Deep Turbulence as a Novel Main Driver for Multi-Specific Toxic Algal Blooms: The Case of an Anoxic and Heavy Metal-Polluted Submarine Canyon That Harbors Toxic Dinoflagellate Resting Cysts
Abstract
1. Introduction
2. Materials and Methods
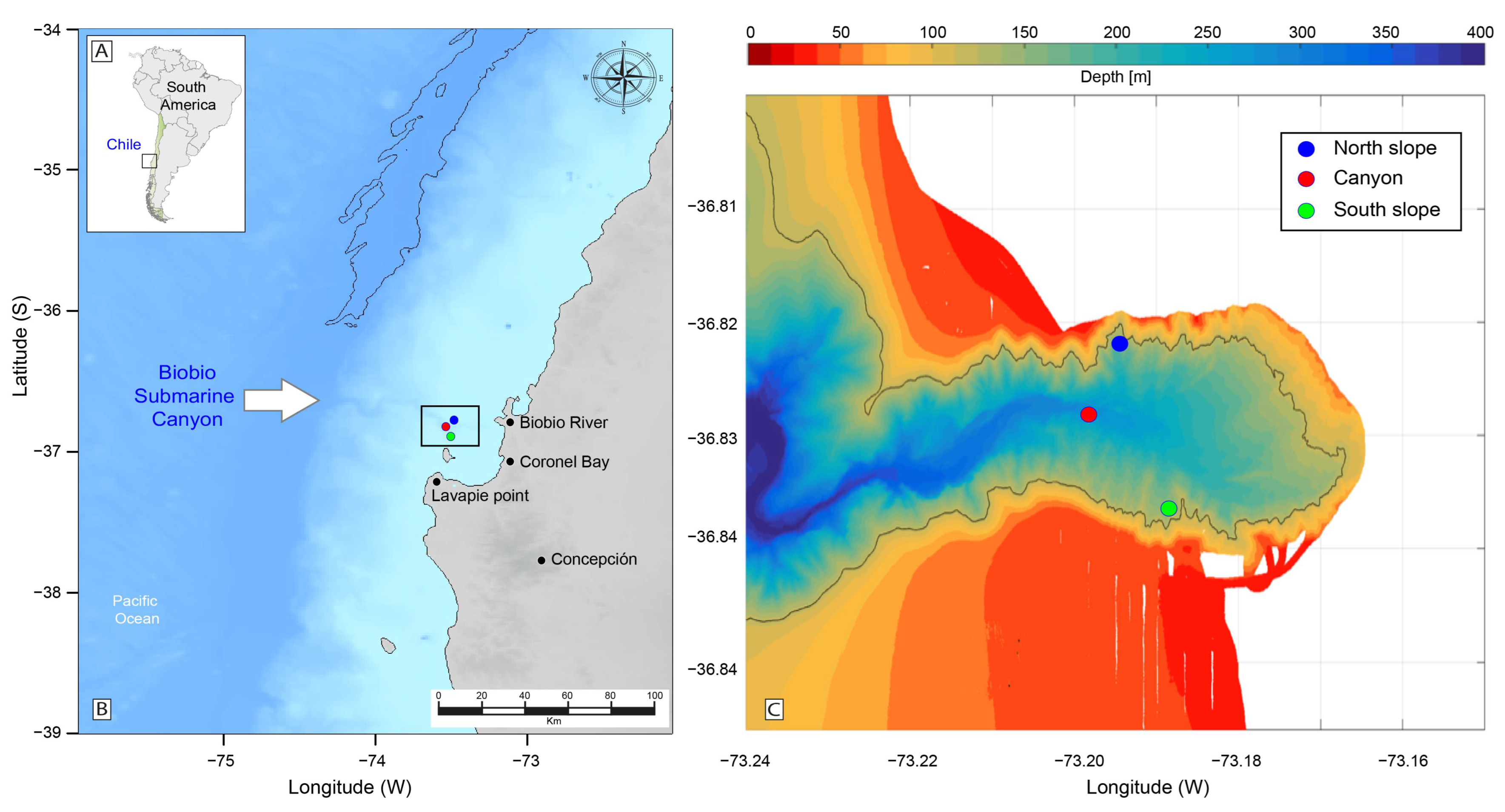
2.1. Survey Area
2.2. Water Sampling
2.2.1. Hydrographic and Turbulence Data
2.2.2. Phytoplankton Characterization
2.3. Sediment Sampling
Sediment Grain Size, Total Organic Content, Pigments, and Heavy Metals
2.4. Sediment Processing, Dinoflagellate Resting Cyst Counts, and Excystment Tests
2.5. Data Analysis
3. Results
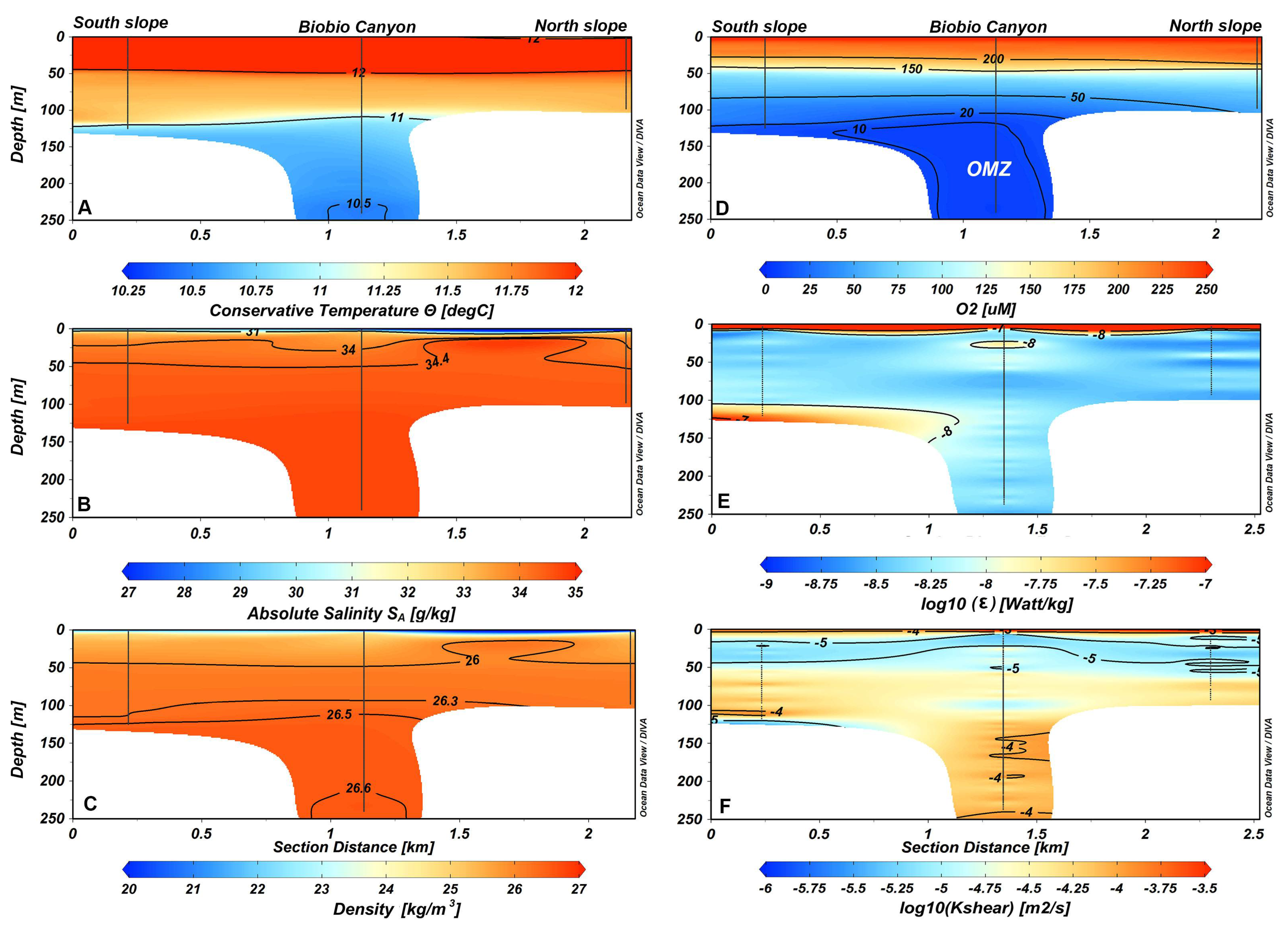
3.1. Hydrography and Turbulence
3.2. Phytoplankton Density
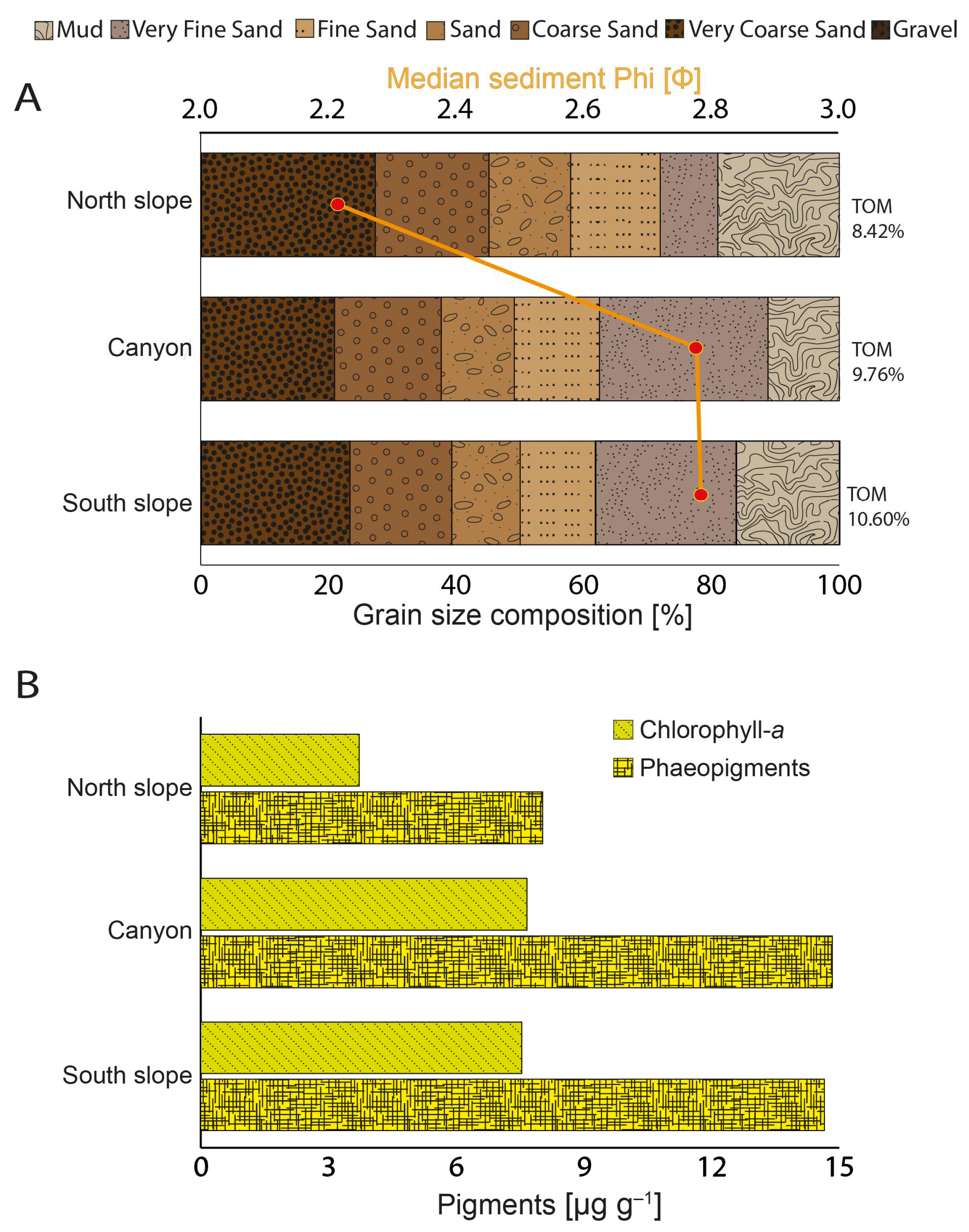
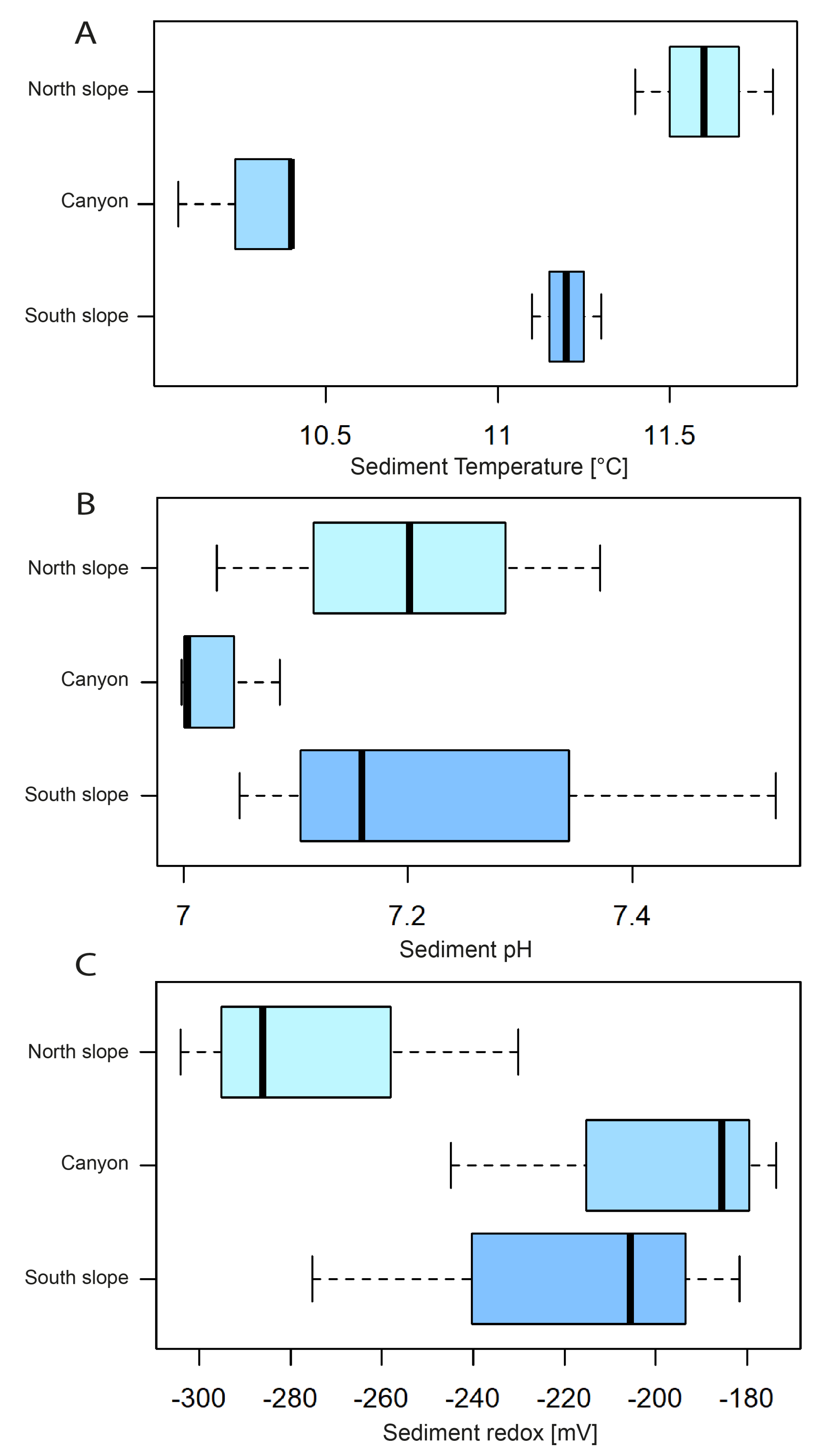
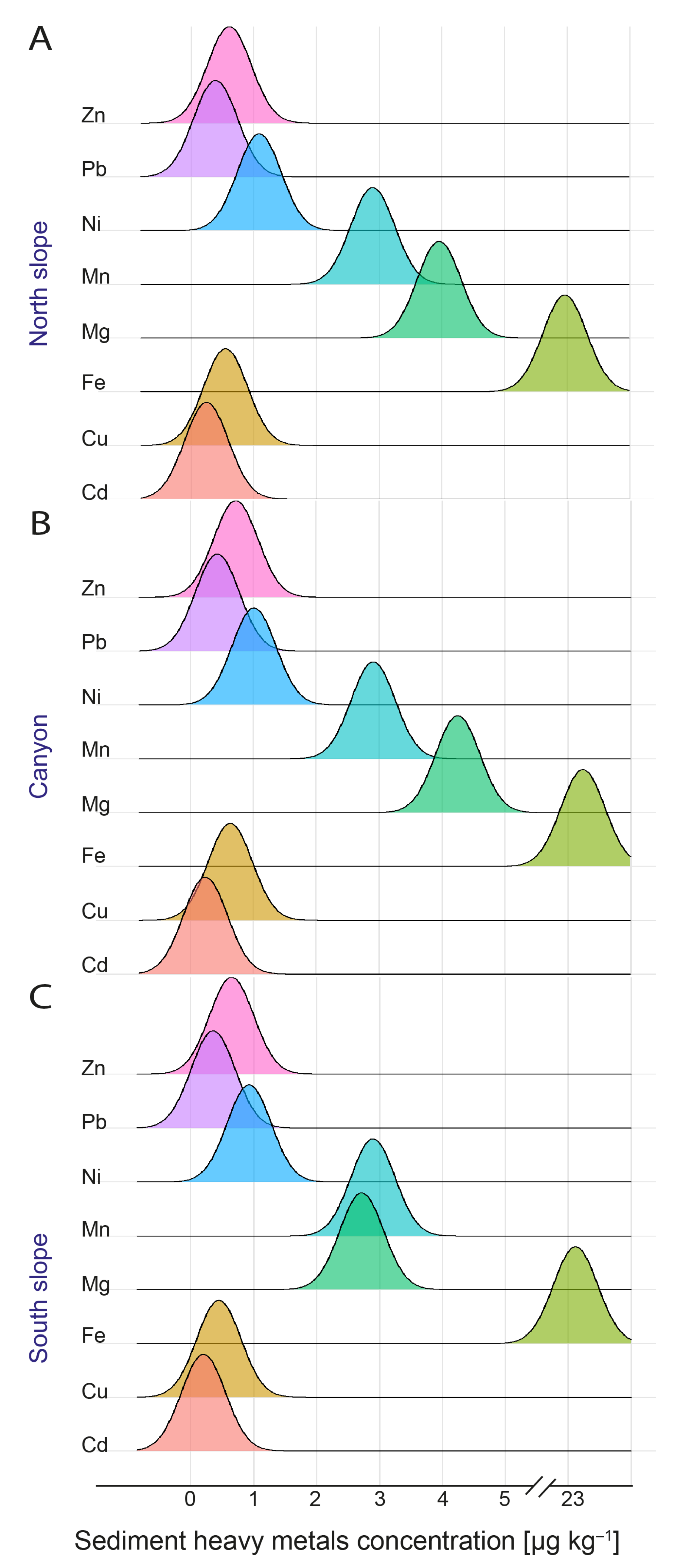
3.3. Sediment Physical–Chemical Parameters, Phaeopigments, and Heavy Metals
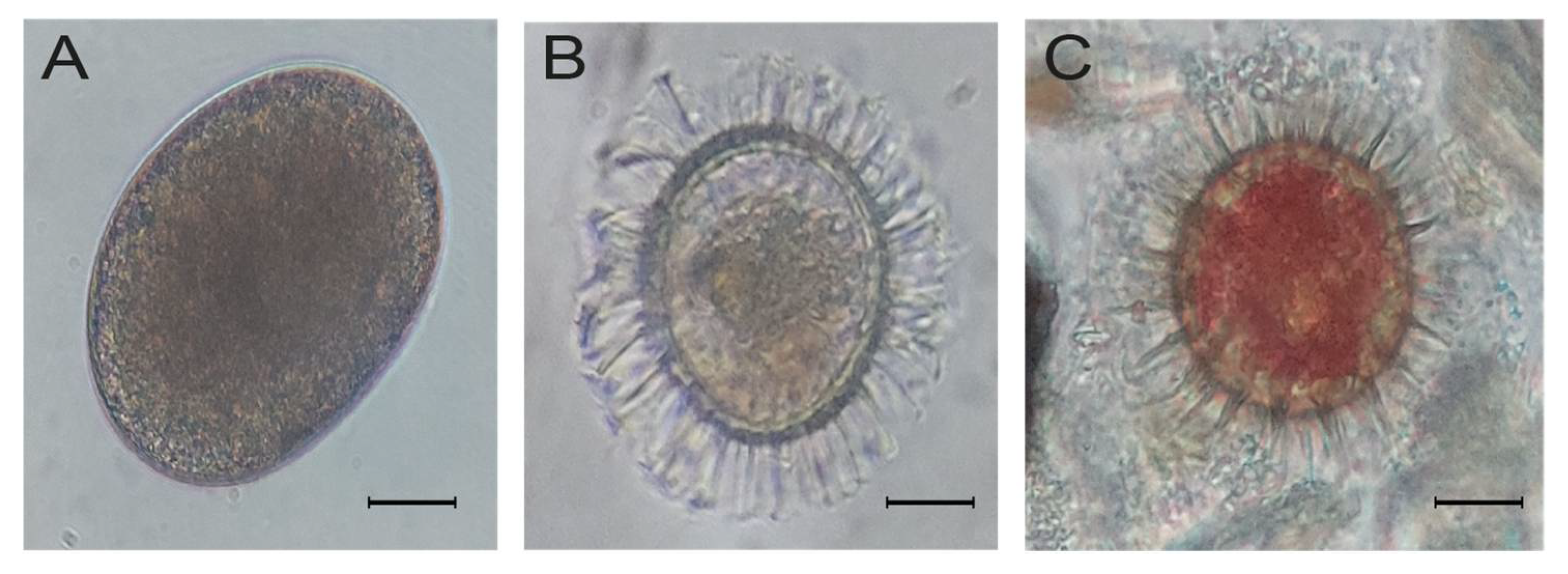
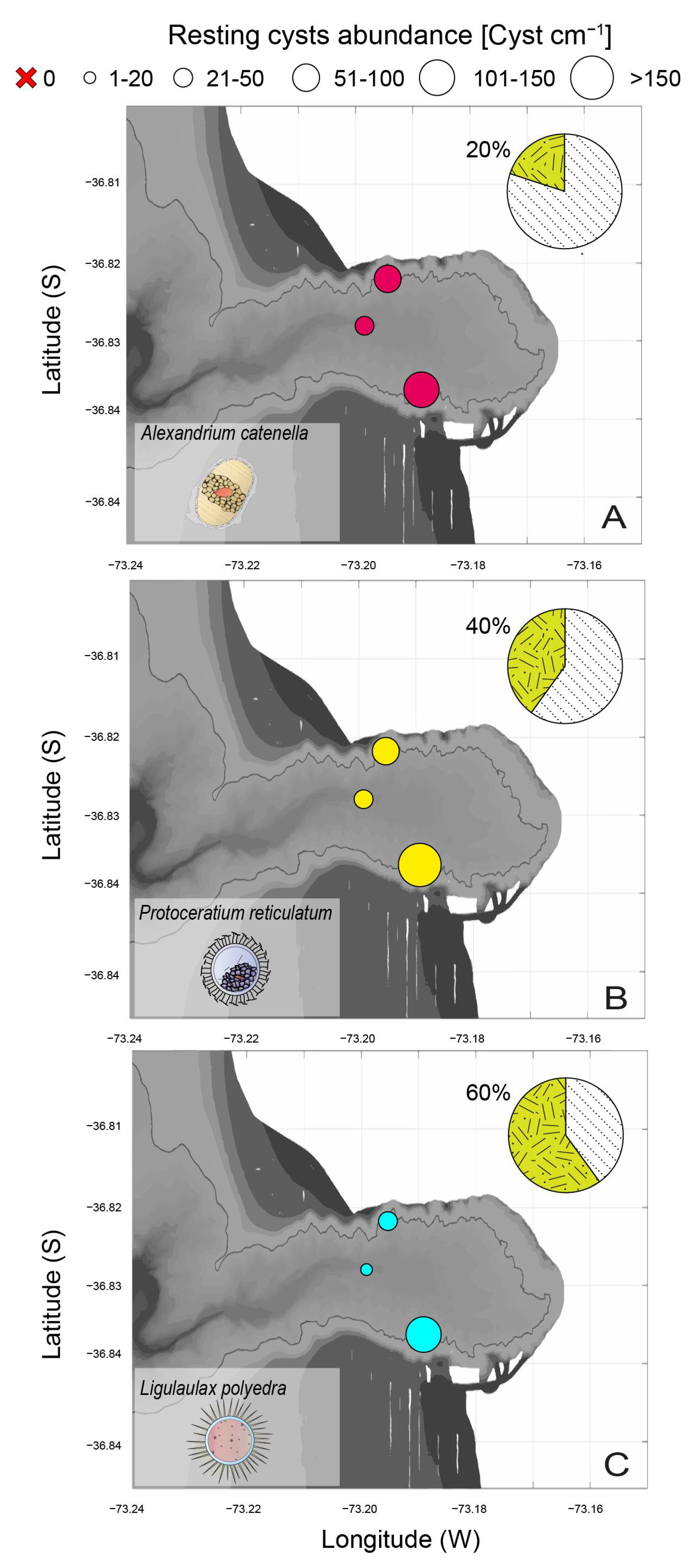
3.4. Toxigenic Dinoflagellate Resting Cysts Abundance
4. Discussion
4.1. Origin of Alexandrium catenella Resting Cysts: A Successful Colonizer?
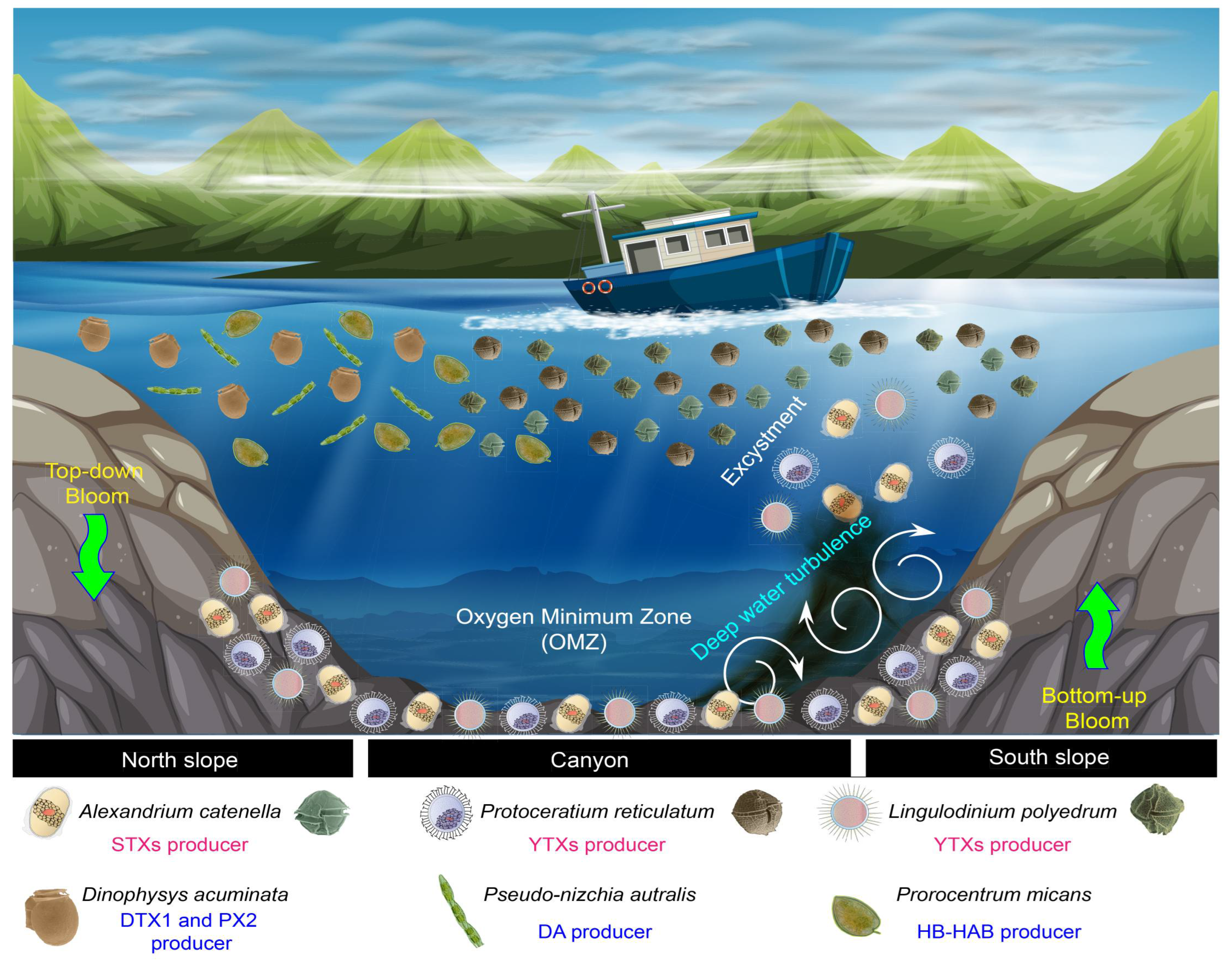
4.2. Bottom-up and Top-down Regulation of Multi-Specific HABs: The Influence of Deep Turbulence on Resting Cyst Resuspension and Excystment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britton, E. Ocean and Human Health: Can We Be Well in a Sick Sea? In The Ocean and Us; Springer: Boston, MA, USA, 2023; pp. 297–305. [Google Scholar]
- Morales, E.; Wincker, P.; Araya, M.H. Costas de Chile: Medio Natural, Cambio Climático, Ingeniería Oceánica, Gestión Costera; Servicio Hidrográfico y Oceanográfico de la Armada de Chile: Valparaíso, Chile, 2019. [Google Scholar]
- Reimann, L.; Vafeidis, A.T.; Honsel, L.E. Population development as a driver of coastal risk: Current trends and future pathways. Camb. Prism. Coast. Futures 2023, 1, e14. [Google Scholar] [CrossRef]
- Creel, L. Ripple Effects: Population and Coastal Regions; Population Reference Bureau: Washington, DC, USA, 2003. [Google Scholar]
- Castilla, J.C.; Neill, P.E. Marine bioinvasions in the southeastern Pacific: Status, ecology, economic impacts, conservation and management. In Biological Invasions in Marine Ecosystems; Springer: Boston, MA, USA, 2009; pp. 439–457. [Google Scholar]
- Baldrich, Á.M.; Díaz, P.A.; Rosales, S.A.; Rodríguez-Villegas, C.; Álvarez, G.; Pérez-Santos, I.; Díaz, M.; Schwerter, C.; Araya, M.; Reguera, B. An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia. Toxins 2024, 16, 77. [Google Scholar]
