ. 2021, 759, 143549. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Taherzadeh, M.J. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Microbial community changes in methanogenic granules during the transition from mesophilic to thermophilic conditions. Appl. Microbiol. Biotechnol. 2016, 101, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 14851:2019; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2019.
- Rich, N.; Bharti, A. Assessment of different types of in-vessel composters and its effect on stabilization of MSW compost. Int. Res. J. Eng. Techno. 2015, 2, 37–42. [Google Scholar]
- Steiniger, B.; Hupfauf, S.; Insam, H.; Schaum, C. Exploring Anaerobic Digestion from Mesophilic to Thermophilic Temperatures—Operational and Microbial Aspects. Fermentation 2023, 9, 798. [Google Scholar] [CrossRef]
- Wang, P.; Han, S.; Lin, Y. Chapter 8—Role of microbes and microbial dynamics during composting. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–220. [Google Scholar]
- Tannouri, A.; Rizk, Z.; Daccache, M.; Ghanem, C.; Azzi, V.; Maroun, R.G.; Hobaika, Z.; Salameh, D. Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency. Agronomy 2023, 13, 2048. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Metcalf & Eddy. In Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Higher Education: New York, NY, USA, 2014. [Google Scholar]
- The new Italian standard UNI/TS 11703:2018 on the BMP assay. In Method for the Measurement of the Potential Production of Methane from Wet Anaerobic Digestion—Matrices in Feed; Comitato Termotecnico Italiano (CTI): Milan, Italy, 2018.
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- ISO 14853:2016; Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in an Aqueous System—Method by Measurement of Biogas Production. International Organization for Standardization: Geneva, Switzerland, 2016.
- Sameena, B.; Sudharshan, J.; Gangagni, R.A.; Nicky, E. Comparison of mesophilic and thermophilic methane production potential of acids rich and high-strength landfill leachate at different initial organic loadings and food to inoculum ratios. Sci. Total Environ. 2020, 715, 136658. [Google Scholar]
- Kang, J.-H.; Kang, S.-W.; Kim, W.-J.; Kim, D.-H.; Im, S.-W. Anaerobic Co-Digestion of Bioplastics and Food Waste under Mesophilic and Thermophilic Conditions: Synergistic Effect and Biodegradation. Fermentation 2022, 8, 638. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic Digestion of Solid Organic Substrates in Batch Mode: An Overview Relating to Methane Yields and Experimental Procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- ASTM D1252-06(2020); Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. ASTM International: West Conshohocken, PA, USA, 2020.