- Rodríguez-Villegas, C.; Díaz, P.A.; Pizarro, G.; Salgado, P.; Pérez-Santos, I.; Díaz, M.; Seguel, M.; Baldrich, Á.; Bravo, I.; Iriarte, L.; et al. Alexandrium catenella cyst accumulation by passive and active dispersal agents: Implications for the potential spreading risk in Chilean Patagonian fjords. Harmful Algae 2020, 96, 101832. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Lomolino, M.V. Biogeography, 2nd ed.; Sinuer Associates Publishers: Sunderland, MA, USA, 1998. [Google Scholar]
- Hanski, I.; Gilpin, M. Metapopulation dynamics: Brief history and conceptual domain. Biol. J. Linn. Soc. 1991, 42, 3–16. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, R. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2014, 2, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.I.; Dapena, C.; Bravo, I.; Cuadrado, A. The hidden sexuality of Alexandrium minutum: An example of overlooked sex in dinoflagellates. PLoS ONE 2015, 10, e0142667. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Bravo, I.; Garcés, E. Effects of nutritional factors and different parental crosses on the encystment and excystment of Alexandrium catenella (Dinophyceae) in culture. Phycologia 2005, 44, 658–670. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Díaz, P.A.; Riobó, P.; Rossignol, A.E.; Rodríguez, F.; Loures, P.; Baldrich, Á.M.; Varela, D.; Sandoval-Sanhueza, A.; Figueroa, R. Latitudinal Variation in the Toxicity and Sexual Compatibility of Alexandrium catenella Strains from Southern Chile. Toxins 2021, 13, 900. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Estrada, M.; Garcés, E. Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 2018, 73, 44–57. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Figueroa, R.; Baldrich, Á.; Pérez-Santos, I.; Díaz, M.; Tomasetti, S.; Seguel, M.; Álvarez, G.; Salgado, P.; Díaz, P. Small and patchy is enough: An example about how toxic HAB events can spread through low resting cyst loads. Harmful Algae 2023, 129, 102495. [Google Scholar] [CrossRef]
- Díaz, P.A.; Figueroa, R.I. Toxic Algal Bloom Recurrence in the Era of Global Change: Lessons from the Chilean Patagonian Fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Mella, J.; Mardones, J.I.; Norambuena, L.; Fuenzalida, G.; Labra, G.; Espinoza-González, O.; Guzmán, L. Toxic Alexandrium catenella expanding northward along the Chilean coast: New risk of paralytic shellfish poisoning off the Bío-Bío region (36° S). Mar. Pollut. Bull. 2021, 172, 112783. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Díaz, P.A.; Molinet, C.; Seguel, M. Exceptional climate anomalies and north—wards expansion of Paralytic Shellfish Poisoning outbreaks in Southern Chile. Harmful Algae News 2016, 54, 1–2. [Google Scholar]
- Mardones, J.I.; Bolch, C.; Guzmán, L.; Paredes, J.; Varela, D.; Hallegraeff, G.M. Role of resting cysts in Chilean Alexandrium catenella dinoflagellate blooms revisited. Harmful Algae 2016, 55, 238–249. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Lee, M.R.; Salgado, P.; Figueroa, R.I.; Baldrich, Á.M.; Pérez-Santos, I.; Tomasetti, S.J.; Niklitschek, E.; Díaz, M.; Álvarez, G.; et al. Drivers of dinoflagellate benthic cyst assemblages in the NW Patagonian fjords system and its adjacent oceanic shelf, with a focus on harmful species. Sci. Total Environ. 2021, 785, 147378. [Google Scholar] [CrossRef]
- Persson, A. Possible predation of cysts—A gap in the knowledge of dinoflagellate ecology? J. Plankton Res. 2000, 22, 803–809. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Villegas, C.; Figueroa, R.I.; Pérez-Santos, I.; Molinet, C.; Saldías, G.S.; Rosales, S.A.; Álvarez, G.; Linford, P.; Díaz, P.A. Continental shelf off northern Chilean Patagonia: A potential risk zone for the onset of Alexandrium catenella toxic bloom? Mar. Pollut. Bull. 2022, 184, 114103. [Google Scholar] [CrossRef]
- Prego, R.; Bao, R.; Varela, M.; Carballeira, R. Naturally and Anthropogenically Induced Lingulaulax polyedra Dinoflagellate Red Tides in the Galician Rias (NW Iberian Peninsula). Toxins 2024, 16, 280. [Google Scholar] [CrossRef]
- Murray, S.; Hallegraeff, G. Harmful Algae Introductions: Vectors of Transfer, Mitigation, and Management. In Harmful Algal Blooms: A Compendium Desk Reference; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 493–506. [Google Scholar]
- Connolly, T.P.; Hickey, B.M. Regional impact of submarine canyons during seasonal upwelling. J. Geophys. Res. Ocean. 2014, 119, 953–975. [Google Scholar] [CrossRef]
- Saldías, G.S.; Allen, S.E. The Influence of a Submarine Canyon on the Circulation and Cross-Shore Exchanges around an Upwelling Front. J. Phys. Oceanogr. 2020, 50, 1677–1698. [Google Scholar] [CrossRef]
- Cubillos, L.A.; Serra, R.; Fréon, P. Synchronous pattern of fluctuation in three anchovy fisheries in the Humboldt Current System. Aquat. Living Resour. 2007, 20, 69–75. [Google Scholar] [CrossRef]