- Sztupecki, W.; Rhazi, L.; Depeint, F.; Aussenac, T. Functional and Nutritional Characteristics of Natural or Modified Wheat Bran Non-Starch Polysaccharides: A Literature Review. Foods 2023, 12, 2693. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Mahfuz, S.; Piao, X. The Impact of Wheat Bran on the Morphology and Physiology of the Gastrointestinal Tract in Broiler Chickens. Animals 2020, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Yang, M.D.; Fan, X.F. Study on Modification of Dietary Fiber from Wheat Bran. Adv. Mater. Res. 2011, 183–185, 1268–1272. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A Sight to Wheat Bran: High Value-Added Products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R. Microbial Biofilms: Structural Plasticity and Emerging Properties. Microorganisms 2022, 10, 138. [Google Scholar] [CrossRef]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kuhar, S.; Sharma, K.K.; Shrivastava, B. Microorganisms and Enzymes Involved in Lignin Degradation Vis-à-vis Production of Nutritionally Rich Animal Feed: An Overview. In Biotechnology for Environmental Management and Resource Recovery; Kuhad, R., Singh, A., Eds.; Springer: New Delhi, India, 2013. [Google Scholar]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.W.; Charlton, A. Establishing Experimental Conditions to Produce Lignin-Degrading Enzymes on Wheat Bran by Trametes versicolor CM13 Using Solid State Fermentation. Waste 2023, 1, 711–723. [Google Scholar] [CrossRef]
- Li, P.; Liu, D.; Pei, Z.; Zhao, L.; Shi, F.; Yao, Z.; Liu, J. Evaluation of lignin inhibition in anaerobic digestion from the perspective of reducing the hydrolysis rate of holocellulose. Bioresour. Technol. 2021, 333, 125204. [Google Scholar] [CrossRef] [PubMed]
- Blair, E.M.; Dickson, K.L.; O’malley, M.A. Microbial communities and their enzymes facilitate degradation of recalcitrant polymers in anaerobic digestion. Curr. Opin. Microbiol. 2021, 64, 100–108. [Google Scholar] [CrossRef]
- Liu, W.; Sun, C.; Li, W.; Li, T.; Chen, Z.; Wang, J.; Ren, Z.; Wen, X. Sludge composition and characteristics shaped microbial community and further determined process performance: A study on full-scale thermal hydrolysis-anaerobic digestion processes. J. Environ. Sci. 2024, 137, 96–107. [Google Scholar] [CrossRef]
- Duan, N.; Kougias, P.G.; Campanaro, S.; Treu, L.; Angelidaki, I. Evolution of the microbial community structure in biogas reactors inoculated with seeds from different origin. Sci. Total Environ. 2021, 773, 144981. [Google Scholar] [CrossRef]