- Marinovic, B.; Croll, D.; Gong, N.; Benson, S.; Chavez, F. Effects of the 1997–1999 El Niño and La Niña events on zooplankton abundance and euphausiid community composition within the Monterey Bay coastal upwelling system. Prog. Oceanogr. 2002, 54, 265–277. [Google Scholar] [CrossRef]
- Sahu, S.; Allen, S.E.; Saldías, G.S.; Klymak, J.M.; Zhai, L. Spatial and Temporal Origins of the La Perouse Low Oxygen Pool: A Combined Lagrangian Statistical Approach. J. Geophys. Res. Ocean. 2022, 127, e2021JC018135. [Google Scholar] [CrossRef]
- Rodríguez–Villegas, C.; Díaz, P.A.; Salgado, P.; Tomasetti, S.J.; Díaz, M.; Marín, S.L.; Baldrich, Á.M.; Niklitschek, E.; Pino, L.; Matamala, T.; et al. The role of physico-chemical interactions in the seasonality of toxic dinoflagellate cyst assemblages: The case of the NW Patagonian fjords system. Environ. Pollut. 2022, 311, 119901. [Google Scholar] [CrossRef]
- Persson, A.; Smith, B.C. Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments. Phycology 2022, 2, 384–418. [Google Scholar] [CrossRef]
- George-Nascimento, M.; Khan, R.; Garcias, F.; Lobos, V.; Muñoz, G.; Valdebenito, V. Impaired health in flounder, Paralichthys spp. Inhabiting Coastal Chile. Bull. Environ. Contam. Toxicol. 2000, 64, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Triki, H.Z.; Laabir, M.; Lafabrie, C.; Malouche, D.; Bancon-Montigny, C.; Gonzalez, C.; Deidun, A.; Pringault, O.; Daly-Yahia, O.K. Do the levels of industrial pollutants influence the distribution and abundance of dinoflagellate cysts in the recently-deposited sediment of a Mediterranean coastal ecosystem? Sci. Total Environ. 2017, 595, 380–392. [Google Scholar] [CrossRef]
- Allen, S.; Durrieu de Madron, X. A review of the role of submarine canyons in deep-ocean exchange with the shelf. Ocean Sci. 2009, 5, 607–620. [Google Scholar] [CrossRef]
- Rodrigo, C.; Díaz-Naveas, J.; Frutos, J. Cañones submarinos en el margen continental chileno. In Geología Marina de Chile; Pontificia Universidad Católica de Valparaíso-Servicio Nacional de Geología y Minería de Chile-Servicio Hidrográfico de la Armada-Comité Oceanográfico Nacional: Valparaíso, Chile, 2010. [Google Scholar]
- Sobarzo, M.; Saldías, G.S.; Tapia, F.J.; Bravo, L.; Moffat, C.; Largier, J.L. On subsurface cooling associated with the Biobio River Canyon (Chile). J. Geophys. Res. Ocean. 2016, 121, 4568–4584. [Google Scholar] [CrossRef]
- Thornburg, T.M.; Kulm, L.D.; Hussong, D.M. Submarine-fan development in the southern Chile Trench: A dynamic interplay of tectonics and sedimentation. Geol. Soc. Am. Bull. 1990, 102, 1658–1680. [Google Scholar] [CrossRef]
- Vergara, O.A.; Figueroa, P.A.; Salas, C.; Vásquez, S.I.; Muñoz, R.; Saldías, G.S. The influence of the Biobio Canyon on the circulation and coastal upwelling/downwelling off central Chile. Cont. Shelf Res. 2024, 1, 105335. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Paulmier, A.; Ruiz-Pino, D. Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 2009, 80, 113–128. [Google Scholar] [CrossRef]
- Fuenzalida, R.; Schneider, W.; Garcés-Vargas, J.; Bravo, L.; Lange, C. Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 992–1003. [Google Scholar] [CrossRef]
- Tomczak, M.; Godfrey, J.S. Regional Oceanography: An Introduction; Daya Books: Delhi, India, 2003. [Google Scholar]
- Stewart, R.H. Introduction to Physical Oceanography; Robert H. Stewart: Louisville, KY, USA, 2008. [Google Scholar]
- Simpkins, G. Humboldt upwelling. Nat. Clim. Change 2018, 8, 272. [Google Scholar] [CrossRef]
- Oyarzún, D.; Brierley, C.M. The future of coastal upwelling in the Humboldt current from model projections. Clim. Dyn. 2019, 52, 599–615. [Google Scholar] [CrossRef]
- Busecke, J.J.; Resplandy, L.; Ditkovsky, S.J.; John, J.G. Diverging fates of the Pacific Ocean oxygen minimum zone and its core in a warming world. AGU Adv. 2022, 3, e2021AV000470. [Google Scholar] [CrossRef]
- Linford, P.; Pérez-Santos, I.; Montes, I.; Dewitte, B.; Buchan, S.; Narváez, D.; Saldías, G.; Pinilla, E.; Garreaud, R.; Díaz, P.; et al. Recent Deoxygenation of Patagonian Fjord Subsurface Waters Connected to the Peru–Chile Undercurrent and Equatorial Subsurface Water Variability. Glob. Biogeochem. 2023, 37, e2022GB007688. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View. 2019. Available online: https://odv.awi.de (accessed on 23 August 2023).
- Lueck, R. Calculating the Rate of Dissipation of Turbulent Kinetic Energy; Tech. Note TN-028; Rockland Scientific International: Victoria, BC, Canada, 2013; p. 18. [Google Scholar]
- Cuypers, Y.; Bouruet-Aubertot, P.; Marec, C.; Fuda, J.-L. Characterization of turbulence and validation of fine-scale parametrization in the Mediterranean Sea during BOUM experiment. Biogeosci. Disc. 2011, 8, 8961–8998. [Google Scholar]
- Osborn, T.R. Estimates of the local rate of vertical diffusion from dissipation measurements. J. Phys. Oceanogr. 1980, 10, 83–89. [Google Scholar] [CrossRef]