| Substrate ID | Bran Content (%) | Saccharomyces cerevisiae |
|---|---|---|
| B15Y | 15 | Yes |
| B40 | 40 | No |
| B40Y | 40 | Yes |
| VS (Per Unit Mass) | VS/TS | Mass, Material | Mass, VS | COD | Volume of Test Medium | Working Volume | |
|---|---|---|---|---|---|---|---|
| Substrate ID | (gVS, S/kgFM, S) | (%) | (gFM, S/bott) | (gVS, S/bott) | mgO2/bott | (mL) | (mL) |
| B15Y | 940.77 | 99.71 | 1.0025 | 0.94 | 1065.62 | 200 | 217 |
| B40 | 927.09 | 98.92 | 1.0049 | 0.93 | 1076.95 | 200 | 217 |
| B40Y | 932.36 | 99.62 | 1.0019 | 0.93 | 1110.41 | 200 | 217 |
| Cellulose | 960.71 | 99.99 | 1.0027 | 0.96 | 1188.20 | 200 | 217 |
| Inoculum | |||||||
| Compost | 182.27 | 38.15 | 20.00 | 3.65 | - | - | - |
| VS (Per Unit Mass) | VS/TS | Mass, Material | Mass, VS | COD | Volume of Dilution Tap Water | Total Volume of Nutrients | Working Volume | |
|---|---|---|---|---|---|---|---|---|
| Substrate ID | (gVS, S/kgFM, S) | (%) | (gFM, S/bott) | (gVS, S/bott) | mgO2/bott | (mL) | (mL) | (mL) |
| B15Y | 940.77 | 99.71 | 2.42 | 2.28 | 2572.36 | 74.80 | 52.8 | 480.00 |
| B40 | 927.09 | 98.92 | 2.45 | 2.27 | 2625.67 | 74.80 | 52.8 | 480.00 |
| B40Y | 932.36 | 99.62 | 2.44 | 2.27 | 2704.25 | 74.80 | 52.8 | 480.00 |
| Cellulose | 960.71 | 99.99 | 2.39 | 2.30 | 2832.15 | 74.80 | 52.8 | 480.00 |
| Inoculum | ||||||||
| Sludge | 13.77 | 57.35 | 350.00 | 4.82 | - | - | - | - |
| Substrate ID | Initial COD (mgO2/Bott) | COD from Test (mgO2/Bott) | COD Corrected (mgO2/Bott) | Initial COD (mgO2/L) | COD from Test (mgO2/L) | Coefficient of Variation CV(%) | COD Corrected (mgO2/L) | Biodegradation Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| B15Y | 1065.62 | 26.56 | 23.91 | 4910.69 | 122.40 | 7.11 | 110.18 | 2.24 |
| B40 | 1076.95 | 48.64 | 43.78 | 4962.90 | 224.15 | 5.66 | 201.75 | 4.07 |
| B40Y | 1110.41 | 78.26 | 70.43 | 5117.10 | 360.65 | 3.83 | 324.56 | 6.34 |
| Cellulose | 1188.20 | 9.33 | 8.40 | 5475.58 | 43.00 | 17.25 | 38.71 | 0.71 |
| Substrate ID | Initial COD (mgO2/Bott) | NET CH4 Produced (mL/Bott) | CH4 Final (mL/Bott) | Theoretical CH4 (mL/Bott) | Initial COD (mgO2/L | NET CH4 Produced (mL/L) | Coefficient of Variation CV(%) | CH4 Final (mL/L) | Theoretical CH4 (mL/L) | Biodegradation Efficiency (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| B15Y | 2572 | 883.6 | 795.24 | 823.04 | 5358.33 | 1840.83 | 2.34 | 1656.75 | 1714.67 | 96.62 |
| B40 | 2626 | 826.30 | 743.67 | 840.32 | 5470.83 | 1721.46 | 1.41 | 1549.31 | 1750.67 | 88.50 |
| B40Y | 2704 | 847.40 | 762.66 | 865.28 | 5633.33 | 1765.42 | 1.7 | 1588.88 | 1802.67 | 88.14 |
| Cellulose | 2832 | 828.90 | 746.01 | 906.24 | 5900.00 | 1726.88 | 16.7 | 1554.19 | 1888.00 | 82.32 |