- Shih, L.H.; Koseff, J.R.; Ivey, G.N.; Ferziger, J.H. Parameterization of turbulent fluxes and scales using homogeneous sheared stably stratified turbulence simulations. J. Fluid Mech. 2005, 525, 193–214. [Google Scholar] [CrossRef]
- Thorpe, S.A. The Turbulent Ocean; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. ICES J. Mar. Sci. 1960, 25, 279–284. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Für Theor. Und Angew. Limnol. Mitteilungen 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Mardones, J.I.; Clément, A. Manual de Microalgas del Sur de Chile; Plancton Andino SpA: Los Lagos, Chile, 2019. [Google Scholar]
- Tomas, C.R. Identifying Marine Phytoplankton; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Anderson, D.M.; Taylor, C.D.; Armbrust, E.V. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 1987, 32, 340–351. [Google Scholar] [CrossRef]
- De Camargo, M.G. SysGran: Um sistema de código aberto para análises granulométricas do sedimento. Rev. Bras. Geociências 2016, 36, 371–378. [Google Scholar] [CrossRef]
- Donoghue, J.F. Phi Scale. In Encyclopedia of Estuaries; Kennish, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 483–484. [Google Scholar]
- Brito, A.; Newton, A.; Tett, P.; Fernandes, T. Development of an optimal methodology for the extraction of microphytobenthic chlorophyll. J. Int. Environ. Appl. Sci. 2009, 4, 42–54. [Google Scholar]
- EPA, U.S. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Office of Hazardous Waste Test Methods: Washington, DC, USA, 1996. [Google Scholar]
- Genovesi, B.; Mouillot, D.; Vaquer, A.; Laabir, M.; Pastoureaud, A. Towards an optimal sampling strategy for Alexandrium catenella (Dinophyceae) benthic resting cysts. Harmful Algae 2007, 6, 837–848. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y. Taxonomy of cysts. In Manual on Harmful Marine Microalgae; UNESCO: Paris, France, 2003; pp. 563–592. [Google Scholar]
- Anderson, D.; Fukuyo, Y.; Matsuoka, K. Cyst methodologies. In Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Enevoldsen, H.O., Eds.; UNESCO: Paris, France, 2004; Volume 11, pp. 165–190. [Google Scholar]
- Varela, D.; Paredes, J.; Alves-de-Souza, C.; Seguel, M.; Sfeir, A.; Frangópulos, M. Intraregional variation among Alexandrium catenella (Dinophyceae) strains from southern Chile: Morphological, toxicological and genetic diversity. Harmful Algae 2012, 15, 8–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Package ‘Vegan’—Community Ecology Package. 2019.
- Ter Braak, C.J. Canonical community ordination. Part I: Basic theory and linear methods. Ecoscience 1994, 1, 127–140. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Hart, C.; Tobin, E.; Lanphier, K.; Sullivan, K.; Eckert, G.L. Using cyst mapping to uncover trends of PSP toxicity in Southeast Alaska Geoduck harvest areas. In Proceedings of the 10.5 U.S. Symposium on Harmful Algae Emerging Voices and Blooming Careers, Virtual, 25–27 May 2021; Abstract Book, 2021; p. 45. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic Toxins in Chile: History, Producers and Impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Anil, A.; Venkat, K.; Sawant, S.; Dileepkumar, M.; Dhargalkar, V.; Ramaiah, N.; Harkantra, S.; Ansari, Z. Marine bioinvasion: Concern for ecology and shipping. Curr. Sci. 2002, 83, 214–218. [Google Scholar]
- Hallegraeff, G.M.; Bolch, C.J. Transport of toxic dinoflagellate cysts via ships’ ballast water. Mar. Pollut. Bull. 1991, 22, 27–30. [Google Scholar] [CrossRef]
- Blackburn, S.I.; Bolch, C.J.; Haskard, K.A.; Hallegraeff, G.M. Reproductive compatibility among four global populations of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). Phycologia 2001, 40, 78–87. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Varela, D.; Correa, K.; Vera, C.; Olivares, B.; Mayorga, J.; Baldrich, Á. When sex may hold the key: The sexual drivers behind the hidden reproductive niche of the worldwide distributed and bloom-forming microalgae Alexandrium catenella. Centre for Biotechnology and Bioengineering; Hotel Sheraton: Santiago, Chile, 2023. [Google Scholar]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- McGillicuddy, D., Jr.; Townsend, D.W.; He, R.; Keafer, B.A.; Kleindinst, J.L.; Li, Y.; Manning, J.P.; Mountain, D.G.; Thomas, M.A.; Anderson, D.M. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnol. Oceanogr. 2011, 56, 2411–2426. [Google Scholar] [CrossRef]
- Anderson, D.M.; Keafer, B.A.; Kleindinst, J.L.; McGillicuddy, D.J., Jr.; Martin, J.L.; Norton, K.; Pilskaln, C.H.; Smith, J.L.; Sherwood, C.R.; Butman, B. Alexandrium fundyense cysts in the Gulf of Maine: Long-term time series of abundance and distribution, and linkages to past and future blooms. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 103, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Fachon, E.; Pickart, R.S.; Lin, P.; Fischer, A.D.; Richlen, M.L.; Uva, V.; Brosnahan, M.L.; McRaven, L.; Bahr, F.; et al. Evidence for massive and recurrent toxic blooms of Alexandrium catenella in the Alaskan Arctic. Proc. Natl. Acad. Sci. USA 2021, 118, e2107387118. [Google Scholar] [CrossRef]
- Díaz, P.; Molinet, C.; Seguel, M.; Dıaz, M.; Labra, G.; Figueroa, R.I. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamic and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Salgado, P.; Figueroa, R.I.; Ramilo, I.; Bravo, I. The life history of the toxic marine dinoflagellate Protoceratium reticulatum (Gonyaulacales) in culture. Harmful Algae 2017, 68, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.I.; Bravo, I. Sexual reproduction and two different encystment strategies of Lingulaulax polyedrum (Dinophyceae) in culture. J. Phycol. 2005, 41, 370–379. [Google Scholar] [CrossRef]
- Saldías, G.S.; Ramos-Musalem, K.; Allen, S.E. Circulation and Upwelling Induced by Coastal Trapped Waves Over a Submarine Canyon in an Idealized Eastern Boundary Margin. Geophys. Res. Lett. 2021, 48, e2021GL093548. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Seguel, M.; Marín, A.; Krock, B. First detection of pectenotoxin-2 in shellfish associated with an intense spring bloom of Dinophysis acuminata on the central Chilean coast. Mar. Pollut. Bull. 2020, 158, 111414. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Schwerter, C.; Baldrich, Á.M.; Pérez-Santos, I.; Díaz, M.; Araya, M.; Nieves, M.G.; Rosales, S.A.; Mancilla-Gutiérrez, G. Synchronic distribution of the dinoflagellate Protoceratium reticulatum and yessotoxins in a high stratified fjord system: Tidal or light modulation? Harmful Algae 2024, 135, 102649. [Google Scholar] [CrossRef]
- Díaz, P.A.; Araya, M.; Cantarero, B.; Miranda, C.; Varela, D.; Figueroa, R.I.; Basti, L.; Carbonell, P.; Aravena, Á.; Pérez-Santos, I. Are yessotoxins an emerging problem in Chile? Context and perspectives following the first report of YTX levels exceeding the regulatory limit in the Patagonian fjord system. Environ. Pollut. 2024, 361, 124844. [Google Scholar] [CrossRef] [PubMed]
- Nieves, M.G.; Alvarez, G.; López-Carvallo, J.A.; Millanao, P.; Araya, M.; Díaz, R.; Díaz, P.A. Effects of the Toxic Dinoflagellate Protoceratium reticulatum on Physiological Rates of Juvenile Scallops Argopecten purpuratus. Fishes 2024, 9, 331. [Google Scholar] [CrossRef]
- Nieves, M.G.; Díaz, P.A.; Araya, M.; Salgado, P.; Rojas, R.; Quiroga, E.; Pizarro, G.; Álvarez, G. Effects of the toxic dinoflagellate Protoceratium reticulatum and its yessotoxins on the survival and feed ingestion of Argopecten purpuratus veliger larvae. Mar. Pollut. Bull. 2024, 199, 116022. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Foord, C.J.; Macey, B.M.; Mansfield, L.; Mouton, A.; Smith, M.E.; Osmond, S.J.; van der Molen, L. Devastating farmed abalone mortalities attributed to yessotoxin-producing dinoflagellates. Harmful Algae 2019, 81, 30–41. [Google Scholar] [CrossRef]
- Sernapesca. Anuario Estadístico de Pesca; Servicio Nacional de Pesca: Valparaíso, Chile, 2022. [Google Scholar]

| North Slope | Canyon | South Slope | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth (m) | 0 | 5 | 10 | 20 | 30 | 50 | 0 | 5 | 10 | 20 | 30 | 50 | 0 | 5 | 10 | 20 | 30 | 50 | |
| Group | Species | ||||||||||||||||||
| Diatoms | Actinoptychus senarius | 0 | 0 | 600 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira granulata | 500 | 700 | 0 | 0 | 0 | 0 | 0 | 0 | 1300 | 0 | 0 | 0 | 800 | 0 | 0 | 0 | 0 | 0 | |
| Asterionella formosa | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asterionellopsis glacialis | 600 | 700 | 0 | 400 | 0 | 0 | 200 | 1000 | 2100 | 200 | 300 | 0 | 0 | 1800 | 1300 | 0 | 0 | 0 | |
| Cerataulina pelagica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1000 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | |
| Chaetoceros contortus | 0 | 0 | 300 | 0 | 0 | 0 | 0 | 4400 | 0 | 700 | 0 | 0 | 400 | 1500 | 0 | 0 | 0 | 0 | |
| Chaetoceros constrictus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1100 | 0 | 0 | 1400 | 0 | 0 | 600 | 0 | 0 | 0 | 0 | |
| Chaetoceros criophilus | 0 | 0 | 0 | 400 | 0 | 0 | 0 | 300 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | |