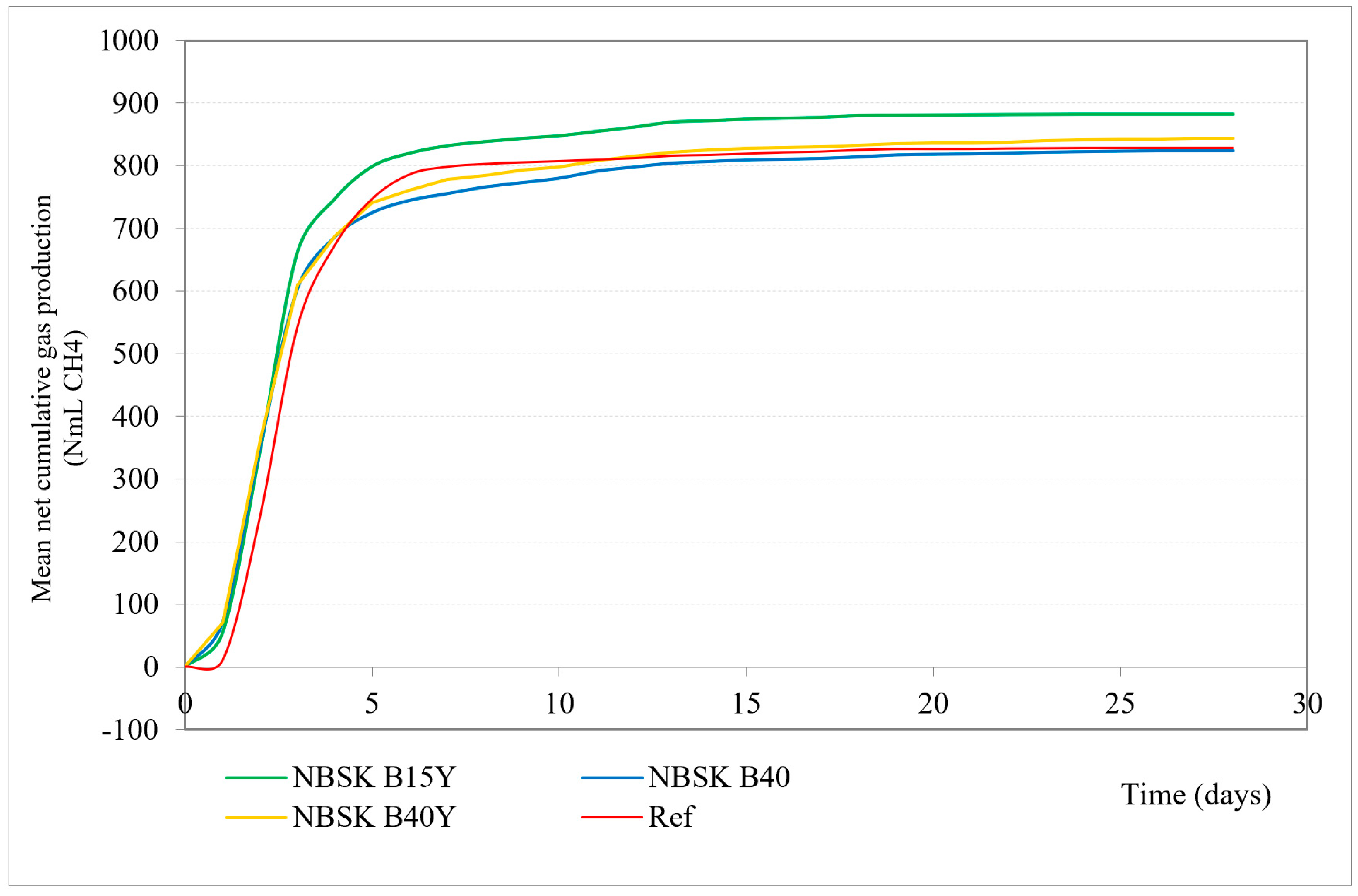
| Day | B40 (NmLCH4/Bott) | B40Y (NmLCH4/Bott) | B15Y (NmLCH4/Bott) | Cellulose, (NmLCH4/Bott) |
|---|---|---|---|---|
| 1 | 69.68 | 70.63 | 52.95 | 10.22 |
| 2 | 347.00 | 361.25 | 340.92 | 239.50 |
| 3 | 601.88 | 609.45 | 660.87 | 542.92 |
| 4 | 686.07 | 687.85 | 747.11 | 673.55 |
| 5 | 724.89 | 741.37 | 799.03 | 747.39 |
| 6 | 744.59 | 761.42 | 820.39 | 786.53 |
| 7 | 755.25 | 778.25 | 832.29 | 798.51 |
| 8 | 765.84 | 784.55 | 838.88 | 803.03 |
| 9 | 772.87 | 793.32 | 844.13 | 805.57 |
| 10 | 780.03 | 797.97 | 848.36 | 807.62 |
| 11 | 791.29 | 807.95 | 855.32 | 810.12 |
| 12 | 797.85 | 815.66 | 862.18 | 812.37 |
| 13 | 804.10 | 821.49 | 870.09 | 816.28 |
| 14 | 806.68 | 825.04 | 872.25 | 817.50 |
| 15 | 809.21 | 827.71 | 874.99 | 819.52 |
| 16 | 810.40 | 829.31 | 876.45 | 821.64 |
| 17 | 811.65 | 830.56 | 877.78 | 822.96 |
| 18 | 814.20 | 832.85 | 880.50 | 825.70 |
| 19 | 817.28 | 835.21 | 880.92 | 827.15 |
| 20 | 818.28 | 836.08 | 881.32 | 827.15 |
| 21 | 818.77 | 836.91 | 881.64 | 827.17 |
| 22 | 819.63 | 838.04 | 882.00 | 827.54 |
| 23 | 820.93 | 839.27 | 882.33 | 827.83 |
| 24 | 821.98 | 840.53 | 882.69 | 828.13 |
| 25 | 822.84 | 841.48 | 882.93 | 828.23 |
| 26 | 823.73 | 842.44 | 883.16 | 828.34 |
| 27 | 824.72 | 843.42 | 883.40 | 828.44 |
| 28 | 825.64 | 844.85 | 883.58 | 828.54 |
| 29 | 826.27 | 846.28 | 883.58 | 828.65 |
| 30 | 826.27 | 847.40 | 883.58 | 828.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markevičiūtė, Z.; Guerreschi, A.; Menin, G.; Malpei, F.; Varžinskas, V. Wheat Bran and Saccharomyces Cerevisiae Biomass’ Effect on Aerobic and Anaerobic Degradation Efficiency of Paper Composite. Microorganisms 2024, 12, 2018. https://doi.org/10.3390/microorganisms12102018
Markevičiūtė Z, Guerreschi A, Menin G, Malpei F, Varžinskas V. Wheat Bran and Saccharomyces Cerevisiae Biomass’ Effect on Aerobic and Anaerobic Degradation Efficiency of Paper Composite. Microorganisms. 2024; 12(10):2018. https://doi.org/10.3390/microorganisms12102018
Chicago/Turabian StyleMarkevičiūtė, Zita, Arianna Guerreschi, Glauco Menin, Francesca Malpei, and Visvaldas Varžinskas. 2024. "Wheat Bran and Saccharomyces Cerevisiae Biomass’ Effect on Aerobic and Anaerobic Degradation Efficiency of Paper Composite" Microorganisms 12, no. 10: 2018. https://doi.org/10.3390/microorganisms12102018
APA StyleMarkevičiūtė, Z., Guerreschi, A., Menin, G., Malpei, F., & Varžinskas, V. (2024). Wheat Bran and Saccharomyces Cerevisiae Biomass’ Effect on Aerobic and Anaerobic Degradation Efficiency of Paper Composite. Microorganisms, 12(10), 2018. https://doi.org/10.3390/microorganisms12102018