| Chaetoceros debilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2500 | 1800 | 0 | 0 | 0 | 0 | 2500 | 0 | 0 | 0 | 0 | |
| Chaetoceros diadema | 0 | 1000 | 0 | 0 | 0 | 0 | 0 | 2200 | 600 | 0 | 0 | 0 | 500 | 300 | 900 | 0 | 0 | 0 | |
| Chaetoceros didymus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 600 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Chaetoceros lorenzianus | 800 | 700 | 0 | 0 | 700 | 0 | 1400 | 4200 | 5600 | 0 | 600 | 0 | 500 | 800 | 0 | 300 | 0 | 0 | |
| Chaetoceros radicans | 0 | 0 | 1400 | 0 | 0 | 0 | 0 | 24,400 | 6200 | 0 | 0 | 0 | 0 | 2800 | 200 | 0 | 0 | 0 | |
| Chaetoceros socialis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2600 | 3100 | 0 | 0 | 0 | 0 | 300 | 0 | 0 | 0 | 0 | |
| Chaetoceros spp. | 0 | 500 | 0 | 400 | 0 | 0 | 0 | 5300 | 200 | 0 | 300 | 100 | 200 | 3300 | 100 | 0 | 0 | 200 | |
| Corethron hystrix | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Coscinodiscus spp. | 0 | 100 | 100 | 100 | 0 | 0 | 0 | 100 | 200 | 0 | 200 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | |
| Cylindrotheca closterium | 300 | 0 | 100 | 100 | 0 | 0 | 0 | 200 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Detonula pumila | 200 | 2100 | 0 | 500 | 0 | 0 | 800 | 18,700 | 4000 | 0 | 0 | 0 | 0 | 2800 | 600 | 0 | 0 | 0 | |
| Eucampia spp. | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 400 | 0 | 0 | 0 | 0 | |
| Fragilaria crotonensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 800 | 400 | 0 | 0 | 0 | 0 | 0 | |
| Guinardia striata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 500 | 0 | 200 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | |
| Leptocylindrus danicus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Leptocylindrus minimus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Odontella longicruris | 0 | 0 | 0 | 200 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 600 | 200 | 0 | 0 | 0 | |
| Pleurosigma directum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 200 | 0 | 0 | |
| Pseudo-nitzschia cf. australis | 0 | 3100 | 0 | 0 | 0 | 0 | 0 | 800 | 0 | 0 | 0 | 0 | 0 | 1300 | 0 | 200 | 0 | 0 | |
| Pseudo-nitzschia spp. | 100 | 0 | 0 | 200 | 100 | 0 | 0 | 100 | 200 | 400 | 300 | 0 | 600 | 100 | 300 | 100 | 0 | 0 | |
| Rhizosolenia setigera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rhizosolenia imbricata | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Skeletonema spp. | 0 | 4200 | 0 | 0 | 0 | 0 | 7900 | 5400 | 2200 | 300 | 0 | 0 | 3100 | 8600 | 3400 | 0 | 0 | 0 | |
| Stephanopyxis turris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | |
| Thalassionema nitzschioides | 1000 | 27,300 | 15,000 | 1900 | 2900 | 1100 | 1300 | 41,900 | 31,600 | 3600 | 3700 | 2300 | 4600 | 38,400 | 15,600 | 2700 | 800 | 300 | |
| Thalassiosira gravida | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 400 | 0 | 0 | 0 | 0 | 200 | 500 | 0 | 0 | 0 | 0 | |
| Thalassiosira subtilis | 0 | 1100 | 200 | 300 | 0 | 0 | 300 | 19,900 | 12,800 | 1600 | 2800 | 400 | 0 | 5500 | 3000 | 3800 | 800 | 800 | |
| Thalassiosira spp. | 300 | 1900 | 300 | 300 | 200 | 200 | 500 | 7100 | 2800 | 300 | 200 | 100 | 200 | 4300 | 1100 | 200 | 100 | 200 | |
| Pennadas | 900 | 500 | 700 | 600 | 200 | 200 | 600 | 800 | 2100 | 1800 | 1100 | 200 | 400 | 600 | 500 | 500 | 200 | 100 | |
| Dinoflagellates | Amphidinium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinophysis acuminata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 200 | 0 | 0 | 0 | 0 | |
| Gyrodinium spp. | 100 | 400 | 0 | 0 | 0 | 0 | 0 | 200 | 100 | 0 | 0 | 100 | 200 | 200 | 0 | 0 | 0 | 0 | |
| Prorocentrum micans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | |
| Protoperidinium spp. | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 100 | 200 | 0 | 100 | 0 | 0 | |
| Torodinium robustum | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tripos pentagonus | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Silicoflagellates | Dictyocha speculum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | Mesodinium rubrum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 300 | 100 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 0 |
| Vectors | RDA 1 | RDA 2 | R2 | Pr (>R) |
|---|---|---|---|---|
| Temperature | 0.320 | 0.947 | 0.940 | 0.0041 |
| pH | 0.734 | 0.6783 | 0.293 | 0.3601 |
| Redox | 0.154 | −0.988 | 0.440 | 0.1912 |
| TOC | 0.723 | −0.690 | 1.000 | 0.0044 |
| Phi | 0.321 | −0.946 | 1.000 | 0.0043 |
| Cu | −0.935 | −0.354 | 0.999 | 0.0042 |
| Zn | −0.265 | −0.964 | 0.994 | 0.0047 |
| Cd | −0.972 | 0.234 | 0.966 | 0.0061 |
| Mg | −0.997 | −0.075 | 1.000 | 0.0042 |
| Ni | −0.827 | 0.562 | 0.996 | 0.0048 |
| Mn | −0.562 | −0.826 | 0.504 | 0.152 |
| Pb | −0.895 | −0.444 | 0.995 | 0.0046 |
| Fe | −0.036 | −0.999 | 0.998 | 0.0040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Villegas, C.; Pérez-Santos, I.; Díaz, P.A.; Baldrich, Á.M.; Lee, M.R.; Saldías, G.S.; Mancilla-Gutiérrez, G.; Urrutia, C.; Navarro, C.R.; Varela, D.A.; et al. Deep Turbulence as a Novel Main Driver for Multi-Specific Toxic Algal Blooms: The Case of an Anoxic and Heavy Metal-Polluted Submarine Canyon That Harbors Toxic Dinoflagellate Resting Cysts. Microorganisms 2024, 12, 2015. https://doi.org/10.3390/microorganisms12102015
Rodríguez-Villegas C, Pérez-Santos I, Díaz PA, Baldrich ÁM, Lee MR, Saldías GS, Mancilla-Gutiérrez G, Urrutia C, Navarro CR, Varela DA, et al. Deep Turbulence as a Novel Main Driver for Multi-Specific Toxic Algal Blooms: The Case of an Anoxic and Heavy Metal-Polluted Submarine Canyon That Harbors Toxic Dinoflagellate Resting Cysts. Microorganisms. 2024; 12(10):2015. https://doi.org/10.3390/microorganisms12102015
Chicago/Turabian StyleRodríguez-Villegas, Camilo, Iván Pérez-Santos, Patricio A. Díaz, Ángela M. Baldrich, Matthew R. Lee, Gonzalo S. Saldías, Guido Mancilla-Gutiérrez, Cynthia Urrutia, Claudio R. Navarro, Daniel A. Varela, and et al. 2024. "Deep Turbulence as a Novel Main Driver for Multi-Specific Toxic Algal Blooms: The Case of an Anoxic and Heavy Metal-Polluted Submarine Canyon That Harbors Toxic Dinoflagellate Resting Cysts" Microorganisms 12, no. 10: 2015. https://doi.org/10.3390/microorganisms12102015
APA StyleRodríguez-Villegas, C., Pérez-Santos, I., Díaz, P. A., Baldrich, Á. M., Lee, M. R., Saldías, G. S., Mancilla-Gutiérrez, G., Urrutia, C., Navarro, C. R., Varela, D. A., Ross, L., & Figueroa, R. I. (2024). Deep Turbulence as a Novel Main Driver for Multi-Specific Toxic Algal Blooms: The Case of an Anoxic and Heavy Metal-Polluted Submarine Canyon That Harbors Toxic Dinoflagellate Resting Cysts. Microorganisms, 12(10), 2015. https://doi.org/10.3390/microorganisms12102015









